Health-related quality of life, work productivity, and other patient-reported outcomes are reported by patients with hepatitis C virus (HCV) genotypes 2 and 3 during treatment with sofosbuvir and ribavirin or sofosbuvir/velpatasvir without ribavirin, and after clearance of HCV infection.
Keywords: health-related quality of life, fatigue, work productivity, hepatitis C, direct-acting antivirals
Abstract
Background. Until recently, the approved treatment regimens for patients with hepatitis C virus (HCV) genotypes (GTs) 2 and 3 contain sofosbuvir (SOF) and ribavirin (RBV) for 12 or 24 weeks. The impact of RBV-free pan-genotypic regimen with SOF and velpatasvir (SOF/VEL) on patient-reported outcomes (PROs) of patients with genotype 2 and 3 has not been described.
Methods. PROs data were collected from participants of ASTRAL-2 and ASTRAL-3 studies before, during, and after treatment using 4 PRO instruments (Short Form-36, Chronic Liver Disease Questionnaire-HCV, Functional Assessment of Chronic Illness Therapy-Fatigue, and Work Productivity and Activity Index: Specific Health Problem), and compared between the SOF/VEL and SOF + RBV groups.
Results. A total of 818 HCV patients were included: 78% treatment naive, 25% cirrhosis. The rates of nearly all adverse events were lower in the RBV-free SOF/VEL group (all P < .03). The SOF/VEL group also experienced improvement of their PROs by treatment week 4 (+1.8% on average across all PROs), which continued throughout treatment (+4.1%) and post-treatment (+5.5%). In contrast, those in the SOF + RBV group had a modest decline in their PROs starting at treatment week 4 (up to −3.7%), which lasted until the end of treatment (up to −6.4%). In multiple regression analysis, the association of a treatment regimen with end-of-treatment PROs was significant for nearly all PROs; the average beta was +5.0% for the use of SOF/VEL (reference: SOF + RBV).
Conclusions. Patients receiving ribavirin-free SOF/VEL reported significantly better PRO scores during treatment compared with those receiving the RBV-containing regimen. Furthermore, the interferon- and ribavirin-free SOF/VEL regimen resulted in a rapid improvement of PROs in HCV GTs 2 and 3 patients during treatment and after achieving sustained virologic response.
Since 2013, treatment of chronic hepatitis C has been revolutionized with the use of new direct-acting antiviral agents (DAAs) [1–13]. In addition, a number of reports have suggested high effectiveness rates in real-world practices, while others have highlighted significant improvement in patient-reported outcomes (PROs) during treatment and after achieving a sustained virologic response (SVR) [14–34]. However, most of these interferon (IFN)-free and ribavirin (RBV)-free regimens originated from the clinical trials of hepatitis C virus (HCV) patients with genotype (GT) 1. In fact, there is little published data about the efficacy and PRO data for the use of IFN- and RBV-free regimens in patients with HCV GTs 2 and 3. Therefore, the purpose of this study was to investigate and determine the impact of sofosbuvir and velpatasvir (SOF/VEL) treatment on patients with HCV GTs 2 and 3.
METHODS
For this study, we used PRO data collected from patients participating in ASTRAL-2 and ASTRAL-3 studies [12]. The 2 open-label studies were similarly designed as multicenter multinational phase 3 clinical trials of SOF/VEL. In ASTRAL-2, HCV GT 2 patients were randomized 1:1 to receive either a fixed-dose combination of SOF and VEL or SOF plus weight-based RBV, once daily for 12 weeks. In ASTRAL-3, patients with HCV GT 3 were similarly randomized 1:1 to receive SOF/VEL for 12 weeks or SOF + RBV for 24 weeks. In both trials, eligibility criteria included being aged ≥18 years, otherwise healthy (which means no clinically significant illness other than chronic HCV infection), and not having hepatitis B virus or human immunodeficiency virus coinfection; patients with compensated cirrhosis and/or a history of unsuccessful IFN-based HCV treatment were eligible. ASTRAL-2 was conducted in the United States only, while ASTRAL-3 also included patients enrolled in Canada, Australia, New Zealand, Germany, France, Great Britain, and Italy. Other details of study design, safety, and efficacy results of both trials have been published [12].
Using extensive medical history collected at screening for all enrolled participants for the purpose of this study, we identified patients who reported having a history of depression or mood disorders, fatigue or asthenia, anxiety or panic disorders, insomnia or sleep disorders, and type 2 diabetes or hyperglycemia. Treatment-related adverse events recorded by the investigators during the study were grouped based on the body system or organ class as previously described [27].
Patient-Reported Outcomes
PROs were systematically collected as exploratory endpoints in both ASTRAL-2 and ASTRAL-3. Specifically, participants of both trials self-administered 4 PRO instruments: the short-form-36 (SF-36), the functional assessment of chronic illness therapy-fatigue (FACIT-F), the chronic liver disease questionnaire-HCV version (CLDQ-HCV), and the work productivity activity index:specific health problem (WPAI:SHP) [35–38] in their native languages. All PRO instruments were administered at baseline (the first day of treatment), at treatment weeks 4, 8, 12 (24 where applicable) visits, and at weeks 4 and 12 follow-up visits. Patients who achieved SVR-12 (undetectable HCV RNA at follow-up week 12 visit) were also invited to a week 24 follow-up visit.
Combined together, the 4 PRO instruments used in this study measured 20 domain and 5 summary PROs where the summary PROs are linear combinations of independently calculated domain PRO scores. The aspects of patients' well-being and health-related quality of life covered by the studied PROs include physical health, bodily pain, fatigue, mental and emotional health, social well-being, worry, and work productivity [35–38]. In all instruments, higher scores would reflect a better health status, except for the work productivity and activity domains of WPAI:SHP where a higher impairment score would indicate a poorer health status. Where stated explicitly, for presentation purposes, we transformed all PROs from their original scales to a universal 0–100 scale as previously described [25–28].
Statistical Analyses
Patients receiving SOF + RBV were included in 1 group regardless of treatment duration and were compared with patients receiving RBV-free SOF/VEL. Clinico-demographic parameters, baseline, end-of-treatment, and post-treatment PROs were compared between the 2 groups using a Pearson χ2 test for independence or a Wilcoxon nonparametric test; the latter was used for continuous and pseudo-continuous variables such as PRO scores. Also, at all study time points, we calculated the changes (decrements or improvements) in the PRO scores with reference to patients' own baseline levels and used a Wilcoxon sign rank test for matched pairs to identify significant changes. Only P values ≤.05 were considered potentially statistically significant.
The association of the treatment regimen (SOF/VEL as opposed to SOF + RBV) with PRO scores was assessed using mixed repeated-measures regression models. These models included time and treatment regimen as interacted fixed effects, and patient identification as a random effect and were adjusted for baseline PRO scores and for demographic and clinical PRO predictors. Those clinico-demographic PRO predictors adjusted for in the regression models were location (United States vs non-United States), age, gender, being treatment naive, history of psychiatric diseases (as defined above), compensated cirrhosis, HCV GT, obesity and type 2 diabetes, and having achieved SVR.
All analyses were run using SAS 9.3 (SAS Institute, Cary, North Carolina). Each site's institutional review board approved the study.
RESULTS
In ASTRAL-2, 134 HCV GT 2 patients received SOF/VEL and 132 received SOF + RBV for 12 weeks. In ASTRAL-3, 277 GT 3 patients received SOF/VEL for 12 weeks and 275 patients received SOF + RBV for 24 weeks. The SVR-12 rates were as follows: SOF/VEL in HCV genotype 2–99.3%; SOF/VEL in HCV genotype 3–95.3%; SOF + RBV in HCV genotype 2–93.9%; and SOF + RBV in HCV genotype 3–80.7%.
Baseline demographic parameters and relevant elements of medical history for the study cohort are shown in Table 1. Demographic characteristics were balanced between the SOF + RBV and SOF/VEL treatment groups (Table 1) with exception of race; patients assigned to receive SOF + RBV were less frequently white and more frequently Asian (P = .02). The baseline PRO scores of HCV patients from the 2 study arms were also similar (all P > .015; Supplementary Table 1).
Table 1.
Demographics and Baseline Medical History of ASTRAL-2 and ASTRAL-3 Participants
| Parameter | SOF + RBV (%) | SOF/VEL (%) | P Value |
|---|---|---|---|
| N | 407 | 411 | |
| Age, y | 52.5 ± 10.3 | 51.8 ± 11.0 | .36 |
| Male gender | 246 (60.4) | 256 (62.3) | .59 |
| Race White |
350 (86.0) | 374 (91.0) | .0249 |
| African-American | 13 (3.2) | 9 (2.2) | .37 |
| Asian | 34 (8.4) | 24 (5.8) | .16 |
| Enrolled in the United States | 190 (46.7) | 188 (45.7) | .79 |
| Employed at baseline | 215 (59.1) | 229 (61.2) | .55 |
| Hemoglobin, g/dL | 14.9 ± 1.4 | 14.9 ± 1.3 | .89 |
| Treatment naive | 316 (77.6) | 321 (78.1) | .87 |
| Cirrhosis | 102 (25.4) | 99 (24.1) | .66 |
| HCV genotype 2 | 132 (32.4) | 134 (32.6) | .96 |
| HCV genotype 3 | 275 (67.6) | 277 (67.4) | .96 |
| ALT > 1.5 × ULN | 238 (58.5) | 236 (57.4) | .76 |
| HCV RNA > 6 log 10/mL | 282 (69.3) | 286 (69.6) | .93 |
| History of: | |||
| Anxiety or panic disorders | 60 (14.7) | 73 (17.8) | .24 |
| Depression | 95 (23.3) | 116 (28.2) | .11 |
| Clinically overt fatigue | 63 (15.5) | 59 (14.4) | .65 |
| Sleep disorders | 80 (19.7) | 70 (17.0) | .33 |
| Type 2 diabetes or hyperglycemia | 37 (9.1) | 28 (6.8) | .23 |
| Body mass index, kg/m2 | 27.4 ± 6.0 | 26.9 ± 5.1 | .41 |
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus; RBV, ribavirin; SOF, sofosbuvir; ULN, upper limit of the norm; VEL, velpatasvir.
Treatment-related adverse events are summarized in Table 2. As shown, the rates of nearly all adverse events were significantly higher in the SOF + RBV arm (all P < .03) with the only exception of flu-like symptoms, which were infrequent (<2%–3%) in both treatment arms. The excessive rates of adverse events in the SOF + RBV group in comparison to the RBV-free SOF/VEL group ranged from 5% more patients having musculoskeletal symptoms (which were primarily muscle and joint pain) to approximately 16% more patients having treatment-related fatigue and psychiatric symptoms (primarily sleep disorders followed by anxiety, irritability, and mood swings). No treatment-related adverse events were experienced by 28.7% of patients during treatment with SOF + RBV and 47.7% with SOF/VEL (P < .0001; Table 2).
Table 2.
Treatment-related Adverse Events in ASTRAL-2 and ASTRAL-3
| Adverse Event | SOF + RBV (%) | SOF/VEL (%) | P Value |
|---|---|---|---|
| Blood related or anemia | 33 (8.1) | 1 (0.2) | <.0001 |
| Fatigue or asthenia | 143 (35.1) | 80 (19.5) | <.0001 |
| Flu-like symptoms | 10 (2.5) | 6 (1.5) | .30 |
| Gastrointestinal symptoms | 107 (26.3) | 81 (19.7) | .0253 |
| Musculoskeletal symptoms | 50 (12.3) | 30 (7.3) | .0164 |
| Nervous system symptoms | 138 (33.9) | 100 (24.3) | .0026 |
| Psychiatric symptoms | 137 (33.7) | 72 (17.5) | <.0001 |
| Skin-related symptoms | 80 (19.7) | 34 (8.3) | <.0001 |
| Other adverse events | 105 (25.8) | 54 (13.1) | <.0001 |
| No adverse events | 117 (28.7) | 196 (47.7) | <.0001 |
Abbreviations: RBV, ribavirin; SOF, sofosbuvir; VEL, velpatasvir.
At the study time points, between 79% and 90% of patients completed their PRO questionnaires. Also, at the post-SVR week 24 visit, PRO data were available for 74% of patients who achieved SVR.
Patient-Reported Outcomes During Treatment with SOF/VEL and SOF+RBV
Four weeks after treatment initiation, improvements in some PROs were noted in patients receiving SOF/VEL; in particular, improvements in bodily pain, general health, and physical component summary scores of SF-36; physical and emotional well-being, fatigue scale, and total scores of FACIT-F; and in all domains of CLDQ-HCV (from +1.8 to +8.7 on a universal 0–100 PRO scale) were statistically significant (P < .02 for 12 of 25 studied PROs; Supplementary Figure 1A). Furthermore, no statistically significant decrements in any PROs were observed among patients receiving RBV-free SOF/VEL at this point or at any later time point during treatment or in follow-up. On the other hand, in the SOF + RBV arm at the same time point, improvements in a few PRO scores (+1.7 to +9.2 in bodily pain of SF-36; emotional well-being of FACIT-F; emotional health, worry, and total CLDQ-HCV) were accompanied by decrements in others (−1.4 to −3.7 in physical functioning and role physical of SF-36, as well as work productivity and its absenteeism component; all P < .05; Supplementary Figure 1A).
By treatment week 8, both trends continued (Supplementary Figure 1B). In particular, significant improvements from baseline in patients treated with SOF/VEL were observed in all the PRO scores described above, and the average magnitude of improvement across these PROs, which improved significantly (P < .05 when compared with baseline), increased from +3.3 at treatment week 4 to +3.8 points at treatment week 8. In contrast, at this time point, in addition to the PRO decrements described above, the decrements in social functioning and role emotional of SF-36 and in physical and functional well-being of FACIT-F became statistically significant, and the average decrement magnitude also increased from −2.6 to −3.4.
By the end of 12 weeks of treatment with SOF/VEL, significant improvements in nearly all PROs were observed. In fact, the only 3 exceptions were social functioning and role emotional of SF-36 and absenteeism of WPAI:SHP, which remained at their baseline levels (all P > .05). The average magnitude of improvement ranged from +1.7 to +13.0 (P < .015). On the other hand, the PRO changes from patients' own baseline levels in the SOF + RBV arm remained similar to those at the previously described time points (Figure 1 for summary PROs, Supplementary Figure 2 for all studied PROs) and included improvements in bodily pain and general health of SF-36, emotional and social well-being of FACIT-F; worry, systemic and total of CLDQ-HCV (+1.5 to +11.9), accompanied by decrements in role physical, social functioning, role emotional, and mental component summary of SF-36; and total work productivity and absenteeism of WPAI:SHP (−3.0 to −6.4; all P < .05). Notably, at the last day of treatment, there was no difference in PROs between those who completed a 12- and a 24-week-long SOF + RBV regimen (all P > .10).
Figure 1.
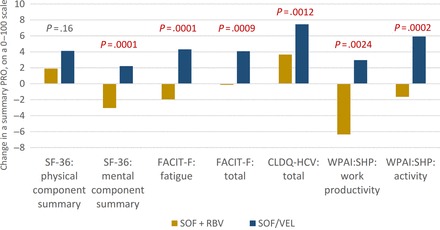
End-of-treatment changes in summary patient-reported outcomes (PROs) from patients' own baseline levels in patients treated with sofosbuvir (SOF)/velpatasvir and SOF + ribavirin regardless of duration. P values reflect differences between the 2 treatment groups. Changes exceeding 2 points were also statistically significant (P < .05) when compared with zero change (ie, no change from patient's own baseline PRO level). Abbreviations: CLDQ-HCV, chronic liver disease questionnaire-hepatitis C virus version; FACIT-F, functional assessment of chronic illness therapy-fatigue; PRO, patient-reported outcome; RBV, ribavirin; SF-36, short-form-36; SOF, sofosbuvir; VEL, velpatasvir; WPAI:SHP, work productivity activity index:specific health problem.
Despite moderate PRO decrements at the end of treatment with SOF + RBV, by post-treatment week 4, the only significant decrement from patients' own baseline level in that treatment arm was in the absenteeism component of work productivity (−4.6, P = .0065), while improvements in 14 of 25 studied PROs were statistically significant (from +2.9 to +12.7, all P < .005; Figure 2). At the same time point, significant improvements from the baseline levels in all but 3 PROs were again observed in patients who completed the SOF/VEL treatment (from +3.1 to +13.7, all P < .05). In that treatment arm, the average magnitude of improvement across all PROs also increased from +4.5 points at the end of treatment to +5.1 at post-treatment week 4 (Figure 2).
Figure 2.
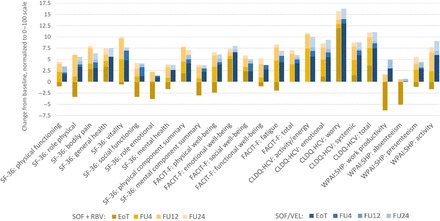
Post-treatment incremental changes in patient-reported outcomes (PROs) in patients treated with sofosbuvir (SOF) + ribavirin and SOF/velpatasvir . At end of treatment, P < .05 between the 2 regimens for all but 6 PROs (SF-36: bodily pain, general health, physical component summary; FACIT-F: emotional well-being, social well-being; CLDQ-HCV: worry). At post-treatment follow-up (FU)4, P < .05 for only 3 PROs (SF-36: mental component summary; FACIT-F: functional well-being; CLDQ-HCV: systemic). At FU12 and FU24, all P > .05 between the 2 regimens. Abbreviations: CLDQ-HCV, chronic liver disease questionnaire-hepatitis C virus version; EoT, end of treatment; FACIT-F, functional assessment of chronic illness therapy-fatigue; FU, post-treatment follow-up; RBV, ribavirin; SF-36, short-form-36; SOF, sofosbuvir; VEL, velpatasvir; WPAI:SHP, work productivity activity index:specific health problem.
At post-treatment week 12, changes from patients' own baseline levels were no longer different between the 2 treatment arms (all P > .05; Figure 2) and included significant improvements in all studied PROs except for the role emotional domain of SF-36 and the total work productivity score and its absenteeism component included in WPAI:SHP (P > .05). This trend also persisted throughout post-treatment week 24 (Figure 2), but the average magnitude of improvement from baseline increased from +5.8 at post-treatment week 12 to +6.9 at post-treatment week 24 (P < .05).
In multiple regression analysis, the association of a treatment regimen with end-of-treatment PROs was highly significant for all but 6 PROs (P > .05 for bodily pain, general health, and physical component summary of SF-36; emotional and social well-being of FACIT-F; and worry of CLDQ-HCV; Figure 3). The average magnitude of such association was +5.0 points to end-of-treatment PROs for the use of the SOF/VEL regimen in comparison to the SOF + RBV regimen, when all other clinico-demographic predictors were held equal. Other predictors of higher PRO scores before, during, and after treatment were similar to those reported previously for patients with HCV [16–20, 22, 23, 25–28, 30, 31, 33] and included younger age, male gender, US location, lower body mass index, the absence of psychiatric comorbidities, fatigue, and cirrhosis (all P < .05). There was no association of treatment duration with PROs in those who received the SOF + RBV regimen (P > .05). In the regression model, which included regimen-with-predictor interaction terms, the treatment regimen significantly interacted with cirrhosis for some PROs (resulting in the effect of the regimen being less pronounced in cirrhotic patients) but not with HCV GT (Supplementary Figure 3).
Figure 3.
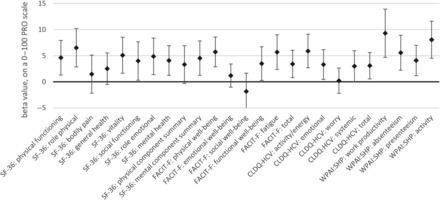
Independent association of the use of an ribavirin-free regimen (the reference regimen: sofosbuvir + ribavirin) with the end-of-treatment patient-reported outcomes (PROs) after adjustment for clinical and demographic PRO predictors. Multiple regression beta values are shown with their 95% confidence intervals. P < .05 for all PROs except for 6 PROs (SF-36: bodily pain, general health, physical component summary; FACIT-F: emotional well-being, social well-being; CLDQ-HCV: worry). Abbreviations: CLDQ-HCV, chronic liver disease questionnaire-hepatitis C virus version; FACIT-F, functional assessment of chronic illness therapy-fatigue; PRO, patient-reported outcome; SF-36, short-form-36; WPAI:SHP, work productivity activity index:specific health problem.
DISCUSSION
The purpose of this study was to assess the impact of the new IFN- and RBV-free SOF/VEL regimen on PROs among HCV GT2 and GT3 patients who were treated as a part of ASTRAL-2 and -3 clinical trials. In these studies, the majority of enrolled patients were infected with HCV GT3 who are known to be more difficult to treat in terms of both generally lower SVR rates and greater rates of hepatic complications as well as some extrahepatic manifestations of HCV (such as HCV-induced insulin resistance, steatosis, and fibrosis) [1, 4, 12, 39–41]. It is important to note that despite this, in contrast to differences in clinical outcomes between GT2 and GT3, HCV GT does not seem to impact PROs [28, 31].
Our results show that at baseline, there were no differences in demographics and PROs across all 4 questionnaires between the 2 treatment groups. However, after treatment began, the PRO scores decreased in the SOF + RBV group. Although most of these reductions in PRO scores were modest, some exceeded the minimal clinically important difference (MCID), which is commonly believed to be in the 3%–5% range for a PRO [42, 43]; this difference also roughly coincides with an impairment in PRO scores attributed to having compensated cirrhosis compared with the absence of cirrhosis [20]. This decrease in the PRO scores also persisted throughout treatment, although for the 24-week-long SOF + RBV group, there was no further decline in the scores past 12 weeks into treatment.
The most likely explanation for the reported decline in PRO scores may be related to known side effects associated with the use of RBV. Indeed, 35% of the patients receiving the RBV-containing regimen experienced treatment-related fatigue and more than 30% experienced neuro-psychiatric symptoms; both rates were significantly higher in comparison with the studied RBV-free regimen. Despite this, as soon as treatment with RBV-containing regimens was completed, all PRO scores began to improve. Furthermore, the post-treatment PRO scores were significantly higher than patients' own baseline levels regardless of the regimen used to achieve SVR. This observation suggests that the impact associated with RBV on PROs is moderate and often resolves quickly. This is in contrast to previous reports that have shown that the impact of IFN on PROs could be profound and long lasting [31].
Our data show that treatment with the RBV-free regimen (SOF/VEL) was associated with an improvement in most PRO scores as early as 4 weeks into treatment. Furthermore, these early improvements exceeded the above-mentioned MCID, which would make these changes in PRO scores not only statistically significant but also clinically meaningful. In fact, MCID is defined as “the smallest difference in score in the domain of interest which patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient's management” [43]. It is also important to note that throughout treatment and up to the last point of follow-up at 24 weeks post-treatment, all PRO scores continue to increase substantially, except for only the absenteeism component of WPAI:SHP, which did not differ much from baseline.
Study results indicate that SOF/VEL is not only highly effective (with SVR12 rates of 99% for GT2 and 95% for GT3) but is also associated with an immediate improvement in quality of life, including among patients with GT3 infection and those with cirrhosis. Our data add to the evidence that indicates that SOF-based DAA regimens are well tolerated and, at the same time, improve PROs [13, 17, 19, 31]. This may help clinicians and patients to manage treatment expectations by identifying at what point a gradual improvement in PROs may be expected and how long the PRO improvement may continue, which potentially may signal an improvement in hepatic function [16, 20, 44]. However, it is also important to note that achievement of SVR does not necessarily make an HCV patient “healthy.” Rather, it is possible that some patients who cleared their HCV infection can still experience clinical and fatigue-related issues that would require monitoring and potential intervention.
It is important to note that our results did not show improvement in the absenteeism component of work productivity over time despite patients reporting that all other areas of their PROs improved. In fact, improvement of absenteeism can be associated with significant economic gains [26, 45]. It is possible that the absenteeism component of work productivity may be affected by the extrahepatic manifestations of HCV or socioeconomic factors that may take longer to improve. This is certainly an area in need of further exploration, as more and more patients are being treated with the newer DAAs.
The main study limitation is based on the fact that all patients were participants of clinical trials without major nonhepatic comorbidities, were primarily white, and all had an opportunity for close monitoring throughout treatment and in follow-up. Thus, it is critical that our results are confirmed in studies performed in the real-world setting of clinical practices. Another limitation of the study is its open-label design, which could have biased emotional health-related PROs in patients receiving RBV who could be aware of its side effects. Additionally, due to small sample size, we were unable to study the effect of virologic failure on reported PROs.
In conclusion, the use of SOF/VEL has been shown to be successful in improving PROs in addition to its reported high cure rate. These PRO improvements occur early into treatment and are sustained for months after treatment completion. Although these findings are consistent with prior reports of PROs before, during, and after HCV treatment, this is the first study to assess the impact of IFN- and RBV-free regimens upon PROs of this patient population. Given a generally favorable PRO profile accompanied by high efficacy, we suggest that that this regimen is a treatment option that should be offered in this hard-to-treat population.
Supplementary Material
Notes
Author contributions. Z. Y.: study concept and design; critical revision of the manuscript for important intellectual content; obtained funding; study supervision. M. S.: analysis and interpretation of data; drafting of the manuscript. M. S.: acquisition of data; critical revision of the manuscript for important intellectual content. G. R. F.: acquisition of data; critical revision of the manuscript for important intellectual content. N. R.: acquisition of data; critical revision of the manuscript for important intellectual content. A. M.: acquisition of data; critical revision of the manuscript for important intellectual content. K. P.: acquisition of data; critical revision of the manuscript for important intellectual content. N. B.: acquisition of data; critical revision of the manuscript for important intellectual content. S. K. R.: acquisition of data; critical revision of the manuscript for important intellectual content. N. A.: acquisition of data; critical revision of the manuscript for important intellectual content. F. N.: acquisition of data; critical revision of the manuscript for important intellectual content. L. H.: drafting of the manuscript. S. H.:- acquisition of data; critical revision of the manuscript for important intellectual content.
Financial support. M. S., G. F., N. R., A. M., K. P., N. B., S. K. R., and N. A. have received research support and/or on advisory board or consultant to Gilead Sciences.
Potential conflict of interest. Z. M. Y. is a consultant and/or advisory board member for Abbvie, Intercept, Gilead Sciences, Salix, GlaxoSmithKline, BMS, and Janssen. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jacobson IM, Gordon SC, Kowdley KV et al. . Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013; 368:1867–77. [DOI] [PubMed] [Google Scholar]
- 2. Afdhal N, Zeuzem S, Kwo P et al. . Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–98. [DOI] [PubMed] [Google Scholar]
- 3. Afdhal N, Reddy KR, Nelson DR et al. . Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014; 370:1483–93. [DOI] [PubMed] [Google Scholar]
- 4. Zeuzem S, Dusheiko GM, Salupere R et al. . Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014; 370:1993–2001. [DOI] [PubMed] [Google Scholar]
- 5. Kowdley KV, Gordon SC, Reddy KR et al. . Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370:1879–88. [DOI] [PubMed] [Google Scholar]
- 6. Andreone P, Colombo MG, Enejosa JV et al. . ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology 2014; 147:359–65. (PEARL II). [DOI] [PubMed] [Google Scholar]
- 7. Poordad F, Hezode C, Trinh R et al. . ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014; 370:1973–82. (TURQUOISE II). [DOI] [PubMed] [Google Scholar]
- 8. Zeuzem S, Jacobson IM, Baykal T et al. . Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014; 370:1604–14. (SAPPHIRE II). [DOI] [PubMed] [Google Scholar]
- 9. Smith MA, Regal RE, Mohammad RA. Daclatasvir: A NS5A replication complex inhibitor for hepatitis C infection. Ann Pharmacother 2016; 50:39–46. [DOI] [PubMed] [Google Scholar]
- 10. Nelson DR, Cooper JN, Lalezari JP et al. . ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015; 61:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lawitz E, Sulkowski MS, Ghalib R et al. . Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 2014; 384:1756–65. [DOI] [PubMed] [Google Scholar]
- 12. Foster GR, Afdhal N, Roberts SK et al. . Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015; 373:2608–17. [DOI] [PubMed] [Google Scholar]
- 13. Lam B, Henry L, Younossi Z. Sofosbuvir (Sovaldi) for the treatment of hepatitis C. Expert Rev Clin Pharmacol 2014; 7:555–66. [DOI] [PubMed] [Google Scholar]
- 14. Cheetham TC, Niu F, Chiang K et al. . Factors associated with failure to achieve SVR in hepatitis C genotype 3 patients within an integrated care delivery system. J Manag Care Spec Pharm 2015; 21:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Effectiveness of sofosbuvir-based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. Veterans. Aliment Pharmacol Ther 2015; 42:559–73. [DOI] [PubMed] [Google Scholar]
- 16. Younossi ZM, Stepanova M, Afdhal N et al. . Improvement of health-related quality of life and work productivity in chronic hepatitis C patients with early and advanced fibrosis treated with ledipasvir and sofosbuvir. J Hepatol 2015; 63:337–45. [DOI] [PubMed] [Google Scholar]
- 17. Younossi Z, Henry L. Systematic review: patient-reported outcomes in chronic hepatitis C—the impact of liver disease and new treatment regimens. Aliment Pharmacol Ther 2015; 41:497–520. [DOI] [PubMed] [Google Scholar]
- 18. Younossi ZM, Stepanova M, Sulkowski M et al. . Sofosbuvir and ribavirin for treatment of chronic hepatitis C in patients coinfected with hepatitis C virus and HIV: the impact on patient-reported outcomes. J Infect Dis 2015; 212:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Younossi Z, Henry L. The impact of the new antiviral regimens on patient reported outcomes and health economics of patients with chronic hepatitis C. Dig Liver Dis 2014; 46(suppl 5):S186–96. [DOI] [PubMed] [Google Scholar]
- 20. Younossi ZM, Stepanova M, Nader F et al. . Patient-reported outcomes in chronic hepatitis C patients with cirrhosis treated with sofosbuvir-containing regimens. Hepatology 2014; 59:2161–9. [DOI] [PubMed] [Google Scholar]
- 21. Vera-Llonch M, Martin M, Aggarwal J et al. . Health-related quality of life in genotype 1 treatment-naïve chronic hepatitis C patients receiving telaprevir combination treatment in the ADVANCE study. Aliment Pharmacol Ther 2013; 38:124–33. [DOI] [PubMed] [Google Scholar]
- 22. Younossi ZM, Stepanova M, Henry L et al. . Minimal impact of sofosbuvir and ribavirin on health related quality of life in chronic hepatitis C (CH-C). J Hepatol 2014; 60:741–7. [DOI] [PubMed] [Google Scholar]
- 23. Younossi ZM, Stepanova M, Henry L et al. . Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin Gastroenterol Hepatol 2014; 12:1349–59.e13. [DOI] [PubMed] [Google Scholar]
- 24. Gerber L, Estep M, Stepanova M, Escheik C, Weinstein A, Younossi ZM. Effects of viral eradication with ledipasvir and sofosbuvir, with or without ribavirin, on measures of fatigue in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol 2016; 14:156–64.e3. [DOI] [PubMed] [Google Scholar]
- 25. Younossi ZM, Stepanova M, Pol S, Bronowicki JP, Carrieri MP, Bourlière M. The impact of ledipasvir/sofosbuvir on patient-reported outcomes in cirrhotic patients with chronic hepatitis C: the SIRIUS study. Liver Int 2016; 36:42–8. [DOI] [PubMed] [Google Scholar]
- 26. Younossi ZM, Stepanova M, Marcellin P et al. . Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: results from the ION-1, -2, and -3 clinical trials. Hepatology 2015; 61:1798–808. [DOI] [PubMed] [Google Scholar]
- 27. Younossi ZM, Stepanova M, Zeuzem S et al. . Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. J Hepatol 2014; 61:228–34. [DOI] [PubMed] [Google Scholar]
- 28. Younossi Z, Stepanova M, Henry L, Nader F, Hunt S. An in-depth analysis of patient-reported outcomes in patients with chronic hepatitis C treated with different anti-viral regimens. Am J Gastroenterol 2016; 111:808–16. [DOI] [PubMed] [Google Scholar]
- 29. Aggarwal J, Vera-Llonch M, Donepudi M, Suthoff E, Younossi Z, Goss TF. Work productivity among treatment-naïve patients with genotype 1 chronic hepatitis C infection receiving telaprevir combination treatment. J Viral Hepat 2015; 22:8–17. [DOI] [PubMed] [Google Scholar]
- 30. Younossi Z, Stepanova M, Nader F, Henry L. Patient-reported outcomes in elderly patients with chronic hepatitis C treated with interferon- and ribavirin-free regimens. J Am Geriatr Soc 2016; 64:386–93. [DOI] [PubMed] [Google Scholar]
- 31. Younossi ZM, Stepanova M, Nader F, Lam B, Hunt S. The patient's journey with chronic hepatitis C from interferon plus ribavirin to interferon- and ribavirin-free regimens: a study of health-related quality of life. Aliment Pharmacol Ther 2015; 42:286–95. [DOI] [PubMed] [Google Scholar]
- 32. Baran RW, Samp JC, Walker DR et al. . Costs and absence of HCV-infected employees by disease stage. J Med Econ 2015; 18:691–703. [DOI] [PubMed] [Google Scholar]
- 33. Younossi ZM, Stepanova M, Feld J et al. . Sofosbuvir/velpatasvir improves patient-reported outcomes in HCV patients: results from Astral-1 placebo-controlled trial. J Hepatol 2016; 65:33–9. [DOI] [PubMed] [Google Scholar]
- 34. Younossi ZM, Stepanova M, Charlton M et al. . Patient-reported outcomes with sofosbuvir and velpatasvir with or without ribavirin for hepatitis C virus-related decompensated cirrhosis: an exploratory analysis from the randomised open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol Hepatol 2016; 10.1016/S2468-1253(16)30020-6. [DOI] [PubMed] [Google Scholar]
- 35. Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res 2001; 10:405–13. [DOI] [PubMed] [Google Scholar]
- 36. Webster K, Odom L, Peterman A, Lent L, Cella D. The functional assessment of chronic illness therapy (FACIT) measurement system: validation of version 4 of the core questionnaire. Qual Life Res 1999; 8:604. [Google Scholar]
- 37. Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 1999; 45:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmaco Economics 1993; 4:353–65. [DOI] [PubMed] [Google Scholar]
- 39. Negro F. Steatosis and insulin resistance in response to treatment of chronic hepatitis C. J Viral Hepat 2012; 19(suppl 1):42–7. [DOI] [PubMed] [Google Scholar]
- 40. Bochud PY, Cai T, Overbeck K et al. . Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol 2009; 51:655–66. [DOI] [PubMed] [Google Scholar]
- 41. Abenavoli L, Masarone M, Peta V et al. . Insulin resistance and liver steatosis in chronic hepatitis C infection genotype 3. World J Gastroenterol 2014; 20:15233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spiegel BM, Younossi ZM, Hays RD, Revicki D, Robbins S, Kanwal F. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology 2005; 41:790–800. [DOI] [PubMed] [Google Scholar]
- 43. Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF-6D. Health Qual Life Outcomes 2003; 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Teuber G, Schäfer A, Rimpel J et al. . Deterioration of health-related quality of life and fatigue in patients with chronic hepatitis C: association with demographic factors, inflammatory activity, and degree of fibrosis. J Hepatol 2008; 49:923–9. [DOI] [PubMed] [Google Scholar]
- 45. Younossi Z, Brown A, Buti M et al. . Impact of eradicating hepatitis C virus on the work productivity of chronic hepatitis C (CH-C) patients: an economic model from five European countries. J Viral Hepat 2016; 23:217–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


