Abstract
We examined the impact of the Pediatric Infectious Diseases Society/Infectious Diseases Society of America guidelines that recommend ampicillin or amoxicillin for children hospitalized with community-acquired pneumonia. Prescribing of ampicillin/amoxicillin increased following guideline publication, but remains low. Cephalosporin and macrolide prescribing decreased but remains common. Further studies exploring outcomes of and reasons for compliance with guidelines are warranted.
Keywords: community-acquired pneumonia, antibiotics, guidelines, pediatrics
Pediatric community-acquired pneumonia (CAP) accounts for >500 000 emergency department visits and 7% of pediatric hospitalizations in the United States annually [1, 2]. Pneumonia accounts for more days of antimicrobial therapy than any other indication for admission to US children's hospitals [3].
In October 2011, the Pediatric Infectious Diseases Society (PIDS) and the Infectious Diseases Society of America (IDSA) published guidelines for the management of CAP in children [4]. Because Streptococcus pneumoniae is the most common bacterial cause of pediatric CAP and narrow-spectrum β-lactam antibiotics effectively target S. pneumoniae, these guidelines recommended penicillin or ampicillin/amoxicillin as first-line therapy for most children hospitalized with CAP.
Prior to the publication of the guidelines, <10% of hospitalized children with CAP received penicillin or ampicillin/amoxicillin; most children received cephalosporins, and prescribing varied significantly across physicians and institutions [3, 5–7]. The objective of this study was to investigate the impact of the PIDS/IDSA guidelines on antibiotic prescribing for children hospitalized with nonsevere, uncomplicated CAP.
METHODS
Study Design and Data Source
We performed a longitudinal analysis to examine the effect of PIDS/IDSA CAP guideline publication on antibiotic choice for children hospitalized with CAP. Data were obtained from the Pediatric Health Information System (PHIS), a comparative clinical and administrative database that contains information from 43 freestanding US children's hospitals located in 27 states plus the District of Columbia. The analysis was restricted to 38 hospitals after excluding institutions with data quality issues involving service date and prescribing data.
Study Population
We included patients aged 6 months to 18 years discharged between 1 October 2009 and 31 March 2013 with CAP identified using a previously validated algorithm [8]. The algorithm was validated in the PHIS database using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes and had a positive predictive value (PPV) of 89.6% for identifying CAP using a primary diagnosis of pneumonia, excluding patients with complex chronic conditions [9]. To restrict the population to children with nonsevere, uncomplicated pneumonia, we excluded patients with an ICD-9-CM code for effusion or empyema; 2 or more consecutive days of mechanical ventilation and/or vasopressors; or an ICD-9-CM code for colonization, infection, or history of methicillin-resistant Staphylococcus aureus (MRSA) infection.
Outcomes
The primary outcome was the rate of prescribing of guideline-recommended first-line antibiotic therapy, defined as 1 or more days of amoxicillin, ampicillin, or penicillin. Secondary outcomes included the rate of prescribing of cephalosporins and macrolides.
Statistical Analysis
Using a longitudinal piecewise logistic model with a knot at October 2011 (guideline publication date), we modeled the trajectory of prescribing before and after guideline publication [10]. Patients were grouped by month, which was included as a linear variable. We examined potential confounders including age, sex, and insurance status; none of these variables meaningfully changed the model estimates and thus were not included in final models. Standard errors were adjusted for clustering by hospital. For each antibiotic therapy, we obtained the probabilities of prescribing for each month. For macrolides, probabilities were standardized by season because of seasonal variation.
To investigate the effect of the guidelines by hospital, we fit piecewise logistic models for each of 18 hospitals that provided data for at least 75% of the time period and averaged at least 20 patients per month. The marginal analysis results for recommended first-line therapy of these 18 hospitals were similar to the results of the analysis of all 38 hospitals.
Analyses were conducted using Stata version 12.1 (StataCorp, College Station, Texas).
RESULTS
There were 44 133 admissions for children with CAP across 38 hospitals. After excluding children with effusion, empyema, mechanical ventilation, vasopressor use, or MRSA, 38 819 admissions were included.
Guideline-Recommended First-line Therapy
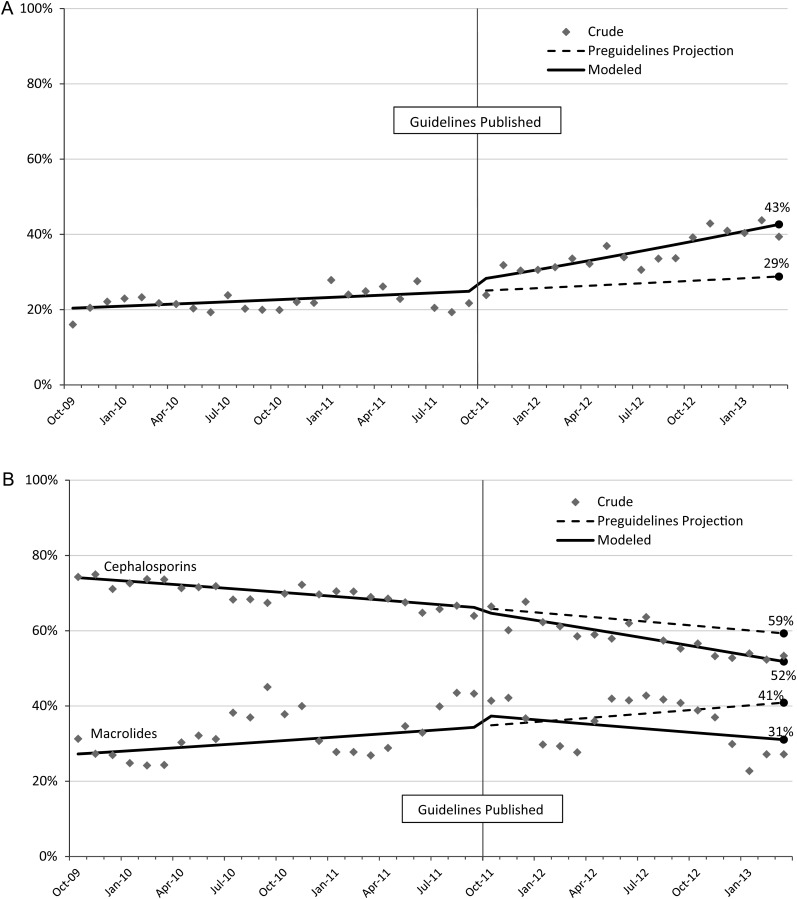
During the 42-month study period, 28% of all hospitalized children received ampicillin or amoxicillin (<0.1% of patients received penicillin). Among patients who received ampicillin or amoxicillin, 78% received it on the first day of antibiotic therapy and 47% did not receive any other antibiotics during their admission. In the 2 years prior to guideline publication, ampicillin/amoxicillin use was low, with a nonsignificant increase over time (P = .068). After guideline publication, there was a significant increase in prescribing (P = .02 comparing preguideline and postguideline trajectories), bringing the overall ampicillin/amoxicillin prescribing rate 18 months after publication to 43% (Figure 1A). If the preguideline trajectory had been maintained, however, the projected rate of prescribing 18 months after publication would have been 29%.
Figure 1.
Crude, modeled, and projected trajectories of rates of prescribing of antibiotics for hospitalized children with community-acquired pneumonia across 38 US children's hospitals, October 2009 to March 2013. A, Guideline-recommended first-line therapy (amoxicillin, ampicillin, or penicillin). B, Cephalosporins and macrolides. Prescribing rates for macrolides have been standardized for season.
Other Antibiotic Therapy
During the 42-month study period, 66% of children received cephalosporins (90% of which were cefotaxime or ceftriaxone), and 32% received macrolides (99% of which were azithromycin). Cephalosporin prescribing decreased significantly over the entire study period (P<.001), but the trajectory was not significantly impacted by guideline publication (P = .12; Figure 1B). Whereas the rate of macrolide prescribing was increasing significantly prior to October 2011 (P < .001), macrolide use declined significantly after guideline publication (P = .003), leading to a prescribing rate 18 months after publication of 31% (Figure 1B). If the preguideline trajectory had been maintained, however, the projected prescribing rate would have been 41% at 18 months after publication.
Hospital-Specific Analysis
Examination of the 18 centers that provided data for at least 75% of the time period and averaged at least 20 patients per month revealed considerable variation in ampicillin/amoxicillin prescribing from the beginning (October 2009: 1%–77%) to the end (March 2013: 5%–77%) of the study period. Initially, only 3 hospitals used ampicillin/amoxicillin for a majority of children, whereas 9 hospitals used ampicillin/amoxicillin for more than half of cases by study's end. Fifteen hospitals increased their use over time, 8 by >25 percentage points. Of the 3 hospitals that decreased use, 2 had high baseline prescribing rates (77% and 76%, dropping to 72% and 74%, respectively).
DISCUSSION
We examined the impact of the PIDS/IDSA guidelines on antibiotic prescribing for children hospitalized with CAP. Following guideline publication, use of recommended first-line therapy, including ampicillin or amoxicillin, increased significantly over time. Eighteen months after guideline release, however, the majority of patients still did not receive ampicillin or amoxicillin. Use of cephalosporins and macrolides remained substantial but was decreasing significantly.
Studies conducted prior to 2011 demonstrated marked variability in antibiotic use for children hospitalized with CAP. This lack of standardization highlighted the need for clinical practice guidelines [3, 7]. Furthermore, >70% of pediatric infectious diseases specialists surveyed recommended cephalosporins for children with uncomplicated CAP [7], consistent with our findings, despite evidence that cephalosporins are not superior to narrower-spectrum β-lactams [5, 11].
Practice guidelines are intended to improve quality of care by encouraging prescribing practice that follows the best evidence available and expert consensus [12, 13]. Guidelines targeting antimicrobial prescribing can be an important tool for reducing unnecessary and inappropriate antibiotic use in the effort to improve clinical outcomes while minimizing the emergence of antimicrobial resistance. Guidelines for the treatment of CAP can be effective in improving quality of care: multiple studies have shown that adherence to IDSA and American Thoracic Society guidelines for treatment of CAP in adults is associated with improved clinical outcomes and reduced costs [14–17]. In the United Kingdom, national clinical guidelines led to significant increases in use of amoxicillin and decreases in length of stay [18].
However, creation and publication of guidelines alone do not ensure uptake of recommendations [19, 20]. We observed significant variability in the adoption of the CAP guidelines by institution, including some institutions where prescribing remained unchanged. Potential barriers to guideline adherence include lack of guideline awareness, clinician attitudes towards standardization, lack of agreement with recommendations, and inertia of previous practice [13].
Implementation of quality improvement initiatives such as institutional clinical pathways can effectively improve adherence to national guidelines and drive behavior change. Clinical pathways based on IDSA/PIDS guidelines have been shown to increase use of ampicillin while decreasing cephalosporin use for patients admitted to children's hospitals with CAP [21–24]. Antimicrobial stewardship programs, recommended to optimize antibiotic prescribing [25, 26], can also be leveraged to successfully facilitate guideline adherence [27].
Our study has limitations. Reliance of ICD-9-CM codes to identify patients with CAP could result in misclassification. However, this algorithm was previously validated within this database (PPV = 89.6%). We also used additional exclusions (MRSA, effusion, empyema, vasopressor use, mechanical ventilation) that were not validated. However, 99% of patients in the final cohort were admitted for ≤7 days, suggesting that we captured patients with uncomplicated disease. We defined therapy with a particular drug as receipt of a single day of the specific agent, which could misclassify patients who switched therapy or received multiple drugs. This misclassification, however, would only overestimate the use of recommended first-line therapy. Finally, given the lack of microbiologic data from this database (and the general lack of microbiologic identification of CAP patients in general), we were unable to distinguish empiric and targeted therapy.
Recommended first-line antibiotic therapy for children hospitalized with CAP has increased following publication of PIDS/IDSA treatment guidelines, but overall use remains low. Cephalosporin and macrolide prescribing is decreasing but remains common. Guideline adoption was not consistent across institutions. Further studies exploring the outcomes of and reasons for compliance with treatment guidelines are warranted.
Note
Potential conflicts of interest. T. E. Z. has received research funding from Merck and Cubist and has served as a consultant for Merck, Pfizer, Astellas Pharma, and Cubist. J. S. G., J. G. N., and A. L. H. have received research funding from Pfizer. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Self WH, Grijalva CG, Zhu Y, et al. Rates of emergency department visits due to pneumonia in the United States, July 2006-June 2009. Acad Emerg Med. 2013;20:957–60. doi: 10.1111/acem.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–63. doi: 10.1056/NEJMoa1209165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children's hospitals. Infect Control Hosp Epidemiol. 2013;34:1252–8. doi: 10.1086/673982. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronman MP, Hersh AL, Feng R, Huang YS, Lee GE, Shah SS. Ambulatory visit rates and antibiotic prescribing for children with pneumonia, 1994–2007. Pediatrics. 2011;127:411–8. doi: 10.1542/peds.2010-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31:1036–41. doi: 10.1097/INF.0b013e31825f2b10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hersh AL, Shapiro DJ, Newland JG, Polgreen PM, Beekmann SE, Shah SS. Variability in pediatric infectious disease consultants’ recommendations for management of community-acquired pneumonia. PLoS One. 2011;6:e20325. doi: 10.1371/journal.pone.0020325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DJ, Shah SS, Myers A, et al. Identifying pediatric community-acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr. 2013;167:851–8. doi: 10.1001/jamapediatrics.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington state, 1980–1997. Pediatrics. 2000;106(1 pt 2):205–9. [PubMed] [Google Scholar]
- 10.Fitzmaurice GM, Laird NM. Applied longitudinal analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc; 2011. [Google Scholar]
- 11.Williams DJ, Hall M, Shah SS, et al. Narrow vs broad-spectrum antimicrobial therapy for children hospitalized with pneumonia. Pediatrics. 2013 doi: 10.1542/peds.2013-1614. 132:e1141–8. doi: 10.1542/peds.2013-1614. Epub 2013 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chassin MR. Practice guidelines: best hope for quality improvement in the 1990s. J Occup Med. 1990;32:1199–206. doi: 10.1097/00043764-199012000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 14.McCabe C, Kirchner C, Zhang H, Daley J, Fisman DN. Guideline-concordant therapy and reduced mortality and length of stay in adults with community-acquired pneumonia: playing by the rules. Arch Intern Med. 2009;169:1525–31. doi: 10.1001/archinternmed.2009.259. [DOI] [PubMed] [Google Scholar]
- 15.Gleason PP, Kapoor WN, Stone RA, et al. Medical outcomes and antimicrobial costs with the use of the American Thoracic Society guidelines for outpatients with community-acquired pneumonia. JAMA. 1997;278:32–9. [PubMed] [Google Scholar]
- 16.Frei CR, Restrepo MI, Mortensen EM, Burgess DS. Impact of guideline-concordant empiric antibiotic therapy in community-acquired pneumonia. Am J Med. 2006;119:865–71. doi: 10.1016/j.amjmed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Mortensen EM, Restrepo M, Anzueto A, Pugh J. Effects of guideline-concordant antimicrobial therapy on mortality among patients with community-acquired pneumonia. Am J Med. 2004;117:726–31. doi: 10.1016/j.amjmed.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Elemraid MA, Rushton SP, Thomas MF, et al. Changing clinical practice: management of paediatric community-acquired pneumonia. J Eval Clin Pract. 2013;20:94–9. doi: 10.1111/jep.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coco A, Vernacchio L, Horst M, Anderson A. Management of acute otitis media after publication of the 2004 AAP and AAFP clinical practice guideline. Pediatrics. 2010;125:214–20. doi: 10.1542/peds.2009-1115. [DOI] [PubMed] [Google Scholar]
- 20.Lomas J, Anderson GM, Domnick-Pierre K, Vayda E, Enkin MW, Hannah WJ. Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med. 1989;321:1306–11. doi: 10.1056/NEJM198911093211906. [DOI] [PubMed] [Google Scholar]
- 21.Dean NC, Bateman KA, Donnelly SM, Silver MP, Snow GL, Hale D. Improved clinical outcomes with utilization of a community-acquired pneumonia guideline. Chest. 2006;130:794–9. doi: 10.1378/chest.130.3.794. [DOI] [PubMed] [Google Scholar]
- 22.Capelastegui A, Espana PP, Quintana JM, et al. Improvement of process-of-care and outcomes after implementing a guideline for the management of community-acquired pneumonia: a controlled before-and-after design study. Clin Infect Dis. 2004;39:955–63. doi: 10.1086/423960. [DOI] [PubMed] [Google Scholar]
- 23.Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129:e597–604. doi: 10.1542/peds.2011-1533. [DOI] [PubMed] [Google Scholar]
- 24.Clements H, Stephenson T, Gabriel V, et al. Rationalised prescribing for community acquired pneumonia: a closed loop audit. Arch Dis Child. 2000;83:320–4. doi: 10.1136/adc.83.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shlaes DM, Gerding DN, John JF, Jr, et al. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25:584–99. doi: 10.1086/513766. [DOI] [PubMed] [Google Scholar]
- 26.Newland JG, Hersh AL. Purpose and design of antimicrobial stewardship programs in pediatrics. Pediatr Infect Dis J. 2010;29:862–3. doi: 10.1097/INF.0b013e3181ef2507. [DOI] [PubMed] [Google Scholar]
- 27.Smith MJ, Kong M, Cambon A, Woods CR. Effectiveness of antimicrobial guidelines for community-acquired pneumonia in children. Pediatrics. 2012;129:e1326–33. doi: 10.1542/peds.2011-2412. [DOI] [PubMed] [Google Scholar]