In a study of a clinic human immunodeficiency virus cohort, we found that hypertension incidence has increased in recent years. Although traditional risk factors were associated with hypertension, we found that low CD4 nadir may also be independently associated with incident hypertension.
Keywords: HIV, hypertension, cardiovascular disease, epidemiology, UCHCC
Abstract
Background. Persons infected with human immunodeficiency virus (HIV) are at higher risk for major cardiovascular disease (CVD) events than uninfected persons. Understanding the epidemiology of major traditional CVD risk determinants, particularly hypertension, in this population is needed.
Methods. The study population included HIV-infected patients participating in the UNC CFAR HIV Clinical Cohort from 1996 to 2013. Annual incidence rates of hypertension were calculated. Multivariable Poisson models were fit to identify factors associated with incident hypertension.
Results. 3141 patients contributed 21 956 person-years (PY) of follow-up. Overall, 57% patients were black, 28% were women, and the median age was 35 years. Hypertension age-standardized incidence rates increased from 1.68 cases per 100 PYs in 1996 to 5.35 cases per 100 PYs in 2013 (P < .001). In adjusted analyses, hypertension rates were higher among obese patients (incidence rate ratio [IRR] 1.70, 95% confidence interval [CI], 1.43–2.02), and those with diabetes mellitus (IRR 1.44, 95% CI, 1.14–1.83) and renal insufficiency (IRR 1.36, 95% CI, 1.16–1.61), but lower among patients with a CD4 nadir of ≥500 cells/mm3 (IRR 0.73, 95% CI, .53–1.01).
Conclusions. The incidence of hypertension increased from 1996 to 2013, alongside increases in traditional hypertension risk determinants. Notably, HIV-related immunosuppression and ongoing viral replication may contribute to an increased hypertension risk. Aggressive CVD risk factor management, early HIV diagnosis, linkage to care, antiretroviral therapy initiation, and durable viral suppression, will be important components of a comprehensive primary CVD prevention strategy in HIV-infected persons.
(See the Major Article by van Zoest et al on pages 205–13.)
Cardiovascular disease (CVD) is a leading cause of death among persons infected with human immunodeficiency virus (HIV) in North America and Europe [1, 2]. With aging of the HIV-infected population as a whole, the incidence of major cardiovascular events (eg, acute coronary syndromes, stroke), strongly associated with advancing age, will likely continue to rise. In addition to the expected increases in major CVD events among HIV-infected persons given changing population demographics, evidence also suggests that HIV infection itself may contribute to an increased risk of major CVD events [3, 4]. For these reasons, improving our understanding of the epidemiology of established CVD risk determinants in HIV-infected persons, and how these determinants impact cardiovascular health in the context of the chronic immune activation unique to HIV infection is of heightened importance.
Hypertension is an “established” CVD risk factor affecting 22% of adults worldwide, contributing to over 7.5 million deaths annually [5]. Despite its prevalence in the general population, hypertension among HIV-infected persons has not been well characterized. Reports assessing hypertension among HIV-infected patients are available prior to the use of contemporary, metabolically safer antiretroviral therapy (ART) [6–10]. Whether the epidemiology of hypertension has changed in more recent years with availability of more potent and well-tolerated ART and longer-term survival remains unclear. Therefore, the objectives of this study were to characterize the annual incidence rates of hypertension and identify patient characteristics associated with hypertension using a well-characterized HIV clinical cohort from 1996 through 2013.
METHODS
Study Population
All HIV-infected patients participating in the UNC Center for AIDS Research HIV Clinical Cohort (UCHCC) between 1 January 1996 and 31 December 2013 were included in the study. The UCHCC collects comprehensive and granular data from institutionally available electronic health and administrative records and performs expansive medical record reviews. The data cover demographic factors, clinical diagnoses, laboratory findings, and medication provision. Comprehensive details of the UCHCC have been described previously [11]. Of the eligible 4290 patients receiving care in the study period, we excluded 678 patients with missing data for key analysis covariates, including unavailable vital signs (n = 193) and CD4 count (n = 474). Overall, 3612 were included in the baseline analysis. The UNC Institutional Review Board approved the study protocol.
Hypertension
The primary outcome of interest was incident hypertension defined as having a documented diagnosis of hypertension with a diagnosis date at least 90 days after start of follow-up. All persons diagnosed with hypertension prior to 90 days after entry into HIV care at UNC were excluded from the final study cohort. Only hypertension documented by the provider (regardless of available ambulatory blood pressures) was used to determine the outcome diagnosis. All diagnoses of hypertension were reviewed based on the clinical record. The date of diagnosis was the date determined by the clinician that the patient met criteria for hypertension.
Covariates
We included data on basic demographics (age, sex, race/ethnicity), insurance status, behavioral risk determinants (alcohol, tobacco), CVD risk diagnoses (hyperlipidemia, diabetes, renal insufficiency), anthropomorphic data, HIV transmission risk factors (men who have sex with men [MSM], IV drug use), detailed substance abuse history, Hepatitis C co-infection and ART use, collected at baseline. Hyperlipidemia, diabetes and Hepatitis C were by the provider-documented diagnosis in the medical record. HIV viral loads (VLs) and CD4 nadir reported in the analysis were based on values obtained prior to an incident event (or the end of follow-up). Obesity was defined as a body mass index ≥30 kg/m2. Renal insufficiency was defined as a maximum creatinine of ≥1.4 mg/dL. ART duration was defined as the time from ART initiation until end of follow-up. HIV-1 VL suppression was defined as two consecutive VL measures <400 copies/mL.
Statistical Methods
Patients were followed from the latter of first clinic visit or 1 January 1996 (baseline) until an incident hypertension diagnosis, loss to follow-up (defined as no clinical visit for 18 months), death or administrative censoring (31 December 2013). Demographic and clinical characteristics of patients with and without prevalent hypertension at baseline were compared using Wilcoxon-Mann–Whitney test for continuous variables and Pearson's χ2 for categorical variables. We estimated hypertension incidence rates by taking the number of events divided by person-time, and calculated 95% confidence intervals (CI) based on a Poisson distribution. For the analysis of incident hypertension by year of diagnosis, calendar year incidence rates were standardized to the age distribution of the analysis cohort at the midpoint of observation.
Unadjusted and adjusted incidence rate ratios (IRRs) and measures of precision were estimated using Poisson regression. Additional models were fit with interaction terms for key covariates to test for effect measure modification. Models with and without interaction terms were assessed for goodness-of-fit using likelihood ratio tests and by comparing Akaike information criteria for each model. A P-value <.1 for interaction was considered statistically significant, and an alpha of 0.05 was used for all other statistical hypothesis testing. All analyses were performed using SAS software, Version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
Study Population
Overall, 3612 cohort members were eligible for analysis. The median age of the group was 36 years (interquartile range [IQR] 29–44). Seventy-one percent of these patients were male, 59% were non-Hispanic Blacks, 41% were MSM, and 42% were uninsured. The median CD4 nadir for the entire cohort was 173 cells/mm3 (IQR 38–360). Forty-one percent of patients were current or former tobacco users, and 18% were coinfected with hepatitis C (Table 1).
Table 1.
Patient Characteristics Stratified by Hypertension at Baseline
| Characteristic | All Patients (n = 3612) | No Hypertension (n = 3141) | Prevalent Hypertension (n = 471) | P Value |
|---|---|---|---|---|
| Age (y), median (IQR) | 36 (29–44) | 35 (29–42) | 43 (36–49) | <.001 |
| Male sex, n (%) | 2575 (71) | 2269 (72) | 306 (65) | .002 |
| Race/ethnicity, n (%) | .01 | |||
| White, non-Hispanic | 1141 (32) | 1018 (32) | 123 (26) | |
| Black, non-Hispanic | 2133 (59) | 1800 (57) | 333 (71) | |
| Hispanic | 212 (6) | 208 (7) | 4 (1) | |
| Other | 126 (3) | 115 (4) | 11 (2) | |
| Insurance, n (%) | .46 | |||
| Medicare/Medicaid | 1072 (30) | 917 (29) | 155 (33) | |
| Private | 1081 (30) | 940 (29) | 141 (31) | |
| Uninsured | 1519 (42) | 1344 (42) | 175 (36) | |
| Body mass index (kg/m2), Median (IQR) | 23.6 (21.7–25.8) | 23.1 (21.7–25.2) | 25.3 (21.9–28.5) | n/a |
| Serum creatinine (mg/dL) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 1.0 (0.8–1.2) | .03 |
| Ever tobacco use, n (%) | 1495 (41) | 1355 (43) | 140 (30) | <.001 |
| Alcohol abuse, n (%) | 1037 (29) | 926 (29) | 111 (24) | .01 |
| Hyperlipidemia, n (%) | 645 (18) | 518 (16) | 127 (27) | <.001 |
| Diabetes, n (%) | 243 (7) | 167 (5) | 76 (16) | <.001 |
| Hepatitis C, n (%) | 636 (18) | 552 (18) | 84 (18) | .89 |
| ART exposed | 3266 (90) | 2836 (90) | 430 (91) | .49 |
| MSM | 1467 (41) | 1311 (42) | 156 (33) | <.001 |
| IVDU | 488 (14) | 412 (13) | 76 (16) | .07 |
| Cocaine use | 1068 (30) | 943 (30) | 125 (27) | .12 |
| Amphetamine use | 141 (4) | 128 (4) | 13 (3) | .16 |
| CD4 nadir, cells/mm3, median (IQR) | 173 (38–360) | 168 (36–352) | 200 (61–390) | n/a |
All characteristics measured at baseline.
P-values were calculated using Wilcoxon-Mann-Test for continuous variables and Pearson's χ2 for categorical variables.
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; IVDU, intravenous drug use; MSM, men who have sex with men; n/a, not applicable.
At baseline analysis, 471 patients had a documented diagnosis of hypertension. Patients with prevalent hypertension at baseline were older, more likely women, and non-Hispanic black, than those without prevalent hypertension (all P < .01) (Table 1). Patients with hypertension at baseline were also more likely to be obese (31% vs 18%), have hyperlipidemia (27% vs 16%), and have diabetes (16% vs 5%) (all P <.01).
The 3141 patients without hypertension at baseline contributed 21 956 person years (PY), with a median follow-up time of 5.5 years (IQR 2.7–10.0). The median nadir CD4 during follow-up was 168 cells/mm3 (IQR 36–352), and the median CD4 at time of hypertension diagnosis or end of follow-up was 436 cells/mm3 (IQR 208–682) (Table 2). Fifty-nine percent of patients were virologically suppressed at end of follow-up; the median time of ART exposure was 4.8 years (IQR 2.2–9.0 years) (Table 2). By the end of the study period, 612 patients died, 942 were lost to follow-up, and 756 cases of incident hypertension were diagnosed.
Table 2.
Clinical Characteristics During Follow-up Stratified by Incident Hypertension
| Characteristic | All Patients (n = 3141) | Hypertension (n = 756) | No Hypertension (n = 2385) |
|---|---|---|---|
| BMI maximum, kg/m2 | |||
| Median (IQR) | 27.7 (24.5–30.2) | 28.5 (25.4–33.1) | 27.5 (24.2–29.5) |
| >30, n (%) | 859 (27) | 308 (41) | 551 (23) |
| BMI meana, kg/m2 | |||
| Median (IQR) | 26.2 (22.7–28.7) | 26.8 (23.6–30.3) | 25.9 (22.3–28.3) |
| >30, n (%) | 565 (18) | 209 (28) | 356 (15) |
| Creatinine maximum ≥1.4, n, (%) | 708 (23) | 230 (30) | 478 (20) |
| Creatinine most recent ≥1.4, n (%) | 232 (7) | 78 (10) | 154 (6) |
| CD4 nadir, cells/mm3 | |||
| Median (IQR) | 168 (36–352) | 153 (30–325) | 175 (38–362) |
| CD4 most recent, cells/mm3 | |||
| Median (IQR) | 436 (208–682) | 446 (266–664) | 433 (190–688) |
| HIV-1 VL most recent, <400 copies/mL, n (%) | 1859 (59) | 485 (64) | 1374 (57) |
| Ever on ART, n (%) | 692 (92) | 2144 (90) | |
| ART exposure (y)b, Median (IQR) | 4.80 (2.21–9.01) | 4.95 (2.38–9.15) | 4.26 (1.74, 8.41) |
| PI use ever, n (%) | 2774 (88) | 685 (91) | 2089 (88) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range; PI, protease inhibitor; VL, viral load.
a Average body mass index over follow-up period.
b Among persons initiated on ART.
Hypertension Incidence by Calendar Year
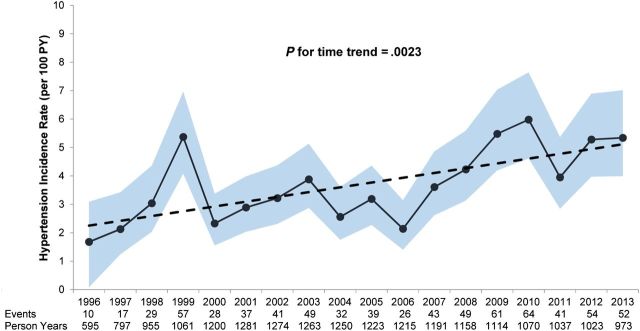
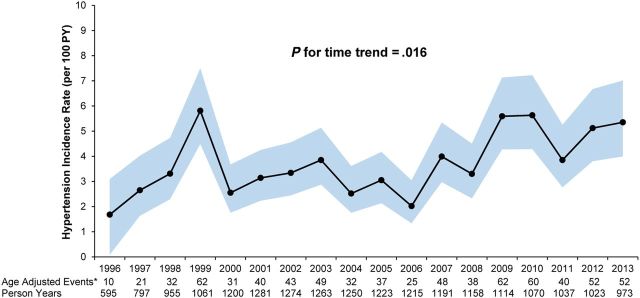
The crude incidence rate of hypertension was 3.44 cases per 100 PYs (95% CI, 3.20–3.70). Overall, unadjusted hypertension incidence rates were observed to increase across calendar years from 1996 to 2010 (p for trend = 0.002 Figure 1). The incidence rate was 1.68 cases per 100 PYs in 1996 rising to 5.98 cases per 100 PY in 2010 and subsequently falling to 5.38 cases per 100 PYs in 2013. Similar results were observed for age-standardized annual estimates (p for trend = 0.02; Figure 2).
Figure 1.
Annual unadjusted hypertension incidence rate with trendline by calendar year. Solid line is the annual unadjusted incidence rate; 95% confidence intervals are shaded area, and dotted line is the linear regression line with slope of 0.17. Abbreviation: PY, person years.
Figure 2.
Age-adjusted incidence rates and 95% confidence intervals of hypertension stratified by calendar year- 1996–2013. *Standardized to the age distribution at the midpoint of participant follow-up. Abbreviation: PY, person years.
Duration of Antiretroviral Therapy at Time of Hypertension Diagnosis
During the study period, 90% of all study participants were initiated on ART. The median duration of ART use was 4.8 years [IQR 2.2–9.1] prior to the end of study follow-up. Among persons eventually diagnosed with hypertension, the median duration on ART prior to diagnosis was 4.3 years [IQR 1.7, 8.4]. When cohort members were stratified by duration of exposure to ART, persons who were on ART for 5 years or more had the highest incidence rates of hypertension (age-adjusted IR 11.16 cases per 100 PY; 95% CI, 9.91–12.41). Hypertension incidence rates were lowest among persons who never started ART (age-adjusted IR 0.95 per 100 PY; 95% CI, .71–1.18). To assess whether total duration in care influenced ART-exposure stratified hypertension IRs, we determined hypertension IRs by total duration of follow-up. Persons who were followed for 5 years or more had higher age-adjusted incidence rates or hypertension than persons who were observed for 2 years or less (4.62 vs 3.36 cases per 100 PY; Table 3). However, the variation in IRs by ART exposure strata was stronger than the variation in IRs by follow-up time strata.
Table 3.
Incidence Rates of Hypertension by Duration of Antiretroviral Therapy and Duration of Follow-up
| Duration of ART | Number of Events | Person Years | Crude Incidence Rate per 100 PY (95% CI) | Age-Adjusted Incidence Rate per 100 PY (95% CI) |
|---|---|---|---|---|
| No ART | 64 | 7298.7 | 0.88 (.66–1.10) | 0.95 (.71–1.18) |
| Less than 2 y | 192 | 5002.1 | 3.83 (3.29–4.37) | 3.84 (3.30–4.38) |
| 2–5 y | 193 | 6932.0 | 2.78 (2.39–3.17) | 2.72 (2.34–3.10) |
| More than 5 y | 307 | 2723.3 | 11.27 (10.01–12.53) | 11.16 (9.91–12.41) |
| Duration of follow-up | ||||
| Less than 2 y | 194 | 5866.3 | 3.31 (2.84–3.78) | 3.37 (2.90–3.84) |
| 2–5 y | 189 | 8105.8 | 2.33 (2.00–2.67) | 2.35 (2.01–2.69) |
| More than 5 y | 373 | 7984.0 | 4.67 (4.20–5.14) | 4.62 (4.15–5.09) |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; PY, person years.
Factors Associated With Incident Hypertension
In multivariable analyses, for every 10 year increase in age at start of follow-up, there was a 34% increase in the incidence rate of hypertension (IRR 1.34, 95% CI, 1.24–1.44) (Table 4). Persons who met criteria for obesity at the time of study entry were diagnosed with hypertension at a significantly higher rate than persons who were not obese (IRR 1.70, 95% CI, 1.43–2.02). Renal insufficiency and diabetes were also significant risk determinants for incident hypertension (IRR 1.36, 95% CI, 1.16–1.61, and IRR 1.44, 95% 1.14–1.83, respectively). There was a trend toward increased incidence of hypertension in blacks (P = .10), and sex was not associated with incident hypertension (P = .76).
Table 4.
Patient Characteristics Associated With Incident Hypertension
| Characteristic | Bivariable |
Multivariable |
|||
|---|---|---|---|---|---|
| IRR | 95% CI | IRR | 95% CI | P Value | |
| Age, per 10 y increase | 1.37 | 1.29–1.48 | 1.34 | 1.24–1.44 | <.001 |
| Female sex | 1.11 | .95–1.28 | 1.03 | .84–1.26 | .76 |
| Race/ethnicity | |||||
| Black | 1.40 | 1.20–1.61 | 1.15 | .97–1.37 | .10 |
| Hispanic | 0.43 | .29–0.65 | 0.54 | .35–.84 | .006 |
| White | Ref | Ref | |||
| No insurance | 0.90 | .78–1.05 | 0.76 | .62–.90 | .003 |
| BMI ≥ 30 | 1.92 | 1.63–2.26 | 1.70 | 1.43–2.02 | <.001 |
| Serum creatinine ≥ 1.4 (mg/dL) | 1.23 | 1.06–1.43 | 1.36 | 1.16–1.61 | <.001 |
| Tobacco use | 0.68 | .58–0.78 | 0.73 | .62–.86 | <.001 |
| Hyperlipidemia | 0.97 | .83–1.15 | 1.06 | .89–1.27 | .73 |
| Diabetes | 1.66 | 1.32–2.09 | 1.44 | 1.14–1.83 | .002 |
| HCV coinfection | 0.98 | .82–1.17 | 1.02 | .81–1.30 | .83 |
| MSM | 0.92 | .81–1.07 | 1.18 | .97–1.42 | .10 |
| IVDU | 1.07 | .87–1.32 | 1.20 | .91–1.58 | .20 |
| Cocaine | 1.12 | .97–1.31 | 1.32 | 1.09–1.59 | .004 |
| Amphetamine | 0.77 | .49–1.20 | 1.01 | .62–1.63 | .97 |
| CD4 nadir, cells/mm3 | |||||
| <200 | 0.90 | .78–1.04 | 1.07 | .92–1.27 | .36 |
| 200–499 | Ref | Ref | |||
| ≥500 | 1.00 | .76–1.29 | 0.73 | .53–1.01 | .06 |
| VL most recent, <400 copies/mL | 0.83 | .71–0.96 | 0.87 | .74–1.03 | .11 |
| Any ART exposure | 0.61 | .47–0.80 | 1.19 | .84–1.70 | .33 |
| History of PI exposure | 0.69 | .54–0.90 | 1.28 | .93–1.77 | .12 |
Incidence rate ratios (and associated confidence intervals, P-values) were derived using multivariable Poisson regression.
Bold represents statistically significant P-value <.05.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; HCV, hepatitis C virus; IRR, incident rate ratio; IVDU, intravenous drug use; MSM, men who have sex with men; PI, protease inhibitor; Ref, reference; VL, viral load.
After adjusting for the aforementioned hypertension risk factors, we observed a strong trend toward a lower incidence in hypertension among patients with high CD4 nadirs (≥500 cells/mm3) (IRR 0.73, 95% CI, .53–1.01). We also observed a trend toward decreased hypertension incidence among persons with a suppressed HIV-1 VL (P = .11) and persons not exposed to protease inhibitors (P = .12) (Table 4).
DISCUSSION
CVD has emerged as a leading cause of mortality among HIV-infected persons, yet our understanding of key CVD risk determinants in this population is inadequate [1, 2]. In a well-characterized clinical HIV cohort, we observed that many of the traditional risk factors for hypertension in uninfected persons (advancing age, obesity, renal insufficiency, diabetes, and black race) also portend risk of incident hypertension in HIV-infected persons [12]. More interestingly, we observed that persons who maintained a CD4 cell count above 500 cells/mm3 appeared to be at lower risk for a subsequent diagnosis of hypertension. Our data also suggest a possible association between durable HIV VL suppression and lower incident hypertension diagnoses among HIV-infected persons in our clinical cohort. Finally, over the course of the last decade, observations from the clinical cohort show that there is a notable increase in the incidence of new hypertension diagnoses among persons living with HIV.
Although there was no definitive linear trend between CD4 nadir count and incident hypertension diagnoses, data from our cohort suggest that persons who maintain CD4 counts above 500 cells/mm3 for the duration of infection have a lower incidence of hypertension. These findings were independent of other known risk factors for hypertension including black race, obesity, renal insufficiency, and sex. Our observations are consistent with findings reported by Manner and others in a Norwegian clinical HIV cohort [13]. CD4 nadir, a biomarker for the degree of HIV-associated immunosuppression and a surrogate for the intensity of ongoing chronic immune activation and persistent inflammation, has been shown to be associated with cardiovascular outcomes in persons living with HIV [14, 15]. Low CD4 nadir have been reported to be associated with both increased subclinical atherosclerosis as assessed by carotid intimal medial thickness and increased risk of myocardial infarction in a large clinical cohorts [16]. Low CD4 nadirs have also been suggested to be associated with the diagnosis of left ventricular hypertrophy in asymptomatic HIV infected persons, a condition for which hypertension is the preeminent risk factor [17].
Though the etiology of essential hypertension is complex and incompletely understood, a growing body of evidence suggests that there may be an immunologic basis for this disease pathophysiology [18, 19]. Data from experimental mouse models have shown that T-cells are essential to the development of angiotensin-II induced hypertension [20]. Experimental mouse models have shown that on immune reconstitution of T-cell depleted mice, their increase in blood pressure in response to angiotensin-II is restored. Interestingly, endothelial-dependent vasodilatation remained impaired and vascular superoxide levels were increased compared to wild-type mice potentially predisposing the reconstituted mice to subsequent hypertension [21]. Pro-inflammatory cytokines like C-reactive protein (CRP) and interleukin (IL)-6 are also elevated in HIV-infected persons with low CD4 nadir [16, 22]. Elevated levels of CRP and IL-6 have also been shown to be independently associated with hypertension in humans [23]. Most recently, investigators in Korea showed that T-cell enumeration in persons with newly diagnosed hypertension revealed increased circulating CD28neg CD57pos T-cells, a sign of T-lymphocyte aging [24]. Immunosenscence is a well-established consequence of chronic immune activation known to occur at a much higher level in persons with low CD4 nadirs compared to higher CD4 nadirs [14, 25, 26]. Although definitive human studies are needed, the current state of evidence linking CD4 nadir with the risk of incident hypertension is compelling. Our findings also suggest an association between viral suppression and incident hypertension; however, achieving viral suppression may be strongly associated with favorable health behaviors related to lower hypertension risk.
In this analysis, we also observed that the incidence rate of hypertension continues to increase in the ART era. Although the aging of HIV-infected population as a whole may account for some of this observation, the age-standardized incidence rates presented in our study indicate other potential explanations for this trend. First, the lower rates of hypertension in the earlier years of observation may be due to the higher prevalence of severely immunosuppressed persons during that period. The clinical association of AIDS- associated severe immunosuppression, low body weight and generally poor health with low blood pressure is well established. This likely also explains the low incidence rates of hypertension among persons not on ART. In addition, given the relative higher acuity of patients presenting to the clinic during that time period, there is a possibility of underreporting during that time period that was slowly reduced as more patients achieved clinical stability on ART with each passing year On the cellular level, the potential role of T-lymphocytes in the pathobiology of essential hypertension further supports the observed lower incidence rates of elevated blood pressure during that time period.
Another consideration is that persons with previously severe immunosuppression and CD4 were returned to health often presaged by a rapid gain in weight and other dynamic changes in metabolism that may unmask longstanding predilections (genetic, demographic, or behavioral) toward hypertension. As supported by our observations and the aforementioned reports, immune reconstitution itself may be associated with a higher risk of hypertension. Finally, our observation of an increase in hypertension incidence rates may be a consequence of delayed effects of metabolic adverse antiretroviral medications, including early generation protease inhibitors and nucleoside reverse transcriptase inhibitors (D4T, DDI, AZT, etc). This study did not address the association between the chronologic distance between exposure to these drugs and the incident diagnosis of hypertension, although will be addressed in future analyses.
We did not observe a definitive link between duration of ART therapy, protease inhibitor exposure and incident hypertension as indicated in previous reports [9, 12]. Although an association was observed between hypertension incidence and duration of ART exposure, this is likely confounded by duration of follow-up time given the comparable results of our stratified analysis on both variables (Table 3). Accordingly, the seemingly protective effect of ART on incident hypertension noted by our point estimates is likely driven primarily by persons with less than 5 years of ART exposure and therefore hard to interpret (Table 4). When ART exposure was considered as a binary variable in our regression model, there was no clear association with incident hypertension (IRR 1.19, 95% CI, .84–1.70, P-value = .33). Further studies are needed to better define the relationship between ART and hypertension incidence.
This study has limitations. This study is an observational clinical cohort, and therefore data on individual risk determinants are dependent on the quality of clinical care documentation at the time of the event. The definition of hypertension, the outcome of interest, was strictly limited to physician documentation and medical record review by the study team. We acknowledge that this approach although practical may lead to underreporting of hypertension since detection completely depends on both clinician assessment and documentation. As mentioned above, our study did not address the association of hypertension with individual antiretroviral agents, highly relevant given the current discourse of the cardiovascular risk profile of newer protease inhibitors and recently re-emerging ART agents like abacavir.
In conclusion, our data suggest that though risk determinants for hypertension in uninfected persons also predict risk in persons living with HIV, a history of severe HIV related immunosuppression and ongoing viremia may be independently associated with hypertension as well. Though the differential risk of immune vs conventional risk factors is still not well defined, it is clear that further consideration must be given to the long-term deleterious impact of immunosuppression, immune recovery and persistent immune activation on vascular physiology. Our data also indicate a continued increase in the incidence rate of hypertension in HIV-infected persons, a concerning trend in light the novel recognition of people living with HIV as a high-CVD risk group. Finally, our study suggests no clear association between protease inhibitors, duration of exposure to ART, and the risk of incident hypertension. Taken together, our findings present more evidence in support of early ART for all persons with HIV and further emphasizes the importance of high quality CVD primary preventive care in this high-risk population.
Notes
Financial support. This work was supported by the Duke Interdisciplinary Research Training Program in AIDS (T32 AI007392), the Duke Center for AIDS Research (P30 AI064518), and the University of North Carolina Center for AIDS Research (P30 AI50410), and R01 HS18731.
Potential conflicts of interest. J. J. E. has received research support from ViiV Healthcare, Janssen, Bristol-Myers Squibb, Gilead Sciences and Abbvie. He has served as consultant for Abbvie, Merck, BMS, Viiv, Gilead, Tibotec/Janssen, Tobira and has been on a DSMB for Vertex. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Smith CJ, Ryom L, Weber R et al. . Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014; 384:241–8. [DOI] [PubMed] [Google Scholar]
- 2. Crum-Cianflone NF, Grandits G, Echols S et al. . Trends and causes of hospitalizations among HIV-infected persons during the late HAART era: what is the impact of CD4 counts and HAART use? J Acquir Immune Defic Syndr 2010; 54:248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silverberg MJ, Leyden WA, Xu L et al. . Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr 2014; 65:160–6. [DOI] [PubMed] [Google Scholar]
- 4. Freiberg MS, Chang CC, Kuller LH et al. . HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Raised Blood Pressure - Global Health Observatory Data Repository. Available at: http://apps.who.int/gho/data/node.main.A875?lang=en Accessed 6 June 2015.
- 6. Danner SA, Carr A, Leonard JM et al. . A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. European-Australian Collaborative Ritonavir Study Group. N Engl J Med 1995; 333:1528–33. [DOI] [PubMed] [Google Scholar]
- 7. Fontas E, van Leth F, Sabin CA et al. . Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis 2004; 189:1056–74. [DOI] [PubMed] [Google Scholar]
- 8. Shafran SD, Mashinter LD, Roberts SE. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med 2005; 6:421–5. [DOI] [PubMed] [Google Scholar]
- 9. Seaberg EC, Munoz A, Lu M et al. . Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS 2005; 19:953–60. [DOI] [PubMed] [Google Scholar]
- 10. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Napravnik S, Eron JJ Jr, McKaig RG, Heine AD, Menezes P, Quinlivan E. Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care 2006; 18 (suppl 1):S45–50. [DOI] [PubMed] [Google Scholar]
- 12. Thibaut R, El-Sadr WM, Friis-Moller N et al. . Predictors of hypertension and changes of blood pressure in HIV-infected patients. Antiviral Ther 2005; 10:811–23. [DOI] [PubMed] [Google Scholar]
- 13. Manner IW, Troseid M, Oektedalen O, Baekken M, Os I. Low nadir CD4 cell count predicts sustained hypertension in HIV-infected individuals. J Clin Hypertens (Greenwich) 2013; 15:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hunt PW, Martin JN, Sinclair E et al. . T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43. [DOI] [PubMed] [Google Scholar]
- 15. Robbins GK, Spritzler JG, Chan ES et al. . Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group protocol 384. Clin Infect Dis 2009; 48:350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsue PY, Lo JC, Franklin A et al. . Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004; 109:1603–8. [DOI] [PubMed] [Google Scholar]
- 17. Hsue PY, Hunt PW, Ho JE et al. . Impact of HIV infection on diastolic function and left ventricular mass. Circ Heart Fail 2010; 3:132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrison DG, Guzik TJ, Lob HE et al. . Inflammation, immunity, and hypertension. Hypertension 2011; 57:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryan MJ. An update on immune system activation in the pathogenesis of hypertension. Hypertension 2013; 62:226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 2010; 298:R1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guzik TJ, Hoch NE, Brown KA et al. . Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 2007; 204:2449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borges ÁH, O'Connor JL, Phillips AN et al. . Factors Associated With Plasma IL-6 Levels During HIV Infection. J Infect Dis 2015; 212:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens 2005; 19:149–54. [DOI] [PubMed] [Google Scholar]
- 24. Youn JC, Yu HT, Lim BJ et al. . Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 2013; 62:126–33. [DOI] [PubMed] [Google Scholar]
- 25. Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Ann Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]