In a systematic review and metaanalysis of trials involving treatment-naive human immunodeficiency virus–positive persons, we found no difference in clinical outcomes between nonnucleoside reverse-transcriptase inhibitors– and ritonavir-boosted protease inhibitors–based combination antiretroviral therapy. Furthermore, we found that participants who initiated either regimen had comparable viro-immunologic outcomes at week 48.
Keywords: HIV, antiretroviral therapy, protease inhibitor, nonnucleoside reverse transcriptase inhibitor, metaanalysis
Abstract
Background. Previous studies suggest that nonnucleoside reverse-transcriptase inhibitors (NNRTIs) cause faster virologic suppression, while ritonavir-boosted protease inhibitors (PI/r) recover more CD4 cells. However, individual trials have not been powered to compare clinical outcomes.
Methods. We searched databases to identify randomized trials that compared NNRTI- vs PI/r-based initial therapy. A metaanalysis calculated risk ratios (RRs) or mean differences (MDs), as appropriate. Primary outcome was death or progression to AIDS. Secondary outcomes were death, progression to AIDS, and treatment discontinuation. We calculated RR of virologic suppression and MD for an increase in CD4 cells at week 48.
Results. We included 29 trials with 9047 participants. Death or progression to AIDS occurred in 226 participants in the NNRTI arm and in 221 in the PI/r arm (RR, 1.03; 95% confidence interval, .87–1.22; 12 trials; n = 3825), death in 205 participants in the NNRTI arm vs 198 in the PI/r arm (1.04; 0.86–1.25; 22 trials; n = 8311), and progression to AIDS in 140 participants in the NNRTI arm vs 144 in the PI/r arm (1.00; 0.80–1.25; 13 trials; n = 4740). Overall treatment discontinuation (1.12; 0.93–1.35; 24 trials; n = 8249) and from toxicity (1.21; 0.87–1.68; 21 trials; n = 6195) were comparable, but discontinuation due to virologic failure was more common with NNRTI (1.58; 0.91–2.74; 17 trials; n = 5371). At week 48, there was no difference between NNRTI and PI/r in virologic suppression (RR, 1.03; 0.98–1.09) or CD4+ recovery (MD, −4.7 cells; −14.2 to 4.8).
Conclusions. We found no difference in clinical and viro-immunologic outcomes between NNRTI- and PI/r-based therapy.
The advent of combination antiretroviral therapy (cART) was a major breakthrough for human immunodeficiency virus–positive (HIV+) persons, leading to a dramatic reduction in morbidity and mortality [1, 2]. Standard cART regimens consist of 3 or more medications from at least 2 drug classes. In resource-rich settings, guidelines for initial therapy recommend 2 nucleoside reverse-transcriptase inhibitors (NRTI) with a non-NRTI (NNRTI), ritonavir-boosted protease inhibitor (PI/r) or integrase inhibitor for adults [3–5]. Treatment options are limited in resource-constrained settings, with generic NNRTI-based regimens being the preferred choice [6].
As current cART regimens are effective and well tolerated, clinical outcomes such as death and AIDS can only be assessed in randomized clinical trials (RCTs) with very large sample sizes and many years of follow-up. As a result, the majority of RCTs that compared NNRTI- to PI/r-based regimens used virologic suppression and CD4+ count recovery as surrogate outcomes [7–9], lacking statistical power to detect differences in clinical outcomes. The surrogacy of HIV RNA suppression as a proxy to demonstrate clinical benefit following cART initiation was established in 1996/1997 [10] and accepted by regulators thereafter. However, this is not the case for changes in CD4+ counts whose impact on clinical outcomes is less clear. On the one hand, some studies [11, 12], but not all [13], demonstrated a faster CD4+ count recovery in participants who received PI/r. On the other hand, individuals treated with NNRTI as the third drug were shown to be less likely to suffer virologic failure [11, 14] and more rapidly suppress HIV RNA to undetectable levels in some studies [12,15]. However, it remains unclear whether reported differences in viro-immunologic outcomes do translate into clinical benefit.
Results of several RCTs were reported in the last 2 decades [7–9, 11–13]. A systematic review to summarize results using metaanalysis could help to elucidate if NNRTI-based cART regimens are associated with different clinical viro-immunologic outcomes among treatment-naive individuals when compared with PI/r-based regimens.
METHODS
Search
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE for eligible trials (up to February 2016). A combination of free text words and indexing terms was combined with the sensitivity- and precision-maximizing version of the Cochrane search filter for identifying RCTs [16] (see Supplementary Appendix). We searched relevant review articles and reference lists of included articles for additional trials. Web of Science (February 2016) was searched for any articles that cited the included articles. To identify ongoing or unpublished trials, we searched the National Institutes of Health and World Health Organization (WHO) trial registries (February 2013) and proceedings of relevant conferences held in the past 5 years (Conference on Retroviruses and Opportunistic Infections, International AIDS Conference European Congress of Clinical Microbiology and Infectious Diseases, and Interscience Conference on Antimicrobial Agents and Chemotherapy; through March 2013).
Eligibility
We included RCTs, in any language, published or unpublished, involving treatment-naive HIV+ participants who were randomized to receive an NRTI backbone with either NNRTI or PI/r. Treatment naive was defined as absent or limited previous exposure to cART. We excluded trials that were restricted to pediatric participants; included older medications no longer recommended as the third drug, namely, unboosted protease inhibitors, soft gel saquinavir capsules, and delavirdine; and whose primary aim was to reduce mother-to-child transmission in pregnant women by using intermittent regimens.
Study Selection
Two investigators (AHB, AL) independently assessed articles for study inclusion. Titles and abstracts were first screened for includable articles. Full text versions of potential articles were then screened for final inclusion. Disagreement was resolved by discussion.
Data Extraction
Two investigators independently extracted data from included trials. We collected data on trial characteristics (corresponding author, publication year, journal, setting, inclusion and exclusion criteria, length of follow-up, and funding source), participants (number, country, age, gender, race, HIV transmission risk, proportion with AIDS, CD4+ counts, HIV RNA levels), interventions (name and dosage of drugs), and outcomes (event data, outcome assessor, timing of outcomes). To access unpublished data and resolve queries concerning reporting of data, corresponding authors of included trials were contacted and invited to participate as members of the writing group.
Risk of Bias Assessment
Two investigators independently assessed each trial and outcome for risk of bias using the Cochrane Risk of Bias tool [16]. We assessed the following domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors-objective outcomes (eg, CD4+ cell counts), blinding of outcome assessors-subjective outcomes (eg, discontinuation due to toxicity), incomplete outcome data, and selective outcome reporting. We contacted authors for clarifications.
Outcomes
The primary outcome was a composite outcome comprised of death or progression to AIDS. Progression to AIDS was defined as the occurrence of a new or recurrent Centers for Disease control and Prevention stage B or C event [17] or WHO stage 3 or 4 condition [18]. Secondary outcomes were all-cause death, progression to AIDS, treatment discontinuation, CD4+ counts, and HIV RNA levels. We defined treatment discontinuation as the proportion of participants who discontinued the cART regimen they were randomized to, irrespective of cause (ie, this included participants lost to follow-up and withdrawals). To broadly assess differences in adverse events and potency, we reported treatment discontinuations due to cART toxicity and study-defined virologic failure, respectively.
Outcomes were assessed until the end of study follow-up or until a predefined time point at which a switch from triple therapy to a simplified regimen with no NRTI backbone [19, 20] was allowed by study protocol. In factorial [12, 21, 22] and multiarm trials, we assessed outcomes combining trial arms on the basis of whether the third drug was an NNRTI [12, 23–25] or PI/r [12, 26, 27] irrespective of the NRTI backbone. We excluded trial arms in which NNRTI and PI/r were not prescribed [28, 29] or prescribed in combination [11, 30]. In one trial where participants could receive unboosted and boosted PI [13], we included data for a subset of participants who received boosted PI because the decision to treat this subset with either NNRTI- or PI/r-based cART was made as part of the randomization.
Data on HIV RNA levels and CD4+ changes were reported 48 weeks after cART initiation in most trials. Therefore, we reported percentage of participants reaching virologic suppression and the changes in CD4+ counts at this time point. When more than 1 HIV RNA threshold was reported to define virologic suppression in a trial, the lowest was used. In some cases we estimated virologic suppression from Kaplan–Meier curves in published papers. Because most of the trials did not report distributions of changes in CD4+ counts, we contacted corresponding authors for clarifications. We used data on mean changes preferentially and medians if means were unavailable. Data on standard deviation of mean CD4+ count changes could be obtained in 5 trials only; therefore, we imputed standard deviations similar to methods used by the Cochrane HIV/AIDS group [31].
Statistical Analyses
For dichotomous outcomes (death, disease progression, treatment discontinuation, and virologic suppression at week 48), we calculated pooled risk ratios (RRs; NNRTI/PI/r) with 95% confidence intervals (CIs) using the random-effects model with the Mantel–Haenszel method [16]. For CD4+ changes at week 48, we calculated pooled mean differences (NNRTI – PI/r) with 95% CI using the random-effects model with the inverse variance method [16]. We assumed that individual trials did not all estimate the same intervention effect; therefore, we used a random-effects model to estimate RRs and mean differences. Data were analyzed according to the intention-to-treat principle. Denominators consisted of all randomized participants who received at least 1 dose of medication irrespective of treatment adherence, protocol violation, arm crossover, or length of follow-up.
Heterogeneity was assessed using I2 [16]; substantial heterogeneity was defined as I2 above 60%. A funnel plot for the primary outcome was used to detect publication bias.
All statistical analyses were performed using Review Manager 5.3.5., the Nordic Cochrane Centre, Copenhagen 2014.
Secondary Analyses
To compare the effect of NNRTI- and PI/r-based regimens on non-AIDS–defining conditions, we estimated RRs with 95% CIs of non-AIDS death in trials with reliable information on causes of death. Furthermore, we estimated RRs of death and progression to AIDS in preplanned secondary analyses restricted to the following: (1) trials using abacavir or tenofovir in combination with lamivudine or emtricitabine as the NRTI backbone; (2) trials conducted in low- and middle-income countries; (3) trials in which the mean/median CD4+ cell count was 200 cells/mm3 or less at study entry.
Analysis 1 was done to exclude the potential effect of toxic and less potent NRTI drugs, such as didanosine, stavudine, and zidovudine, on our primary outcome. Analysis 2 was done to determine whether results from trials were consistent when restricted to trials from poorer regions that bear the highest burden of HIV. Analysis 3 was performed to assess whether results were consistent when restricted to study participants who initiated cART with severe immunosuppression.
RESULTS
Our literature search identified 7470 citations, leading to the inclusion of 33 articles. We included 6 articles from other sources, leading to the inclusion of 39 articles related to 29 individual trials, with a total of 9047 participants (Table 1; Supplementary Appendix and Figure 1).
Table 1.
Included Randomised Controlled Trials Involving Treatment-Naive Human Immunodeficiency Virus–Positive Persons
| Trial Name and Referencesa | No. Participants | Country | Mean (SD) or Median (IQR) CD4+ Cell Count at Baseline (cells/mm3) | Age (y)-Mean (SD) or Median (IQR) | Male Gender (%) | Race (%) | Length of Follow-up | NRTI Backbone | NNRTI Initiated | Boosted PI Initiated |
|---|---|---|---|---|---|---|---|---|---|---|
| ACTG A5202 [3, 21, 22, S1–3] | 1857 randomized (8 did not receive medication) | United States and Puerto Rico | 230 (90–334) overall | 38 (31–45) overall | 83 | 40 white 33 black 23 Hispanic 2 Asian 2 other |
Median: 138 (106–169) wk | Abacavir/lamivudine or Tenofovir/emtricitabine | Efavirenz | Atazanavir/ritonavir |
| ACTG A5142 [11, S4] | 503 | Unclear, at least 1 South African site | 191 overall | 38 overall | 80 | 36 white 42 black 19 Latino 2 Asian 1 unknown |
112 wk | Lamivudine+ 42% Zidovudine 24% Stavudine 34% Tenofovir |
Efavirenz | Lopinavir/ritonavir |
| Advanz [S5] | 65 | Spain | 41 (25–67) overall | 43 (28–66) NNRTI arm 43 (29–75) PI/r arm |
76 NNRTI arm 84 PI/r arm |
Not reported | 144 wk | Zidovudine/lamivudine | Efavirenz | Indinavir/ritonavir |
| Advanz-3 [27, S6] | 89 | Spain | 32 (20–59) overall | 38 (22–69) overall | 82 | Not reported | 48 wk | Tenofovir/emtricitabine | Efavirenz | Atazanavir/ritonavir Lopinavir/ritonavir |
| ALTAIRb [29, S7] | 219c | Argentina, Australia, Canada, Chile, France, Germany, Hong Kong, Ireland, Malaysia, Israel, Singapore, Mexico, Thailand, Taiwan, United Kingdom | 227(95) NNRTI arm 235 (114) PI/r arm |
37 (9) NNRTI arm 37 (9) PI/r arm |
79 NNRTI arm 71 PI/r arm |
NNRTI: 40 white; 31 Asian; 22 Latino PI/r arm: 41 white; 35 Asian; 19 Latino |
48 wk | Tenofovir/lamivudine | Efavirenz | Atazanavir/ritonavir |
| ARTENd [25, S8] | 569 | Argentina, Germany, Italy, Mexico, Poland, Portugal, Romania, Spain, Switzerland, United Kingdom | 184 (96) overall | 39 (10) overall | 84 | 80 white 12 Asian 8 black |
48 wk | Tenofovir/emtricitabine | Nevirapine 200 mg twice daily or 400 mg once daily (1:1 ratio)d | Atazanavir/ritonavir |
| Camerone [19, S9] | 155 | United States, Canada, Europe | 225 (127–333) overallf | 38 (31–46) overallf | 79 | 66 | 24–48 wke | Zidovudine/lamivudine | Efavirenz | Lopinavir/ritonavir |
| CLASSb [28, S10] | 193 | United States, Canada, Europe, Japan | 307 (184) NNRTI arm 306 (192) PI/r arm |
37 (18–59) NNRTI arm 36 (21–61) PI/r |
83 NNRTI arm 87 PI/r arm |
NNRTI arm 28 white; 35 black; 37 Latino; PI/r arm 27 white; 29 black; 40 Latino; 2 Asian |
96 wk | Abacavir/lamivudine | Efavirenz | Amprenavir/ritonavir initially; participants switched to fosamprenavir/ritonavir when the new drug became available |
| FIRSTg [13, S11] | 168g | United States | 159 (34–292) overall | 39 (32–46) overall | 77 | 55 black 27 white 14 Latino 4 other |
240 wk | 66% Zidovudine/lamivudine 23% Stavudine/lamivudine 5%Abacavir/lamivudine 4% other |
79% efavirenz 21% nevirapine |
49% Indinavir/ritonavir 36% Lopinavir/ritonavir 8% Saquinavir/ritonavir 8% other |
| Hippocampe-ANRS 121h [30, S12] | 57h | France | 216 (119–278) overallf | 39 (32–49) overallf | 75 | 39 black 58 white 4 otherf |
48 wk | 90% Lamivudine/didanosine 7% Lamivudine/didanosine 4% Tenofovir/lamivudine |
79% Efavirenz 21% Nevirapine |
52% lopinavir/ritonavir 48% indinavir/ritonavir |
| Honda [S13] | 71 | Japan | 220 (121–323) NNRTI arm 226 (103–324) PI/r arm |
36 NNRTI arm 35 PI/r arm |
100 | 100 Asian | 96 wk | Abacavir/lamivudine | Efavirenz | Atazanavir/ritonavir |
| INCA [S14] | 91 | Italy | 284 (119) overall | 44 (12) overall | 79 | 99 white 1 black overall |
48 wk | Tenofovir/ritonavir | Efavirenz | Atazanavir/ritonavir |
| KISSd [24, S15] | 187 | Italy | 211 (72–300) overallf | 40 (34–47) overallf | 84 | Not reported | 96 wk | 68% Didanosine/lamivudine 32% Tenofovir/zidovudine |
Efavirenz | Atazanavir/ ritonavir |
| LAKE [7, S16] | 126 | Spain and Italy | 193 (122) NNRTI arm 191 (127) PI/r arm |
39 (8) NNRTI arm 37 (9) PI/r arm |
86 NNRTI arm 87 PI/r arm |
Not reported | 48 wk | Abacavir/lamivudine | Efavirenz | Atazanavir/ ritonavir |
| Lapadula [S17] | 14 | Italy | 190 (101–252) NNRTI arm 132(64–313) PI/r arm |
36 (28–44) NNRTI arm 43 (33–75) PI/r arm |
79 | Not reported | 12 wk | Tenofovir/emtricitabine | Nevirapine | Atazanavir/ritonavir |
| LIPOTAR [S18, S19] | 19 | Spain | 337 overall | 39 overall | 100 | 90 white | 48 wk | Tenofovir/emtricitabine | Efavirenz | Lopinavir/ritonavir |
| Lubumbashi [33, S20, S21] | 425 | Congo DR | 182 (1–711) in males 163 (1–516) in females |
42(19–72) in males 36 (22–67) in females |
23 | 100 black | 96 wk | Tenofovir/emtricitabine or Zidovudine/lamivudine | Nevirapine | Lopinavir/Ritonavir |
| Martin [S22] | 224 | Uganda | 119 overall | 34 overall | 62 NNRTI 50 PI/r arm |
100 black | 48 wk | Tenofovir/emtricitabine | Efavirenz | Lopinavir/ritonavir |
| NEWART [S23] | 152 | United States | 185 overall | 37 (18–66) overall | 90 | 68 white 31 black 1 Asian |
48 wk | Tenofovir/emtricitabine | Nevirapine | Atazanavir/ritonavir |
| NORTHivi [26, S24, S25] | 239 | Norway and Sweden | 150 (80–200) NNRTI arm 150 (80–200) atazanavir/r arm 150 (90–216) lopinavir/r arm |
37 (31–46) NNRTI arm 39 (34–51) atazanavir/r arm 37 (32–45) lopinavir/r arm |
63 NNRTI arm 67 PI/r arms combined |
Not reported | 144 wk | 73% of participants in the NNRTI received
tenofovir/emtricitabine; 67% of participants in the atazanavir arm received tenofovir/emtricitabine; 89% of participants in the lopinavir arm received zidovudine/lamivudine |
Efavirenz | 50% Atazanavir/ritonavir 50% Lopinavir/ritonaviri |
| OCTANE1 [34, S26] | 243 (2 did not initiate medication) | 7 African countries | 139 (48–208) overall | 31 (34–37) overall | 0 | 100 black | 48 wk | Tenofovir/emtricitabine | Nevirapine | Lopinavir/ritonavir |
| OCTANE2 [9, S27] | 502 (2 withdrew consent) | 7 African countries | 121 (38–204) overall | 34 (26–45) overall | 0 | 100 black | 48 wk | Tenofovir/emtricitabine | Nevirapine | Lopinavir/ritonavir |
| Phidisa II [12, S28] | 1771 | South Africa | 107 (48–156) EFV+ZDV+ddI 102 (38–155) EFV+d4t+3TC 104 (43–151) LPV/r+ZDV+ddI 112 (51–154) LPV/r+d4T+3TC: |
35 (5) EFV+ZDV+ddI 36 (6) EFV+d4t+3TC 35 (6) LPV/r+ZDV+ddI 36 (6) LPV/r+d4T+3TC |
67% EFV+ZDV+ddI 69% EFV+d4t+3TC 68% LPV/r+ZDV+ddI 68% LPV/r+d4T++3TC |
Not reported | Median 192 wk; study halted prematurely |
Zidovudine/didanosine odds ratio Stavudine/lamivudine (factorial design) |
Efavirenz | Lopinavir/ritonavir |
| PROMOTE [S29–31] | 391 | Uganda | 374 (270–485) NNRTI arm 368 (282–506) PI/r arm |
30 (5) NNRTI arm 29 (5) PI/r arm |
0 | 100 black | 52 wk postpartum | Zidovudine/lamivudine | Efavirenz | Lopinavir/ritonavir |
| RIFART [S32] | 64 (32 of the 96 eligible participants were treatment experienced)f | Italy (24 centers) | 127 (157) NNRTI arm 100 (76) PI/r armf |
41 (13) NNRTI arm 42 (11) PI/r armf |
73 NNRTI arm 61 PI/r armf |
NNRTI arm: 24.2 white PI/r arm: 23 whitef |
24–36 wk (while receiving tuberculosis treatment) extending to 96 wk in those successfully treated | Tenofovir/emtricitabine | Efavirenz | Lopinavir/ritonavir |
| Sierra-Madero [S33] | 189 | Mexico | 64 (50–79) NNRTI arm 52 (37–67) NNRTI arm |
37 (35–39) NNRTI arm 36 (34–38) PI/r arm |
83 NNRTI arm 87 PI/r |
100 Latino | 48 wk | Zidovudine/lamivudine | Efavirenz | Lopinavir/ritonavir |
| SISTHER [23, S34–36] | 185 enrolled (including the prematurely discontinued arm of 11 patients originally enrolled to receive TDF+ddI+EFV) | Italy | Not reported | Not reported | Not reported | Not reported | 52 wk | Tenofovir/lamivudine (NNRTI
arm) Zidovudine/ lamivudine (PI/r) |
Efavirenz | Lopinavir/ritonavir |
| SUPPORT [S37, S38] | 101 | United States | 272 (19–699) NNRTI arm 237 (19–1061) PI/r arm |
Not reported | 68 NNRTI arm 57 PI/r arm |
NNRTI arm: 66 black 26 Hispanic 8 other PI/r arm 57 black 35 Hispanic 8 other |
96 wk | Abacavir/lamivudine | Efavirenz | Fosamprenavir/ritonavir |
| Trizefale [20, S39] | 209 | Spain | 202 (104–297) overall | 38 (32–43) overall | 80 | Not reported | 24–36 wke | Zidovudine/lamivudine/abacavir | Efavirenz | Lopinavir/ritonavir |
Abbreviations: 3TC, lamivudine; d4T, stavudine; ddI, didanosine; EFV, efavirenz; IQR, interquartile range; LPV/r, ritonavir boosted lopinavir; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitors; SD, standard deviation; ZDV, zidovudine.
a References to included trials are found in Supplementary Appendix.
b One arm receiving NRTI only was not included in the analyses.
c Three randomized participants withdrew consent and never received combination antiretroviral therapy (cART).
d The 2 NNRTI arms were analyzed combined.
e Outcomes assessed until a predefined time point at which a switch from PI/r- or NNRTI-based cART triple therapy to a simplified regimen with no NRTI backbone was allowed.
f Data informed by the corresponding author, trial statistician, or trial sponsor.
g Includes only the subset of participants in whom the decision to treat with either PI/r or NNRTI was made as part of the randomization. Data informed by the corresponding author.
h One arm sparing NRTI backbone was not included in the analyses.
i The 2 PI/r arms were analyzed combined.
Of 4569 participants assigned to an NNRTI-based regimen, 3510 received efavirenz and 1059 received nevirapine. Of 4478 participants assigned to a PI/r-based regimen, 2600 received lopinavir/r, 1627 received atazanavir/r, and 251 received another PI/r (Table 1 and Supplementary Appendix Figure 1).
Risk of Bias
All trials had low risk of selection bias due to sequence generation and allocation concealment, except 2 where it was judged as unclear (Supplementary Appendix Figure 2). All trials used an open label design, leading to high risk of performance bias from lack of blinding of participants and personnel. For objective outcomes, such as CD4+ counts, we judged the lack of blinded outcome assessment as leading to low risk of bias. For subjective outcomes, such as discontinuation due to toxicity, we judged the lack of blinded outcome assessment as leading to high risk of bias. However, 2 trials [12, 13] used a blinded endpoint committee for the assessment of disease progression, leading to low risk of bias for this outcome. The majority of trials had low risk of bias due to incomplete outcome data and selective outcome reporting.
Table 2 summarizes the quality rating of the evidence as proposed by the Grading of Recommendations, Assessment, Development and Evaluation Working Group.
Table 2.
Nonnucleoside Reverse-Transcriptase Inhibitor– Compared to Ritonavir-Boosted Protease Inhibitor–Based Combination Antiretroviral Therapy for Initial Treatment of Human Immunodeficiency Virus Infection
| Patient or Population: Treatment-Naive
Human Immunodeficiency Virus–Positive Persons Enrolled in Randomized Controlled
Trials Settings: Worldwide Intervention: NNRTI-based cART Comparator: PI/r-based cART | |||||||
|---|---|---|---|---|---|---|---|
| Outcomes (Time Frame of Absolute Effect) | Absolute Effects From Studiesa
(95% CI) |
Relative Effect 95% CI | No. of Participants (Studies), Follow-up | Quality of the Evidence GRADE) | Comments | ||
| PI/r | NNRTI | Difference with NNRTI | |||||
| Death or disease progression | 115 per 1000 | 118 per 1000 | 3 more per 1000 (15 fewer-25 more) | RR 1.03 (.87–1.22) | 3825 (12) | High | |
| Death | 48 per 1000 | 50 per 1000 | 2 more per 1000 (7 fewer - 12 more) | RR 1.04 (.86–1.25) | 8311 (22) | High | |
| Progression to AIDS | 60 per 1000 | 60 per 1000 | 0 fewer per 1000 (12 fewer- 15 more) | RR 1.00 (.80–1.25) | 4740 (13) | High | |
| Treatment discontinuation overall | 265 per 1000 | 297 per 1000 | 32 more per 1000 (19 fewer- 93 more) | RR 1.12 (.93–1.35) | 8249 (24) | Moderate | Heterogeneity 80% |
| Treatment discontinuation due to toxicity | 77 per 1000 | 93 per 1000 | 16 more per 1000 (10 fewer- 52 more) | RR 1.21 (.87–1.68) | 6195 (21) | Low | Lack of blinding, wide 95% CI and heterogeneity 60% |
| Treatment discontinuation due to virologic failure | 59 per 1000 | 93 per 1000 | 34 more per 1000 (5 more - 103 more) | RR 1.58 (.91–2.74) | 5371 (17) | Moderate | Heterogeneity 69% |
| Virologic suppression at week 48 | 658 per 1000 | 678 per 1000 | 20 more per 1000 (13 fewer- 59 more) | RR 1.03 (.98–1.09) | 6526 (18) | Moderate | Heterogeneity 68% |
| Mean CD4 increase at week 48 | Mean | Mean | Mean difference 4.7 cells lower (14.2 lower - 5.8 higher) | 6040 (17) | High | ||
| Non-AIDS death | 15 per 1000 | 11 per 1000 | 4 fewer per 1000 (10 fewer - 8 more) | RR 0.71 (.33–1.55) | 2205 (12) | Moderate | <30 events |
GRADE Working Group grades of evidence as follows: High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate.
Abbreviations: cART, combination antiretroviral therapy; CI, confidence interval; GRADE, Grading of Recommendations, Assessment, Development and Evaluation; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI/r, ritonavir-boosted protease inhibitors; RR, risk ratio.
a The basis for the control group absolute risks from the studies is mean risk across study(ies) unless otherwise stated in comments. The intervention absolute risk and difference is based on the risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Primary and Secondary Outcomes
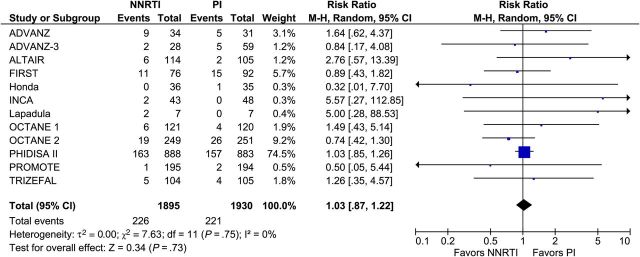
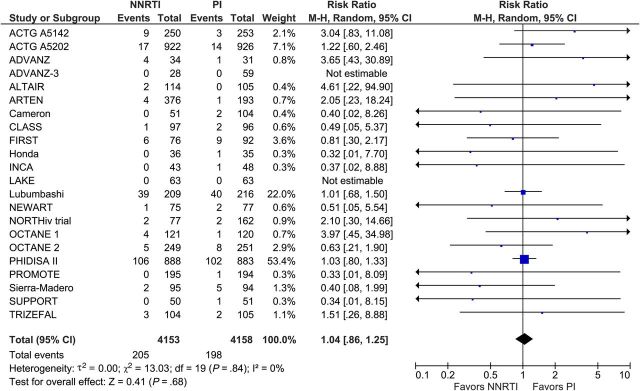
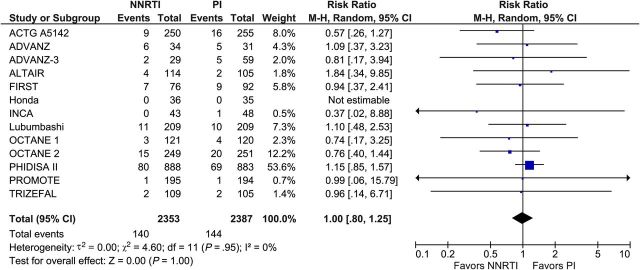
Of the 29 included trials, the primary outcome of death or progression to AIDS was reported in 12 trials involving 3825 participants. There was no difference between the 2 treatment regimens: 226 participants assigned to NNRTI-based cART and 221 participants assigned to PI/r-based cART died or progressed to AIDS (RR, 1.03; 95% CI, .87–1.22; I2, 0%; Figure 1). A funnel plot (Supplementary Appendix Figure 3) for our primary outcome showed no evidence of publication bias. Data on all-cause death was reported by 22 trials involving 8311 participants. We found no difference between NNRTI- and PI/r-based cART. A total of 205 participants in the NNRTI arm and 198 participants in the PI/r arm died during follow-up (RR, 1.04; 95% CI, .86–1.25; I2, 0%; Figure 2). Data on progression to AIDS was reported in 13 trials with 4740 participants. Both regimens were associated with comparable outcomes. A total of 140 participants in the NNRTI arm and 144 participants in the PI/r arm developed an AIDS-defining condition during follow-up (RR, 1.00; 95% CI, .80–1.25; I2, 0%; Figure 3).
Figure 1.
Death or progression to AIDS. Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Figure 2.
All-cause deaths. Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Figure 3.
Progression to AIDS. Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
We obtained data on overall treatment discontinuation from 24 trials with 8249 participants. Overall treatment discontinuation, including switches, was commonly reported; 2321 of 8249 trial participants (28.1%) discontinued medication during follow-up. No statistically significant difference was observed between NNRTI- and PI/r-based cART with respect to treatment discontinuation (RR, 1.12; 95% CI, .93–1.35; I2, 80%; Supplementary Appendix Figure 4). However, there was substantial heterogeneity in results between trials. Twenty-one trials with 6195 participants reported on discontinuation due to toxicity and gave similar results (RR, 1.21; 95% CI, .87–1.68; I, 60%; Supplementary Appendix Figure 5). For the risk of treatment discontinuation due to virologic failure, data were reported in 17 trials with 5371 participants, and discontinuation was more common among those assigned to NNRTI than among those assigned to PI/r (RR, 1.58; 95% CI, .91–2.74; P = .11; I2, 69%; Supplementary Appendix Figure 6). Substantial heterogeneity was also observed for discontinuation due to virologic failure.
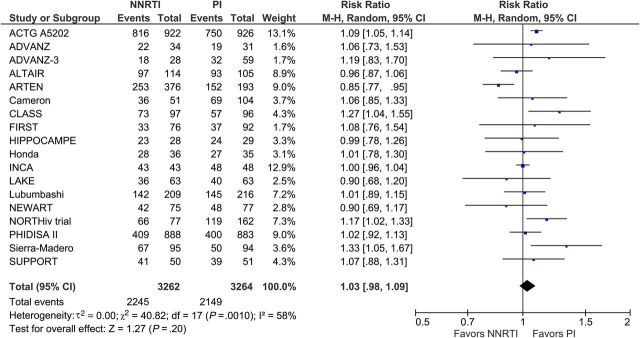
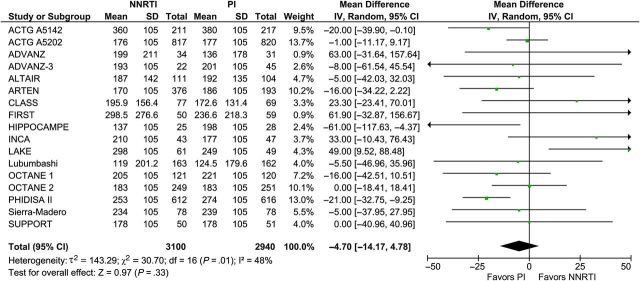
Eighteen trials with 6626 participants reported on virologic suppression at 48 weeks after treatment initiation. The number of participants who reached virologic suppression was similar between NNRTI- and PI/r-based cART (RR, 1.03; 95%: 0.98–1.09; I2, 58%; Figure 4) with moderate heterogeneity. Data from 17 trials with 6040 participants at 48 weeks showed that NNRTI-based cART was associated with a similar mean increase in CD4+ cell counts compared with PI/r-based cART (MD, −4.7 cells/mm3; 95% CI, −14.2 to 4.8; I2, 48%; Figure 5) with moderate heterogeneity.
Figure 4.
Virologic suppression 48 weeks after treatment initiation. Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Figure 5.
Mean CD4+ count difference 48 weeks after treatment initiation. Abbreviations: CI, confidence interval; df, degrees of freedom; IV, inverse variance; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation.
Secondary Analyses
Information on causes of death was reported for 12 trials with 2205 participants. Eleven participants in the NNRTI arm and 17 participants in the PI/r arm died of causes other than AIDS-defining conditions (RR, 0.71; 95% CI, .33–1.55; I2, 0%; Supplementary Appendix Figure 7).
With respect to the primary outcome of death or disease progression, we found consistent results in all preplanned subgroup analyses. No differences were observed between NNRTI- and PI/r-based cART when we restricted our analyses to trials with abacavir or tenofovir associated with lamivudine or emtricitabine as NRTI backbone (RR, 0.90; 95% CI, .56–1.43; I2, 0%; 6 trials; 1004 participants; Supplementary Appendix Figure 8); in trials conducted in low- and middle-income countries (RR, 1.00; 95% CI, .83–1.20; I2, 0%; 4 trials; 2901 participants; Supplementary Appendix Figure 9); and in trials involving participants with severe immunosuppression, as demonstrated by a baseline median/mean CD4+ count below 200 cells/mm3 (RR, 1.02; 95% CI, .85–1.21; I2, 0%; 7 trials; 2846 participants; Supplementary Appendix Figure 10).
DISCUSSION
In this comprehensive systematic review and metaanalysis of RCTs involving treatment-naive HIV+ persons, we found no difference in clinical outcomes between NNRTI- and PI/r-based cART. This was a robust finding consistently observed in analyses restricted to participants who received NRTI backbones recommended as first line and in trials conducted in low- and middle-income countries or enrolling severely immunosuppressed participants. Furthermore, we found that HIV+ persons who initiated either regimen had comparable viro-immunologic outcomes at week 48. Systematic reviews and metaanalyses pooling data from eligible studies increase the sample size and generally produce more precise estimates of effects of interventions than a single randomized trial [16]. This may be the reason why previously reported differences in virologic suppression [11, 12] and immunologic recovery [11, 12] between NNRTI- and PI/r-based cART were not observed in our study.
In resource-rich settings where multiple drugs are available, the choice of the initial cART regimen is determined by the clinician's preferences and participant characteristics. It has been shown that PI/r-based cART may be prescribed preferentially for participants with lower CD4+ counts, high viral load, and AIDS-defining conditions [32]. This is most likely based on a misconception that PI/r-based regimens are more potent than NNRTI-based regimens, which is unsupported by our findings.
We found that treatment discontinuation due to study-defined virologic failure was more common among participants who initiated NNRTI-based cART. This effect was mainly driven by trials that compared nevirapine-based cART to PI/r-based cART [9, 25, 33, 34], one of which recruited HIV+ women previously exposed to single-dose nevirapine to reduce the risk of mother-to-child transmission of HIV [9]. In analysis restricted to trials with efavirenz as the third drug, NNRTI-based cART seemed to be associated with a marginally lower risk of discontinuation due to virologic failure (RR, 0.77; 95% CI, 0.60–1.00; 10 trials; 3302 participants).
The risk of emergence of viral resistance is lower for PI/r than for NNRTI due to higher genetic barrier. However, our results suggest that this does not translate into an immediate excess risk of clinical outcomes. This strengthens the main WHO recommendation to use efavirenz-based cART as first-line therapy [6]. It is possible that accumulated viral resistance against NNRTI may translate into poorer outcomes during life-long treatment, which calls for continued follow-up of participants in RCTs to better understand this. This is particularly the case for settings where cART is used without HIV RNA monitoring. In such settings, virologic failure will not be readily captured as part of routine care since it rarely leads to immediate clinical disease or decline in CD4+ count.
In an attempt to overcome shortcomings of individual trials and to provide evidence to guide treatment decisions, a number of metaanalyses that compared NNRTI- to PI/r-based cART regimens have been performed [14, 35–37]. However, all of these metaanalyses have important limitations, namely, indirect comparisons [35]; pooling of observational and randomized data [37]; inclusion of participants with previous exposure to cART [35]; low numbers of clinical events [14]; limited data from resource-constrained settings [14, 37]; lack of information regarding changes in CD4+ counts [14, 36]; exclusion of unpublished trials [35]; inclusion of trials with old-fashioned regimens that contained delavirdine, saquinavir hard gel, or unboosted PI [14]; and poor representation of new antiretrovirals currently recommended as first-line treatment.
We tried to overcome these limitations, but our study does have other limitations that need to be acknowledged. First, we have no data on ritonavir-boosted darunavir, which is currently recommended as first-line therapy in most guidelines [3–5] and with superior tolerability compared with ritonavir-boosted atazanavir [38]. To our knowledge, however, there has been no head-to-head comparison between darunavir and NNRTI-based cART that could have been included in our metaanalysis. Second, we have no data on emergence of drug resistance after treatment initiation. Third, pooling results from different clinical settings with different regimes and risks of bias may risk oversimplification [16], and heterogeneity was substantial for the outcomes of treatment discontinuation and virologic suppression. The large differences in participant characteristics, trial conduct, loss to follow-up, and handling of missing data between trials are likely the causes of this heterogeneity. We found no single factor that could explain this heterogeneity, and use of a random-effects model was our best approach to summarize data to inform clinical care. Due to the way data were reported, it was not possible to assess differences in covariates on outcomes. However, one approach could be to perform an individual patient data metaanalysis where the influence of covariates, such as specific drug regimes, on individual outcomes could be investigated [16]. In addition, we did not investigate integrase inhibitor–based regimens, which have been shown to be noninferior or, in the case of dolutegavir, superior to NNRTI- and PI/r-based cART in individual trials [39, 40]. Whether this superior virologic suppression will translate into clinical benefit, as demonstrated by a lower incidence of clinical outcomes, warrants further investigation.
To conclude, we found no difference in clinical and viro-immunologic outcomes between treatment-naive HIV+ persons randomized to NNRTI- or PI/r-based cART. Treatment discontinuation due to virologic failure was more common in those who received NNRTI, an effect mainly driven by nevirapine-based regimens. Our findings indicate that NNRTI- and PI/r-based cART are equivalent and support current recommendations to use either regimen for the initial therapy of HIV infection [3–5]. An individual patient data metaanalysis is warranted to further investigate the different adverse event profiles and the dynamics of CD4+ count recovery and virologic suppression after the initiation of NNRTI- or PI/r-based regimens.
Supplementary Material
Notes
Acknowledgments. We thank Robert Cuffe from ViiV for answering our queries related to CLASS and SUPPORT trials and Linda M. Fredrik from AbbVie for answering our queries about the Cameron trial.
Author contributions. A. H. B., A. L., and J. D. L. conceived the study. A. H. B. and A. L. developed the search strategy, searched databases, included trials, extracted data, assessed trials for risk of bias, analyzed data, and drafted the manuscript. All authors contributed to data interpretation, critically revised the manuscript, and approved the final version.
Financial support. This study was supported by the Research Council at Rigshospitalet and by the Danish National Research Foundation (grant DNRF126). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this manuscript.
Potential conflicts of interest. M. G. has received research grants from Abbvie, Bristol-Myers Squibb (BMS), Gilead Sciences, GlaxoSmithKline (GSK)/ViiV, Merck, Pfizer, Roche, and Janssen-Cilag and honoraria as a speaker and/or scientific advisor from Abbvie, Boehringer-Ingelheim, BMS, Gilead Sciences, GSK/ViiV, Janssen-Cilag, Merck, Pfizer, Roche, and Tibotec. P. K. has received grants and research support from Janssen, GSK, and Merck, Gilead; consultant and speakers bureau fees from Janssen and ViiV Healthcare; is a stock shareholder for Merck, Pfizer, Johnson & Johnson, GSK, Gilead; and has received honoraria from Janssen and ViiV Healthcare. J. M. M. has received consulting honoraria and/or research grants from AbbVie, BMS, Cubist, Merck, Novartis, Gilead Sciences, and ViiV Healthcare. S. O. has received honoraria and research grants from MSD and honoraria from ViiV Healthcare, Torii Pharmaceutical, Janssen Pharmaceutical, Abbvie, GSK, and Japan Tobacco Inc. S. A. R. has received a clinical trial grant from Gilead Sciences. P. E. S. has been a consultant or scientific advisory board member for AbbVie, BMS, Gilead, GSK/ViiV, Merck, and Janssen and has received grant support for research from BMS, Gilead, GSK/ViiV, and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Palella FJ Jr, Delaney KM, Moorman AC et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–60. [DOI] [PubMed] [Google Scholar]
- 2. Mocroft A, Vella S, Benfield TL et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet 1998; 352:1725–30. [DOI] [PubMed] [Google Scholar]
- 3. Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf Accessed 30 November 2015.
- 4. European AIDS Clinical Society 2015. European Guidelines for treatment of HIV-infected adults in Europe. Available at: http://www.eacsociety.org/files/2015_eacsguidelines_8.0-english_revised-20151104.pdf Accessed 30 November 2015.
- 5. British HIV Association. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2015. Available at: http://www.bhiva.org/documents/Guidelines/Treatment/2015/2015-treatment-guidelines.pdf Accessed 30 November 2015. [DOI] [PubMed]
- 6. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: what's new? Available at: http://www.who.int/hiv/pub/arv/15249_HIVTreatementandCare_PolicybriefforWEB.pdf?ua=1 Accessed 30 November 2015.
- 7. Echeverría P, Negredo E, Carosi G et al. Similar antiviral efficacy and tolerability between efavirenz and lopinavir/ritonavir, administered with abacavir/lamivudine (Kivexa), in antiretroviral-naïve patients: a 48-week, multicentre, randomized study (Lake Study). Antivir Res 2010; 85:403–8. [DOI] [PubMed] [Google Scholar]
- 8. Daar ES, Tierney C, Fischl MA et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med 2011; 154:445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lockman S, Hughes M, Sawe F et al. Nevirapine-versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in Africa: a randomized trial. PLoS Med 2012; 9:e1001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marschner IC, Collier AC, Coombs RW et al. Uses in changes in plasma levels of human immunodeficiency virus type 1 RNA to assess the clinical benefit of antiretroviral therapy. J Infect Dis 1998; 177:40–7. [DOI] [PubMed] [Google Scholar]
- 11. Riddler SA, Haubrich R, DiRienzo AG et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 2008; 358:2095–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ratsela A, Polis M, Dhlomo S et al. A randomized factorial trial comparing 4 treatment regimens in treatment-naive HIV-infected persons with AIDS and/or a CD4 cell count <200 cells/μL in South Africa. J Infect Dis 2010; 202:1529–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MacArthur RD, Novak RM, Peng G et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): a long-term randomised trial. Lancet 2006; 368:2125–35. [DOI] [PubMed] [Google Scholar]
- 14. Chou R, Fu R, Huffman LH, Korthuis PT. Initial highly-active antiretroviral therapy with a protease inhibitor versus a non-nucleoside reverse transcriptase inhibitor: discrepancies between direct and indirect meta-analyses. Lancet 2006; 368:1503–15. [DOI] [PubMed] [Google Scholar]
- 15. Haubrich RH, Riddler SA, Ribaudo H et al. Initial viral decay to assess the relative antiretroviral potency of protease inhibitor-sparing, nonnucleoside reverse transcriptase inhibitor-sparing, and nucleoside reverse transcriptase inhibitor-sparing regimens for first-line therapy of HIV infection. AIDS 2011; 25:2269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available at: www.cochrane-handbook.org Accessed 30 November 2015. [Google Scholar]
- 17. Centers for Disease Control and Prevention. CDC 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41:1–19. [PubMed] [Google Scholar]
- 18. World Health Organization. WHO Case Definitions of HIV—World Health Organization. Available at: http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf Accessed 30 November 2015.
- 19. Cameron DW, da Silva BA, Arribas JR et al. A 96-week comparison of lopinavir-ritonavir combination therapy followed by lopinavir-ritonavir monotherapy versus efavirenz combination therapy. J Infect Dis 2008; 198:234–40. [DOI] [PubMed] [Google Scholar]
- 20. Mallolas J, Pich J, Peñaranda M et al. Induction therapy with trizivir plus efavirenz or lopinavir/ritonavir followed by trizivir alone in naive HIV-1-infected adults. AIDS 2008; 22:377–84. [DOI] [PubMed] [Google Scholar]
- 21. Sax PE, Tierney C, Collier AC et al. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med 2009; 361:2230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sax PE, Tierney C, Collier AC et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis 2011; 204:1191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torti C, Quiros-Roldan E, Regazzi M et al. Early virologic failure after tenofovir + didanosine + efavirenz combination in HIV-positive patients upon starting antiretroviral therapy. Antivir Ther 2005; 10:505–13. [PubMed] [Google Scholar]
- 24. Maggiolo F, Airoldi M, Ripamonti D et al. TAMs prevention in first-line HAART [abstract MOPEB059]. In: Program and abstracts of 5th IAS Conference on HIV Pathogenesis and Treatment 2009 (Cape Town, South Africa). [Google Scholar]
- 25. Soriano V, Arastéh K, Migrone H et al. Nevirapine versus atazanavir/ritonavir, each combined with tenofovir disoproxil fumarate/emtricitabine, in antiretroviral-naive HIV-1 patients: the ARTEN trial. Antivir Ther 2011; 16:339–48. [DOI] [PubMed] [Google Scholar]
- 26. Andersson LM, Vesterbacka J, Blaxhult A et al. Lopinavir/ritonavir, atazanavir/ritonavir, and efavirenz in antiretroviral-naïve HIV-1-infected individuals over 144 weeks: an open-label randomized controlled trial. Scand J Infect Dis 2013; 45:543–51. [DOI] [PubMed] [Google Scholar]
- 27. Miro JM, Manzardo C, Ferrer E et al. Immune reconstitution in severely immunosuppressed antiretroviral-naive HIV-1-infected patients starting efavirenz, lopinavir-ritonavir, or atazanavir-ritonavir plus tenofovir/emtricitabine: final 48-week results (the Advanz-3 trial). J Acquir Immune Defic Syndr 2015; 69:206–15. [DOI] [PubMed] [Google Scholar]
- 28. Bartlett JA, Johnson J, Herrera G et al. Long-term results of initial therapy with abacavir and lamivudine combined with efavirenz, amprenavir/ritonavir, or stavudine. J Acquir Immune Defic Syndr 2006; 43:284–92. [DOI] [PubMed] [Google Scholar]
- 29. Puls RL, Srasuebkul P, Petoumenos K et al. Efavirenz versus boosted atazanavir or zidovudine and abacavir in antiretroviral treatment-naive, HIV-infected subjects: week 48 data from the Altair study. Clin Infect Dis 2010; 51:855–64. [DOI] [PubMed] [Google Scholar]
- 30. Duvivier C, Ghosn J, Assoumou L et al. Initial therapy with nucleoside reverse transcriptase inhibitor-containing regimens is more effective than with regimens that spare them with no difference in short-term fat distribution: Hippocampe-ANRS 121 trial. J Antimicrob Chemother 2008; 62:797–808. [DOI] [PubMed] [Google Scholar]
- 31. Spaulding A, Rutherford GW, Siegfried N. Stavudine or zidovudine in three-drug combination therapy for initial treatment of HIV infection in antiretroviral-naïve individuals. Cochrane Database Syst Rev 2010; 8:CD008651. [DOI] [PubMed] [Google Scholar]
- 32. Elzi L, Erb S, Furrer H et al. Choice of initial combination antiretroviral therapy in individuals with HIV infection: determinants and outcomes. Arch Intern Med, 2012; 172:1313–21. [DOI] [PubMed] [Google Scholar]
- 33. Clumeck N, Mwamba C, Kabeya K et al. First-line antiretroviral therapy with nevirapine versus lopinavir-ritonavir based regimens in a resource-limited setting. AIDS 2014; 28:1143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lockman S, Hughes MD, McIntyre J et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med 2010; 363:1499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yazdanpanah Y, Sissoko D, Egger M, Mouton Y, Zwahlen M, Chêne G. Clinical efficacy of antiretroviral combination therapy based on protease inhibitors or non-nucleoside analogue reverse transcriptase inhibitors: indirect comparison of controlled trials. BMJ 2004; 328:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawalec P, Kryst J, Mikrut A, Pilc A. Nevirapine-based regimens in HIV-infected antiretroviral-naive patients: systematic review and meta-analysis of randomized controlled trials. PLoS One 2013; 8:e76587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee FJ, Amin J, Carr A. Efficacy of initial antiretroviral therapy for HIV-1 infection in adults: a systematic review and meta-analysis of 114 studies with up to 144 weeks’ follow-up. PLoS One 2014; 9:e97482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lennox JL, Landovitz RJ, Ribaudo HJ et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014; 161:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walmsley SL, Antela A, Clumeck N et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–18. [DOI] [PubMed] [Google Scholar]
- 40. Clotet B, Feinberg J, van Lunzen J et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014; 383:2222–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.