Sir,
It was with great interest that we read the article on advanced access by Sevilla et al. (2015) regarding axonal Charcot-Marie-Tooth (CMT2) disease caused by mutations in the MORC2 gene. Through whole-exome sequencing in a Spanish four generation CMT2 family with autosomal dominant pattern of inheritance, the authors identified the mutation p.R190W in the MORC2 gene as the cause of the disease. It was the only variant detected by whole-exome sequencing that segregated with the disease in the family. Afterwards, they tested an additional 52 patients with axonal Charcot-Marie-Tooth and found mutations in the MORC2 gene in two additional families, one with different de novo mutation and the second with the same p.R190W mutation, also de novo.
Here, we would like to report another patient with the p.R190W mutation in the MORC2 gene and thus confirm the causality of this gene for the severe CMT2 with striking proximal weakness. Also, we would like to show that the p.R190W mutation is a hot spot and probably the most frequent mutation in this gene. Immediately after we became aware of this newly discovered gene, we detected this mutation by re-examining older whole-exome sequencing data in one of our patients. This observation may have consequences for clinical DNA diagnostics because the phenotype, despite being probably rare, seems to be clinically recognizable and selected patients may first be tested for this mutation. This study was approved by the ethics committee of University Hospital Motol and informed consent was obtained from the patient according to the Declaration of Helsinki.
A 39-year-old patient of Czech origin is a sporadic case in the family. He was born with a foot deformity—a prominent dorsal flexion of both feet—which later returned to normal at the age of 12 months. He reached early developmental milestones normally. The first signs of the disease occurred at the age of 6 and these were mainly frequent tripping due to foot and distal leg weakness, difficulties riding a bicycle or skiing. Diagnosis of axonal Charcot-Marie-Tooth was first stated at the age of 10 years.
At examination at the age of 15 years he complained about difficulty with walking. At that time he was able to walk a maximum distance of 1500 m. Foot deformity, hammer toes and calluses on the external side of the foot were noticed. Moreover, he reported having poor handwriting, difficulty using zippers and buttons. Atrophies of thenar and hypothenar muscles and also forearm muscles were observed.
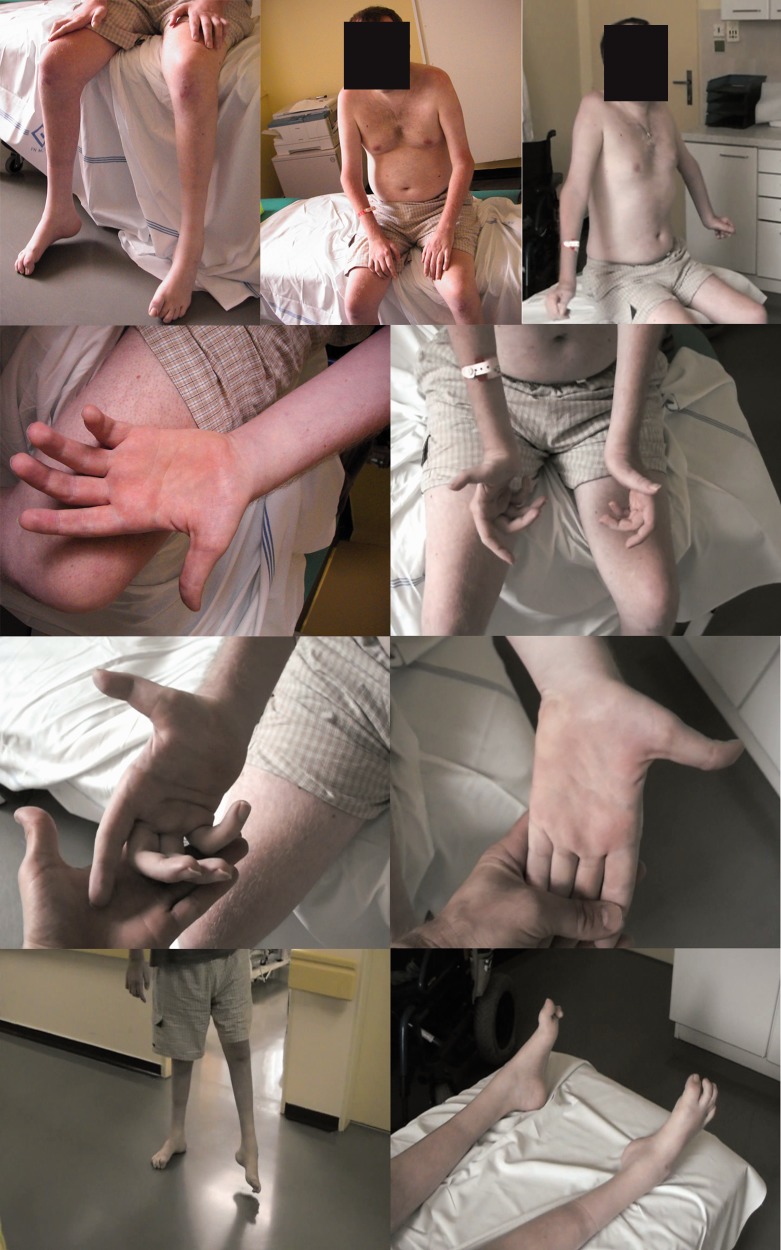
When he was 20 years old, he began using a cane, two canes when he was 23. From the age of 27, he has been confined to a wheelchair. Examination at 30 years of age showed progression of the disease. It is worth mentioning that overall muscle weakness was observed, with flexor muscles (including hip flexors) being weaker than extensor muscles. Clinical presentation is summarized in Table 1 . Photographs of the patient are shown in Fig. 1 . Nerve conduction studies results are summarized in Table 2 .
Table 1.
Clinical presentation
| Test | Results: presentation at the age of 15 | Results: presentation at the age of 30 |
|---|---|---|
| Upper limbs | ||
| Muscle atrophies | Profound muscle atrophies of the forearm and hand muscles are present | Profound muscle atrophies of thenar, hypothenar and interosseous muscles, up to the forearm are present |
| Reflexes | Reflexes C5-C8: seen only with reinforcement (1+) | Reflexes C5–C8: absent (0) |
| Muscle strength (MRC scale): grades 0–5 | Shoulder abduction 3 | Horizontal abduction/adduction of the shoulder and also vertical abduction are not possible |
| Elbow flexion/extension 3 | Elbow flexion 2, elbow extension 4 | |
| Wrist extension/flexion 4 | Wrist extension/flexion 3 | |
| Finger flexion 3 | Finger flexion 2 | |
| Sensory testing | No reported deficit | No reported deficit |
| Pin prick test | He was able to distinguish sharp/blunt stimuli | He was able to distinguish sharp/blunt stimuli |
| Vibration test | Vibration test at fingers was 4–5/8 | Vibration test at fingers was 4–5/8 |
| Lower limbs | ||
| Muscle atrophies | At lower limbs profound atrophies distally are present up to the mid-calf | At lower limbs profound atrophies are present up to the mid-thigh |
| Contractures/ deformities | Foot deformity, calluses | Foot deformity, calluses |
| Reflexes | Knee reflex, ankle reflex absent (0) | Knee reflex, ankle reflex absent (0) |
| Muscle strength (MRC scale): grades 0–5 | A deficit was observed both proximally and distally | A deficit was observed both proximally and distally |
| Hip flexion/extension 2–4 | Hip flexion 1–2/extension 3 | |
| Knee flexion/extension 5 | Knee flexion 1/extension 4–5 | |
| Foot dorsal flexion/plantar flexion 3–4 | Foot dorsal flexion/plantar flexion: plegia | |
| Big toe extension/ toes extension: left 5, right 0 | Big toe extension/ toes extension: left 3, right 1 | |
| Sensory testing | Sensory loss in stockings distribution | Sensory loss in stockings distribution |
| Pin prick test | He was able to distinguish sharp/blunt stimuli | He was able to distinguish sharp/blunt stimuli |
| Vibration test | At toe tips, metatarsal phalangeal joint and tibial tuberosity was 2/8 | At toe tips, metatarsal phalangeal joint and tibial tuberosity was 2/8 |
Figure 1.
Patient at the age of 30 .
Table 2.
Nerve conduction study
| Nerve conduction study (age at EMG 25 years) | ||
|---|---|---|
| Motor nerve | Nerve conduction velocity | CMAP amplitude |
| Median | 50 m/s | 0.5 mV |
| Peroneal | 43.7 m/s | 0.4 mV |
| Tibial | 38.2 m/s | 1.1 mV |
| Sensory nerve | Nerve conduction velocity | SNAP amplitude |
| Median | Not recorded | |
| Sural | Not recorded | |
| Needle EMG | Presence of abnormal spontaneous muscle fiber activity at rest | The assessment of MUAPs during voluntary activation of the muscle |
| Vastus med. | Fibrillations 1+ | Amp 4.0 mV |
| Waves 0, | Duration 10 ms | |
| Fasciculations 0, | ||
| Poly - few | ||
Waves = positive waves; Poly = polyphasic potentials; CMAP = compound muscle action potential; SNAP = sensory nerve action potential; Amp = amplitude; MUAP = motor unit action potential.
The patient has been tested, since 2001, for PMP22 duplication/deletion and Sanger sequencing of MPZ , MFN2 and GDAP1 genes were performed. In 2014 we targeted parallel sequencing with a gene panel of 72 genes, mutations of which were associated with inherited peripheral neuropathies. No significant variants that could explain the cause of CMT2 in this patient were found. Therefore, we sought to determine the genetic basis for CMT2 using exome sequencing. This was done with Agilent SureSelect Human All Exon V5 kit at the John P. Hussman Institute for Human Genomics in Miami, FL, USA. Data were analysed in Genesis application (formerly Gem.app; https://genomics.med.miami.edu/ ) ( Gonzalez et al. , 2015 ). In the second step, data were analysed in SureCall software (Agilent, Santa Clara, CA, USA). Variants were annotated with GenomeTrax ( http://www.biobase-international.com/product/genome-trax ), AlamutBatch (Interactive Biosoftware, http://www.interactive-biosoftware.com/ ) and Annovar software ( http://www.openbioinformatics.org/annovar/ ). Variants were filtered according to recommendations ( MacArthur et al. , 2014 ; Richards et al. , 2015 ). No variants that could explain the cause of the disease were found. The paper by Sevilla et al. (2015) was then published online, and we re-evaluated the whole-exome sequencing data from this patient and the p.R190W mutation was found.
The mutation c.568C>T (p.R190W) in the MORC2 gene (NM_014941.2) was confirmed with Sanger sequencing of exon 10 of the MORC2 gene in the patient, but not in his mother or his father, indicating the de novo origin of the mutation, similarly to that in Family fCMT-197 reported by Sevilla et al. (2015). Maternity/paternity was tested with a set of 10 microsatellite markers and false paternity was not observed.
Considering all of this information together, we concluded that mutation p.R190W is pathogenic and causal for CMT2 in this patient. Moreover, de novo occurrence of the mutation in our patient, but also in a Spanish family, suggest that p.R190W is a mutational hot spot and the most frequent mutation in this gene and that the occurrence in two of the three reported families in the Sevilla et al. (2015) paper is not by chance.
The patient presents with severe axonal form of CMT2. The clinical picture is similar to patients in the original paper by Sevilla et al. , mainly with respect to profound proximal involvement. Several proximal muscles are more affected than the more distal muscles, which is quite unique among the most hereditary neuropathies. Our study corroborates previously published work by Sevilla et al. (2015) and confirms the pathogenicity and causality of MORC2 mutations in CMT2. Our results indicate that mutations in the MORC2 gene may not be so extremely rare as some other causes and this gene should be included in a gene panel design for inherited neuropathies.
Funding
Supported by Ministry of Health of the Czech Republic, AZV CZ 15-33041A and DRO FNM 00064203. The authors declare that there are no conflicts of interest.
References
- Gonzalez MA, Falk MJ, Gai X, Postrel R, Schule R, Zuchner S . Innovative genomic collaboration using the GENESIS (GEM.app) platform . Hum Mutat 2015. ; 36 : 950 – 6 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, et al. . Guidelines for investigating causality of sequence variants in human disease . Nature 2014. ; 508 : 469 – 76 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology . Genet Med 2015. ; 17 : 405 – 24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla T, Lupo V, Martinez-Rubio D, Sancho P, Sivera R, Chumillas MJ, et al. . Mutations in the MORC2 gene cause axonal Charcot-Marie-Tooth disease . Brain 2016. ; 139 : 62 – 72 . [DOI] [PubMed] [Google Scholar]