TO THE EDITOR—We have now embarked on a new era of successful but more complex therapy against hepatitis C virus (HCV) infection [1]. Unfortunately, most healthcare professionals remain poorly aware of treatment options for HCV [2], and more providers are needed to care for patients with this infection.
Generally, gastroenterologists or hepatologists care for HCV-monoinfected patients, whereas infectious-disease specialists care for patients with human immunodeficiency virus (HIV)–HCV coinfection. Which providers should care for special populations of HCV-infected patients, such as those with cancer, is not well defined.
Patients with cancer infected with HCV could benefit from HCV therapy for several reasons: First, HCV treatment prevents progression of liver disease in cancer patients [3] and can eradicate the virus before immunosuppressive therapy is started. This is particularly important as HCV reactivation can occur following chemotherapy and can be fatal alone [4] or by precluding the use of lifesaving chemotherapy [5]. Second, antiviral therapy is associated with lower risk of recurrence and better survival of patients with HCV-related hepatocellular carcinoma [6]. Third, HCV treatment should be considered in all hematopoietic stem-cell transplant recipients, including cancer patients, who meet certain criteria [7], because HCV infection seems to be a significant risk factor for increased mortality after allogeneic hematopoietic stem-cell transplantation [8].
No evidence-based information about care of cancer patients with HCV infection is available, reflecting the limited data published on this patient population [9]. The most likely reason for this lack of information is that trials of pegylated interferon α-2 plus ribavirin typically exclude patients with cancer, in part because baseline hematologic abnormalities commonly seen in cancer patients can be exacerbated by HCV therapy. However, a recent prospective trial demonstrated the feasibility of using pegylated interferon α-2 plus ribavirin in cancer survivors [10].
In 2009, in response to overwhelming demand from our healthcare professionals to see HCV-infected patients, we established an HCV clinic operated by a multidisciplinary team: an experienced infectious-disease physician, a physician assistant, and a nurse. Initially, patients were screened for HCV antibodies, and those with confirmed infection were seen in the HCV clinic to determine whether antiviral therapy was indicated (Figure 1). Patients with indications for treatment were started on pegylated interferon α-2 (a or b) plus ribavirin, following guidelines for noncancer patients [9]. Patients in whom antiviral therapy was not recommended (eg, those unwilling to undergo treatment or with metastatic or terminal cancer, severe anemia, neutropenia, or thrombocytopenia) were referred to gastroenterologists for the management of chronic liver disease.
Figure 1.
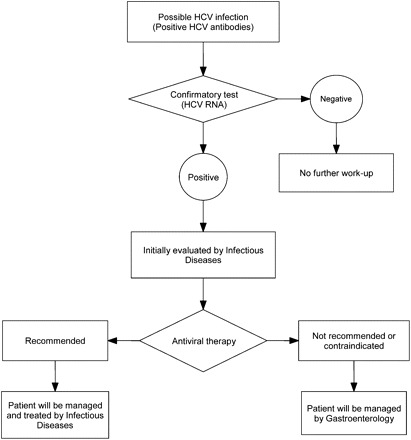
Operational algorithm for the Hepatitis C Clinic operated by the Department of Infectious Diseases. Abbreviation: HCV, hepatitis C virus.
The performance of the Infectious Diseases HCV clinic was then retrospectively analyzed as part of the MD Anderson Quality Improvement program (Clinical Safety and Effectiveness). The study was approved by the Institutional Review Board.
We compared data from the first fully functional year of the HCV clinic (2010) with data from the last year before our clinic was started (2008). We found a significant increase in the number of patients started on antiviral therapy in 2010 (3% vs 19%, P = .03) (Figure 2).
Figure 2.
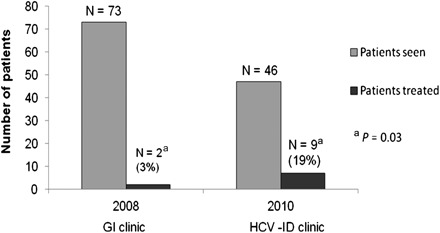
Performance of Hepatitis C Clinic operated by the Department of Infectious Diseases. Abbreviations: GI, gastroenterology; HCV, hepatitis C virus; ID, infectious diseases.
As expected in this difficult-to-treat population, frequent visits to monitor for adverse events were needed, and a multidisciplinary approach with involvement by other services (eg, hematology, psychiatry) was required for patients started on antiviral therapy. Among patients who completed therapy, sustained virological response was observed in more than one-third of patients—a rate similar to that previously observed in other difficult-to-treat populations (eg, HCV–HIV–coinfected) [8]. Establishment of the HCV clinic also led to revenue enhancement as the average number of consults and follow-ups per month increased as more patients were started on treatment. The HCV clinic allowed us to eliminate the patient backlog and give patients prompt access to specialized care.
To our knowledge, ours is the first clinic in the United States devoted to managing HCV infection in cancer patients. Based on our standardized approach, we demonstrate that therapy is possible in this understudied population of HCV-infected patients. Our experience indicates that infectious-disease specialists have the expertise necessary to manage another complicated population and to help the gastroenterology community care for HCV-infected cancer patients. We are up to the challenge.
Note
Potential conflicts of interest.
H. A. T. serves as a consultant for Vertex and Merck and has received grant support from Merck. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Jensen DM. A new era of hepatitis C therapy begins. N Engl J Med. 2011;364:1272–4. doi: 10.1056/NEJMe1100829. [DOI] [PubMed] [Google Scholar]
- 2.Hatzakis A, Wait S, Bruix J, et al. The state of hepatitis B and C in Europe: Report from the hepatitis B and C summit conference. J Viral Hepat. 2011;18:1–16. doi: 10.1111/j.1365-2893.2011.01499.x. [DOI] [PubMed] [Google Scholar]
- 3.Torres HA, Mahale P, Davila M, et al. Abstracts of the 48th Annual Meeting of the Infectious Diseases Society of America. Hepatitis C virus infection in cancer patients: the story of a forgotten population. October 21–24, 2010; Vancouver, Canada. Abstract 729. [Google Scholar]
- 4.Vento S, Cainelli F, Mirandola F, et al. Fulminant hepatitis on withdrawal of chemotherapy in carriers of hepatitis C virus. Lancet. 1996;347:92–3. doi: 10.1016/s0140-6736(96)90212-3. [DOI] [PubMed] [Google Scholar]
- 5.Arcaini L, Merli M, Passamonti F, et al. Impact of treatment-related liver toxicity on the outcome of HCV-positive non-Hodgkin's lymphomas. Am J Hematol. 2010;85:46–50. doi: 10.1002/ajh.21564. [DOI] [PubMed] [Google Scholar]
- 6.Miyatake H, Kobayashi Y, Iwasaki Y, et al. Effect of previous interferon treatment on outcome after curative treatment for hepatitis C virus-related hepatocellular carcinoma [published online ahead of print 12 October 2011] Dig Dis Sci. 2011 doi: 10.1007/s10620-011-1934-1. doi:10.1007/s10620-011-01934-1. [DOI] [PubMed] [Google Scholar]
- 7.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant-ation recipients: A global perspective. Biol Blood Marrow Transplant. 2009;15:1143–238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos CA, Saliba RM, de Pádua L, et al. Impact of hepatitis C virus seropositivity on survival after allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Haematologica. 2009;94:249–57. doi: 10.3324/haematol.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang JF, Yu ML, Huang CF, et al. The efficacy and safety of pegylated interferon plus ribavirin combination therapy in chronic hepatitis C patients with hepatocellular carcinoma post curative therapies—a multicenter prospective trial. J Hepatol. 2011;54:219–26. doi: 10.1016/j.jhep.2010.07.011. [DOI] [PubMed] [Google Scholar]


