Summary
This work shows that Hsp70 participates in mitogenic signaling in intestinal tumors through stabilization of β-catenin, Akt, ERK and ErbB2. This suggests that Hsp70 not only contributes to survival of transformed cells, but that it is also integral to transformation.
Abstract
Colorectal cancer (CRC) develops from colonic epithelial cells that lose expression of key tumor suppressor genes and/or gain expression of proproliferative and antiapoptotic genes like heat shock protein 70 (Hsp70). Heat shock protein 70 is overexpressed in CRC, but it is not known whether this is in response to the proteotoxic stress induced by transformation, or if it contributes to the process of transformation itself. Here, using the ApcMin/+ mouse model of CRC, we show that Hsp70 regulates mitogenic signaling in intestinal epithelial cells through stabilization of proteins involved in the receptor tyrosine kinase (RTK) and WNT signaling pathways. Loss of Hsp70 reduced tumor size with decreased proliferation and increased tumor cell death. Hsp70 loss also led to decreased expression of ErbB2, Akt, ERK and β-catenin along with decreased β-catenin transcriptional activity as measured by c-myc and axin2 expression. Upregulation of RTK or WNT signals are frequent oncogenic events in CRC and many other cancers. Thus, in addition to the role of Hsp70 in cell-survival after transformation, Hsp70 stabilization of β-catenin, Akt, ERK and ErbB2 are predicted to contribute to transformation. This has important implications not only for understanding the pathophysiology of these cancers, but also for treatment since anti-EGFR antibodies are in clinical use for CRC and EGFR is a major ErbB2 heterodimeric partner. Targeting Hsp70, therefore, might provide an alternative or complementary strategy for achieving better outcomes for CRC and other related cancer types.
Introduction
Colorectal cancer (CRC) is the third most frequent type of cancer, with approximately 1.2 million cases diagnosed world-wide each year (1,2). Of those patients, approximately 50% will die of their disease, making this the second most lethal cancer in the United States and the fourth most lethal malignancy in the world (3). CRCs develop from dysplastic colonic epithelium that present as polypoid, flat or multifocal lesions as they accumulate genetic mutations and subsequently metastasize (4). While genetic mutations drive transformation, persistence of transformed cells is also believed to rely on survival functions of wildtype proteins including the stress–response protein Hsp70. Hsp70 is induced by a variety of stresses and overexpression confers a progrowth, antiapoptotic phenotype on cells (5). Human CRCs overexpress Hsp70 and expression levels correlate with poorly differentiated state, advanced clinical stage and worse overall survival (6). However, it is still not known whether increased Hsp70 in tumors is a cause or effect of malignant transformation.
The Hsp70 family of proteins consists of 11 members in humans (7). Of these, Hsp70-1a and Hsp70-1b are thought to perform the stress-induced functions of this family and are generally referred to only as Hsp70 (8). The mouse homologs of these proteins are Hsp70.1 and Hsp70.3 and are also referred to collectively as Hsp70 (8). Heat shock protein 70 is constitutively expressed at low levels and upregulated during the G1 and S phases of the cell cycle or in response to a wide variety of cell stresses including heat shock, oxidative stress and hypoxia (9). During stress, Hsp70 generally promotes cell survival through interaction with conformationally modified proteins to refold them or regulate their association with ubiquitinylation machinery that targets them for destruction (10). In cancer cells, Hsp70 not only alleviates proteotoxic stress via its chaperone function, but also renders cells resistant to normal apoptotic signals (9). In contrast to cell survival mediated by Hsp70 in many tumors, Hsp70 knockdown is lethal in some cancer cell lines and confers senescence in others (11,12). Some of the effects observed with loss of Hsp70 may be mediated by loss of β-catenin activity since colonic tumors in Hsp70−/− mice treated with azoxymethane and dextran sodium sulfate exhibit decreased nuclear β-catenin localization compared to Hsp70+/+ counterparts (13).
Increased β-catenin-mediated transcriptional activity is a feature of most CRC (14). In normal colonocytes, β-catenin is a multifunctional protein found at the cell surfaces in association with α-catenin and E-cadherin as part of the adherens junction (14). Levels of cytosolic β-catenin are regulated by amino-terminal β-catenin phosphorylation and subsequent ubiquination by a destruction complex comprised of axin, casein kinase 1 (CK1), glycogen synthase kinase 3β (GSK-3β) and adenomatous polyposis coli (APC) that targets β-catenin for proteasomal destruction (14). Therefore, under steady-state conditions, very little β-catenin is found in the cytosol. WNT or receptor tyrosine kinase (RTK) signals inactivate the destruction complex, allowing β-catenin to accumulate in the cytosol and undergo transport into the nucleus where β-catenin associates with members of the TCF family of transcription factors and controls the expression of numerous mitogenic and antiapoptotic genes (15,16). In colonic tumor cells, the β-catenin destruction complex is frequently inactivated because of APC loss-of-function mutations or, less frequently, activating mutations in the CTNNB1 gene encoding β-catenin that prevent its destruction (17). While APC or CTNNB1 genetic mutations impair β-catenin destruction contributing to its increased transcriptional activity, there is also evidence that transcriptional activity of wildtype β-catenin may be increased due to constitutive activation of WNT or RTK signaling in CRC (18,19). A major RTK pathway involves the ErbB2/HER2/NEU receptor, which can be upregulated in colon cancer and is known to associate with Hsp90, an Hsp70 co-chaperone (20,21). ErB2 signals through ERK, p38-MAPK or AKT to contribute to β-catenin stability and transcriptional activity (22). Additionally, signaling through Akt or ERK can lead to increased cytosolic β-catenin directly by phosphorylation and release from E-cadherin and indirectly through down-regulation of junctional components or modification of junctional components to decrease their interaction with β-catenin (23–27).
The importance of β-catenin transcriptional activity in CRC and our previous observation that Hsp70 may be involved in this activity in colonic tumors induced by azoxymethane/dextran sodium sulfate led us to investigate the role of Hsp70 in intestinal tumor development in the ApcMin/+ mouse model. The ApcMin/+ model is a well-characterized model for CRC that contains a germline point mutation in the mouse Apc gene, converting codon 850 from a leucine (TTG) to a stop codon (TAG) (28). These mice develop intestinal adenomas throughout their gastrointestinal tract, which invariably show loss of heterozygosity with deletion of the remaining wild type Apc allele (29). Development of adenomas in ApcMin/+ mice resembles familial adenomatous polyposis (FAP), which is caused by germline mutations in the human adenomatous polyposis coli (APC) gene that lead to APC loss of function (29). This model is also thought to be relevant to sporadic CRC since approximately 80% of all colorectal tumors are associated with APC loss-of-function mutations (30).
Materials and methods
Immunoblotting and immunohistochemistry reagents
Antibodies reactive against c-Myc (#9402), PARP (#9542), β-actin (#4967), phospho-Akt (#4051), Akt (#9272), ERK (#4695), phospho-p44/42 MAPK (#4376), p44/42MAPK (#4695), p-38 (#9212) and phospho-p-38 (#9211) as well as PD98059 MEK1 inhibitor (#9900) and cell lysis buffer (#9803) were purchased from Cell Signaling (Technology Danvers, MA). Antibodies reactive against Hsp70 (#SPA-810; #SPA-812) and Hsc70 (#SPA-815) were obtained from Stressgen (Ann Arbor, MI). Mouse monoclonal β-catenin antibody (#610154) was purchased from BD Transduction Laboratories (La Jolla, CA). Mouse monoclonal anti-ubiquitin antibodies (clone FK2, #PW8810) were obtained from Biomol International (Farmingdale, NY). ImmunoPure Immobilized Protein A/G beads (20421) were purchased from Pierce (Rockford, IL). Protease inhibitor cocktail (Cat# 697498) was obtained from Roche Scientific (Indianapolis, IN). The proteasomal inhibitor MG132 (Cat#133407-82-6) was purchased from Tocris Bioscience (Minneapolis, MN).
Human tissue
Surgical resections of normal human colon and colon cancers were obtained from the Surgical Pathology Service at the University of Chicago under an approved IRB. Colon cancer tissue arrays were graciously provided by Dr. Olufunmilayo Olopade. Tissue was fixed in 4% formalin and stained as described below.
Animals
Mutant Apc mice (ApcMin/+) on a C57Bl6/J background were obtained from The Jackson Laboratory (Bar Harbor, ME). Hsp70−/− mice (Hsp70.1−/−, 70.3−/−) on a mixed 129S/C57Bl6 background were obtained from Dr. David Dix (Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, North Carolina). Hsp70−/− mice were interbred 12 generations to a C57Bl6 background using the speed congenic services of The Jackson Laboratory. Hsp70−/− mice were interbred with ApcMin/+ mice to produce ApcMin/+Hsp70+/− and Apc+/+Hsp70+/− F1 progeny mice, which were then mated. To maintain these lines, ApcMin/+Hsp70−/− male mice were interbred with Apc+/+Hsp70+/+ female mice. Mice were maintained under specific pathogen-free conditions. All animal studies were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Chicago.
Tissue harvest
Small intestinal tissue was harvested and divided longitudinally into proximal, middle and distal segments. Tumors, identified with a 10× dissecting scope, were enumerated and their sizes measured. Tumors were fixed in 4% buffered formalin for 24h and paraffin embedded. Sections were stained with hematoxylin and eosin, and histological features analyzed by an expert gastrointestinal pathologist (JH). Some tumors were flash frozen for subsequent protein or RNA extraction.
Cell lines
Hsp70 +/+ and Hsp70−/− mouse embryonic fibroblasts (MEF) were isolated from E13 embryos from Hsp70+/− × Hsp70+/− crosses, and genotyped. MEFs were cultured in Dulbecco’s modified essential medium supplemented with 10% fetal bovine serum, 0.1mM non-essential amino acids and penicillin/streptomycin antibiotics. HCT116 colon cancer cells and C2bbe cells, a Caco-2 colon cancer sub line, were purchased from ATCC (American Type Culture Collection, Virginia) and maintained as recommended. Low passage aliquots of all cell lines were frozen in liquid nitrogen and stocks were replaced periodically so that no cell line was actively passaged for more than 6 months. Cell morphology at low and high density was examined weekly to verify the identity of all cell lines. All cells were routinely tested and found to be negative for mycoplasma contamination.
Immunoblotting
Small intestinal mucosa was isolated by scraping with a glass slide. Mucosa or adenomas were homogenized in 1× cell lysis buffer (Cell Signaling) containing 1mM PMSF, 1× complete protease inhibitor cocktail (Roche), 50U/ml DNase (Amersham, Piscataway, NJ) and 50U/ml RNase (Ambion Inc, Austin, TX). An aliquot (10 μl) was used for protein analysis using the bicinchoninici acid method. To the remainder, 3× Laemmli stop solution was added and samples heated to 65°C for 10 minutes. Mucosal or tumor lysates were subjected to quantitative immunoblotting analysis. Briefly, proteins were separated by SDS-PAGE on 4–10% resolving polyacrylamide gradient gels and electroblotted to PVDF membranes. Blots were incubated overnight at 4°C with specific primary antibodies followed by 1h incubation with appropriate peroxidase coupled secondary antibodies and subsequent detection on X-OMAT film by enhanced chemiluminescence.
Immunohistochemistry
Immunostaining was performed on 4-μm thick paraffin-embedded tissue sections. Tissues were deparaffinized and rehydrated in decreasing concentrations of ethanol. Antigen retrieval for β-catenin and Hsp70 were performed by boiling slides in 10mM sodium citrate (pH 6.0). Endogenous peroxidase activity was quenched by incubating slides for 5min with Peroxidase Block (Dako, Glostrup, Denmark). Slides were incubated overnight at 4ºC with anti-β-catenin (1:200 dilution) or Hsp70 antibodies (1:500 dilution). Slides were then incubated with horseradish peroxidase-conjugated DAKO secondary antibodies for 45min. Epitopes were detected by 5–30sec incubation with 3,3′-diaminobenzidine (DAB) substrate chromogen. Slides were counterstained with Mayer’s hematoxylin. For each of these antibodies, negative controls included isotype-matched antibodies and showed no staining. Images were obtained using the QImaging capture software suite.
RNA interference
CaCo2 or HCT116 cells were transfected with Hsp70 siRNA or non-specific control siRNA according to manufacturer’s instructions (Ambion). We used siLentFect Lipid Reagent for RNAi transfections (Bio-Rad). Briefly, 50–70% confluent cells were transfected with siRNA (100nM final concentration). Four hours after transfection, cells were recovered in 10% serum. Twenty-four hours later, media was changed (without siRNA) and cells transfected with reporter plasmids. siRNA target gene knockdown was assessed by Western blotting and parallel treated cells were used for β-catenin reporter assays.
β-catenin reporter assays
MEF, HCT116 and Caco2 cells were seeded in 12-well plates (50–70% confluence) and tranfected with TOPflash or FOPflash (negative control) β-catenin/TCF reporter plasmids (gift from Dr. T-C He, University of Chicago) using TransIT LT-1 (Mirus; Madison, WI) transfection reagent and following the manufacturer’s recommendations. pRL-TK, that encodes Renilla luciferase, driven by thymidine kinase promoter, was co-transfected to normalize transfection efficiency. Cells were harvested 24h after transfection using 1× lysis buffer (Promega). Firefly and Renilla luciferase activities were determined in lysates using a Dual-Luciferase Reporter assay system according to the manufacturer’s instructions (Promega). Triplicate samples were assayed for Firefly luciferase activity and normalized to Renilla luciferase activity.
Quantitation of β-catenin and ErbB2 mRNA using real-time PCR
RNA was extracted from mouse intestinal mucosa and adenomas in Trizol (Invitrogen, Grand Island, NY). Complementary DNA was synthesized using Transcriptor First Strand cDNA Synthesis Kit (Roche). The primers used were: mouse β-catenin forward, 5′-AGACTGCAGATCTTGGACTGGACA-3′ and reverse 5′ ATCAGGCAGCCCATCAACTGGATA-3′; mouse GAPDH forward, 5′-GGCAAATTCAACGGCACAGT-3′ and reverse GAPDH 5′-AGAT GGTGATGGGCTTCCC-3′; mouse ErbB2 forward, 5′-CAGCTCCAAGTGTTCG AAACC-3′ and reverse ErbB2 5′-TGGCCATGCTGAGATGTACAG-3′; and mouse axin2 forward, 5′-GGCTGCGCTTTGATAAGGTC-3′ and reverse axin2 5′-GCAAAGCTCTCCTTTGGGGA-3′. Real-time PCR was performed using SYBR green I in a Lightcycler 480 II (Roche Diagnostics Indianapolis, IN). cDNA samples were amplified in triplicate using a two-step quantification cycling protocol. Each PCR reaction was repeated at least twice independently. β-catenin Ct values were normalized to Ct values for GAPDH that served as house-keeping reference gene (ΔCt = Ct β-catenin – Ct GAPDH). Transcript abundance was determined using 2exp(−ΔΔCt), where ΔΔCt = ΔCt experimental –ΔCt control.
Ubiquitination assay
Tumors were extracted in RIPA buffer containing protease inhibitors and incubated on ice for 30min. Lysates were centrifuged at 1300× g for 20min at 4°C. The soluble fraction was recovered and protein concentrations measured using the bicinchoninic acid method. The soluble fraction was incubated with protein G Sepharose beads for 30min at 4°C to pre-clear samples. Samples (1mg protein) were incubated overnight with mouse monoclonal anti-β-catenin antibodies followed by 3h incubation with protein G Sepharose beads (100 μl) at 4°C to collect immune complexes. Sepharose G beads were washed three times with lysis buffer containing protease inhibitors. Bound complexes were released by heating to 80°C in SDS sample buffer for 5min. The soluble proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Mouse monoclonal anti-ubiquitin antibodies were used to detect ubiquitinated β-catenin.
BrdU incorporation and TUNEL assay
Formalin-fixed paraffin-embedded tumor sections were analyzed for proliferation and cell death using ZYMED BrdU Staining kit (No.93–3943) and Millipore ApopTag Plus Peroxidase in situ Apoptosis Detection kit (Cat# S7101), respectively, following the manufacturers’ instructions as previously described.
Cellular proliferation assay
Cells grown in a 96-well tissue culture plate for 48h were incubated with 10 μl of WST-1 reagent (Roche #05015944001) for 1h. After this incubation period, the formazan dye formed was quantitated with a scanning multiwell spectrophotometer (ELISA reader) at a wavelength of 450nm. The measured absorbance directly correlates to the number of viable cells.
Statistical analysis
Results are presented as means ± SEM or means ± SD for the indicated number of experiments. The results of multiple experiments were analyzed using two-sided Student’s t-tests and a value of P < 0.05 was considered statistically significant. Tumor multiplicity was compared between groups using negative binomial regression.
Results
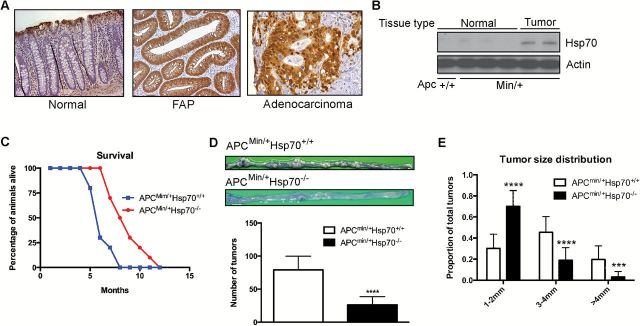
In normal human colon, Hsp70 expression is low except in mature, non-proliferating epithelial cells (Figure 1A). In contrast, Hsp70 is highly expressed in colonic epithelial tumor cells from patients with familial adenomatous polyposis and sporadic CRC, where nuclear localization of Hsp70 is a prominent feature (Figure 1A). Intestinal tumors from ApcMin/+ mice also showed increased expression of Hsp70 versus normal mucosa (Figure 1B). To study the role of Hsp70 in tumor initiation and progression, we crossed Hsp70−/− mice with ApcMin/+ mice to create a model of intestinal tumorigenesis in mice deleted of Hsp70. Hsp70−/− mice lack both Hsp70.1 and Hsp70.3 genes and so are deficient in inducible somatic cell Hsp70 gene expression. We observed that loss of Hsp70 increased survival time compared to ApcMin/+ mice expressing wildtype Hsp70 (Figure 1C). ApcMin/+Hsp70+/+ mice developed many small bowel tumors by 6 months of age, but the number of tumors was decreased in ApcMin/+Hsp70−/− mice (Figure 1D). Heat-shock protein 70 expression also affected tumor size since the average diameter of small bowel adenomas in ApcMin/+Hsp70−/− mice was significantly smaller than ApcMin/+Hsp70+/+ littermates (Figure 1E).
Figure 1.
Hsp70 expression is increased in human CRC and a mouse model of intestinal tumorigenesis and loss of Hsp70 results in fewer and smaller intestinal tumors in ApcMin/+ mice. (A) Immunostaining for Hsp70 (brown) in formalin-fixed, paraffin-embedded (FFPE) normal human colon, a colonic polyp from a patient with familial adenomatous polyposis and sporadic human colonic adenocarcinoma. (B) Immunoblotting for Hsp70 in lysates of normal intestinal mucosa of Apc+/+ and ApcMin/+ mice and tumors isolated from the small intestine of ApcMin/+ mice. Blots are representative of normal mucosa and tumors from individual Apc+/+ (n = 1) and ApcMin/+ (n = 5) mice. (C) Survival curve of ApcMin/+Hsp70+/+ and ApcMin/+Hsp70−/− mice. All mice were on C57Bl6/J background and included 10 mice in each group. (D) Number of tumors per mouse (mean ± S.E.M) in ApcMin/+Hsp70+/+ mice (n = 18) and ApcMin/+Hsp70−/− (n = 29) mice. Mice were sacrificed at 6 months of age. Top panel: representative intestines from indicated mice. (E) Tumor size distribution in 6 month old ApcMin/+Hsp70+/+ (n = 18) and ApcMin/+Hsp70−/− (n = 29) reported as mean ± S.E.M. ***P < 0.005; ****P < 0.001, compared to ApcMin/+Hsp70+/+ mice.
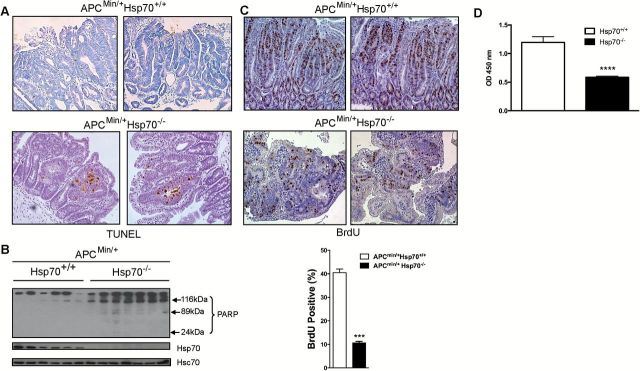
Hsp70 is thought to prevent apoptosis in cancer cells, so we evaluated cell death in tumors from ApcMin/+Hsp70+/+ and ApcMin/+Hsp70−/− mice using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and immunoblotting for cleaved poly (ADP-ribose) polymerase (PARP). These experiments showed that apoptosis was increased in adenomas from ApcMin/+Hsp70−/− mice compared to ApcMin/+Hsp70+/+ mice (Figure 2A and B). We also observed reduced proliferation, as measured by bromodeoxyuridine (BrdU) incorporation in the adenomas from ApcMin/+Hsp70−/− mice compared to controls (Figure 2C). Decreased proliferation was also observed in Hsp70−/− mouse embryonic fibroblasts (Figure 2D), indicating that Hsp70 positively regulates mitogenic pathways in normal and transformed cells. These findings indicate that Hsp70 protects cells from apoptosis and promotes growth during intestinal tumorigenesis and loss of these effects would be predicted to contribute to smaller tumors in ApcMin/+Hsp70−/− mice.
Figure 2.
Hsp70 regulates proliferation and cell death. (A) Apoptosis assessed by TUNEL staining (brown) in tumors from FFPE ApcMin/+Hsp70+/+ (n = 5) and ApcMin/+Hsp70−/− (n = 5) tumors. (B) PARP cleavage assessed by Western blotting in lysates from ApcMin/+Hsp70+/+ (n = 6) and ApcMin/+Hsp70−/− (n = 7) tumors. (C) Proliferation as assessed by BrdU incorporation (brown) after 2h labeling in vivo in ApcMin/+Hsp70+/+ (n = 3) and ApcMin/+Hsp70−/− (n = 3) tumors (mean ± S.E.M), ***P < 0.05 compared to ApcMin/+Hsp70+/+. (D) Hsp70+/+ and Hsp70−/− MEFs were plated in 96-well plates at 5×104 cells/well in two independent experiments and the number of viable cells quantified by WST-1 assay. ****P < 0.001, compared to Hsp70+/+ MEFs.
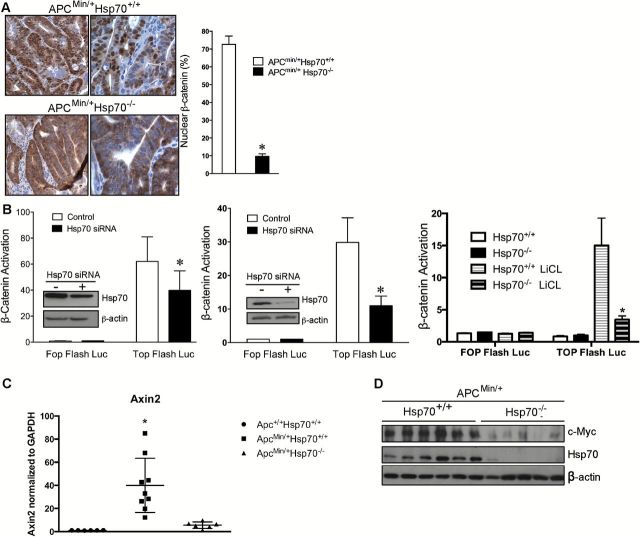
The altered proliferative responses that we measured in Hsp70−/− cells led us to examine β-catenin expression and activity in these cells. By immunohistochemical analysis, we observed reduced nuclear β-catenin in ApcMin/+Hsp70−/− versus ApcMin/+Hsp70+/+ tumors (Figure 3A). β-catenin-mediated transcriptional activity was also compromised in ApcMin/+Hsp70−/− cells. Transfection of Caco-2 (Figure 3B left panel) or HCT116 cells (Figure 3B middle panel) with a Tcf-1 regulated reporter (Top Flash) following knockdown of Hsp70 or in Hsp70−/− MEFs (Figure 3B right panel) showed that loss of Hsp70 decreased activation of this β-catenin reporter gene. Expression of axin2, a target of β-catenin signaling in CRCs was also decreased in the absence of Hsp70 (Figure 3C) (31). Expression of the c-Myc protein was also decreased in ApcMin/+Hsp70−/− cells compared to controls (Figure 3D). We speculate that these results indicate that the proliferative defects detected in Hsp70−/− adenomas are due, at least in part, to loss of β-catenin-mediated mitogenic signals.
Figure 3.
β-catenin transcriptional activity is decreased in Hsp70−/− cells. (A) Immunostaining for β-catenin (brown) in FFPE ApcMin/+Hsp70+/+ (n = 10) and ApcMin/+Hsp70−/− (n = 6) tumors. Nuclear β-catenin was quantified by determining the number of positive nuclei per 100 cells in images of immunostained cells (mean ± S.E.M; P < 0.05 compared to ApcMin/+Hsp70+/+). (B) Human colon cancer cells (Caco-2 in left panel and HCT116 middle panel) were transfected with Hsp70 siRNA or scrambled (control) oligonucleotides for 24h and then transfected with β-catenin/Tcf TOPflash reporter or negative control FOPflash reporter for 24h with values normalized using pRL-TK. Mouse embryonic fibroblasts (right panel) deficient in Hsp70 were transfected with TOPflash or FOPflash reporters. Lysates were assayed for luciferase activity and Hsp70 and β-actin protein levels to verify knockdown in the colon cancer cells (P < 0.05 compared to scrambled siRNA transfected cells). Normalized luciferase activity is reported as β-catenin activation. (C) Axin2 mRNA levels were measured by qRT-PCR on samples from normal small intestine of Apc+/+Hsp70+/+ mice (n = 2) and tumors from ApcMin/+Hsp70+/+ (n = 3) and ApcMin/+Hsp70−/− (n = 2) mice. *P < 0.05. (D) c-Myc as assessed by Western blotting in lysates of tumors from ApcMin/+Hsp70+/+ (n = 7) and ApcMin/+Hsp70−/− (n = 7) mice.
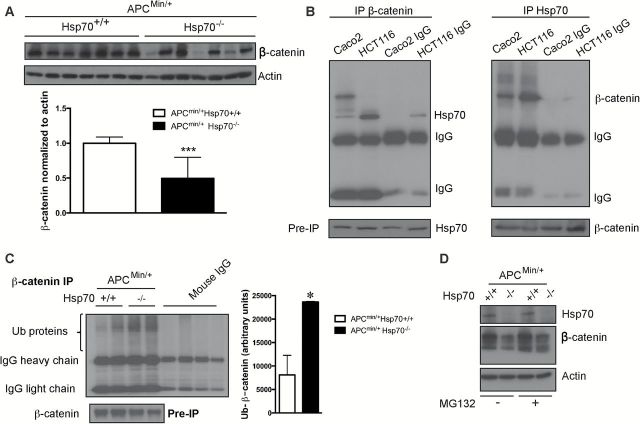
Quantitative real-time PCR showed that mRNA levels for CTNNB1 gene coding for β-catenin in intestinal tumors were comparable between genotypes (Supplementary Figure 1, available at Carcinogenesis Online). However, β-catenin levels in adenomas from ApcMin/+Hsp70−/− mice were reduced as assessed by Western blotting (Figure 4A). This suggests that β-catenin steady-state transcription is unaffected, but protein production and/or degradation differ in tumors between the genotypes. To evaluate this, we first determined that Hsp70 and β-catenin physically interact in cells using immunoprecipitation of cellular lysates from Caco2 and HCT 116 cells (Figure 4B). We next examined β-catenin ubiquitination. Concomitant with reduced β-catenin protein levels in adenomas from ApcMin/+Hsp70−/− mice, levels of ubiquitinated β-catenin were increased (Figure 4C). We confirmed that loss of Hsp70 increases proteasomal degradation of β-catenin by treating tumor cells isolated from ApcMin/+Hsp70+/+ and ApcMin/+Hsp70−/− mice with the proteasomal inhibitor MG132 and immunoblotting for β-catenin (Figure 4D). These data support the hypothesis that β-catenin interacts with Hsp70 and that its degradation is greater in ApcMin/+Hsp70−/− adenomas compared to ApcMin/+Hsp70+/+ adenomas.
Figure 4.
Hsp70 controls β-catenin protein stability in tumor cells. (A) β-catenin levels were measured by Western blotting in lysates from ApcMin/+Hsp70+/+ (n = 7) and ApcMin/+Hsp70−/− (n = 7) tumors. Each lane represents lysates from a different tumor. Densitometry was used to quantify protein expression. (***P < 0.005 compared to ApcMin/+Hsp70+/+). (B) Cell lysates from Caco2 or HCT116 cells were immunoprecipitated with anti-Hsp70 or anti-β-catenin antibodies or IgG alone (negative control) and then probed for Hsp70 or β-catenin. (C) Ubiquitinated β-catenin. β-catenin was immunoprecipitated from tumor lysates and the immunoprecipitates were probed for ubiquitin. Quantification of ubiquitin by densitometry is shown in the right panel. *P < 0.05 compared to ApcMin/+Hsp70+/+. (D) Cells isolated from tumors in ApcMin/+Hsp70+/+ and ApcMin/+Hsp70−/− mice were treated with a vehicle control or 20 μM MG132 for 2h then lysed and immunoblotted for Hsp70, β-catenin and actin.
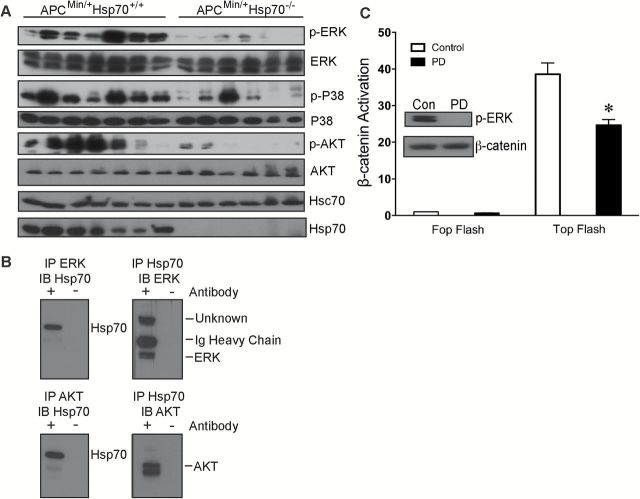
The ERK, p38-MAPK and Akt pathways were all activated in ApcMin/+Hsp70+/+ tumors compared to ApcMin/+Hsp70−/− tumors, where their activations were significantly attenuated or undetectable (Figure 5A). Coimmunoprecipitation of Hsp70 with either ERK or Akt in tumor lysates from ApcMin/+Hsp70+/+ mice showed that Hsp70 interacts with signaling proteins upstream of β-catenin (Figure 5B). We verified that β-catenin-mediated transcriptional activity was regulated by ERK signaling in CRC cells using the MEK inhibitor PD98059 (PD). The PD compound decreased β-catenin activity in Caco-2 cells as measured by the Top Flash reporter plasmid (Figure 5C). Taken together these findings suggest that upstream signals that would be expected to stabilize β-catenin are decreased in ApcMin/+Hsp70−/− adenomas.
Figure 5.
The Ras-Raf-MAPK and PI3K-AKT-mTor signaling cascades are dependent upon Hsp70. (A) Immunoblotting for activated proteins involved in the Ras-Raf-MAPK (ERK and P38) and PI3K-AKT-mTor (AKT) signaling cascades in lysates from ApcMin/+Hsp70+/+ (n = 7) and ApcMin/+Hsp70−/− (n = 7) tumors. (B) Hsp70, ERK or AKT were immunoprecipitated from tumor lysates and the immunoprecipitates were probed for the corresponding proteins. (C) Caco-2 cells were transfected with Top Flash or Fop Flash (negative control) for 24h, then treated with PD98059 (PD) for 2h and lysates were assayed for phospho-ERK (p-ERK) and total β-catenin as well as luciferase activity. Normalized luciferase activity is reported as β-catenin activation. *P < 0.05, compared to vehicle-treated cells.
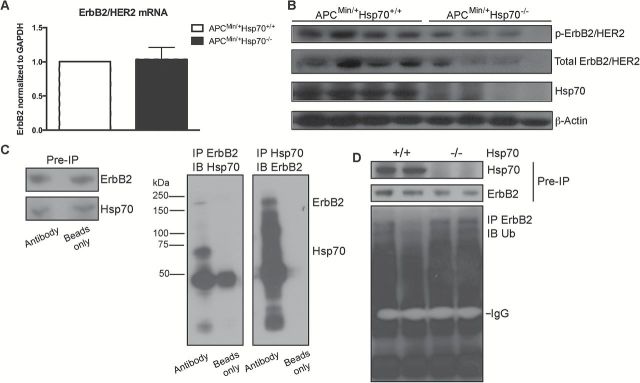
Quantitative RT-PCR revealed comparable levels of ErbB2 mRNA in ApcMin/+Hsp70−/− and ApcMin/+Hsp70+/+ tumors, whereas total and phosphorylated-ErbB2 were decreased in ApcMin/+Hsp70−/− tumors compared to ApcMin/+Hsp70+/+ tumors (Figure 6A and B). Coimmunoprecipitation experiments revealed that Hsp70 and ErbB2 are found in a complex together, suggesting that the chaperone function of Hsp70 may be important for ErbB2 protein stability (Figure 6C). When we examined ubiquitination of ErbB2, there was no significant difference between ApcMin/+Hsp70+/+ and ApcMin/+Hsp70−/− tumor cells (Figure 6D). This may suggest that the difference in ErbB2 levels is not due to differences in degradation, that it is due to degradation independent of the amount of ubiquitination, or it may reflect a failure of the ErbB2 antibody to efficiently immunoprecipitate ubiquitinated ErbB2. Based on our data that Hsp70 likely regulates stability of β-catenin we favor the possibility that ErbB2 degradation is different in Hsp70−/− and Hsp70+/+ tumors, but that our methods are not sufficient to appreciate this difference. Both degradation without ubiquitination and failure of the ErbB2 antibody to efficiently immunoprecipitate ErbB2 have been reported (32,33). We attempted to use an antibody that has been reported to immunoprecipitate human ubiquitinated ErbB2, but it does not appear to immunoprecipitate mouse ErbB2 (data not shown). Our studies indicate that Hsp70 is required for ErbB2 protein expression, and we postulate that Hsp70 also controls β-catenin stabilization by ErbB2 upstream signals. Altogether, these data support a model in which Hsp70 stabilizes ErbB2 and maintains ErbB2 signals that, in turn, upregulate β-catenin to increase mitogenic signals while inhibiting cell death signals. Additionally, increases in axin2 expression also suggest that Hsp70 controls Wnt signaling, another pathway that has been implicated in CRC.
Figure 6.
Hsp70 coassociates with ErbB2 and regulates ErbB2 protein levels. (A) Quantitative RT-PCR for ErbB2 mRNA expression in tumors from ApcMin/+Hsp70+/+ (n = 3) and ApcMin/+Hsp70−/− (n = 3) mice. (B) Total and phosphorylated ErbB2 as assessed by Western blotting in lysates from ApcMin/+Hsp70+/+ (n = 4) and ApcMin/+Hsp70−/− (n = 4) tumors. (C) Co-association of Hsp70 and ErbB2 in lysates of ApcMin/+Hsp70+/+ tumors (n = 6). Tumor lysates were immunoprecipitated with Hsp70 or ErbB2 and the IP’s resolved by SDS-PAGE. The conjugate member (ErbB2 for Hsp70 IP and Hsp70 for ErbB2 IP) were probed by Western blotting. (D) Ubiquitinated-ErbB2. ErbB2 was immunoprecipitated and IPs probed for ubiquitin in ApcMin/+Hsp70+/+ (n = 6) and ApcMin/+Hsp70−/− (n = 6) tumors. The amount of lysate used for immunoprecipitation was adjusted so that levels of ErbB2 were comparable between ApcMin/+Hsp70+/+ and ApcMin/+Hsp70−/− samples.
Discussion
There are several ways in which increased Hsp70 is believed to contribute to aberrant epithelial cell growth and tumor formation in the intestine. First, the canonical Hsp70 chaperone function maintains protein folding during the increased protein synthesis associated with rapid growth (34). Second, Hsp70 has direct and indirect antiapoptotic actions that are predicted to prevent death in these rapidly growing cells (34). Third, we have identified a novel Hsp70 function that supports RTK signaling and β-catenin upregulation. We speculate that this involves interaction with and stabilization of ErbB2, Akt, ERK and β-catenin. The net effect of this function is to promote β-catenin transcriptional activity.
Our data suggests that increased Hsp70 expression in tumor cells supports multiple aspects of the RTK signaling pathway. The ErbB/HER RTK family includes EGFR (ErbB1), ErbB2 (Her2/neu), ErbB3 and ErbB4 (35). These receptors form homo- or heterodimers upon binding ligands that induce phosphorylation, activation and eventual internalization, ubiquitination and destruction of the receptor (36). ErbB2 is unique in this family in that it does not appear to have a high-affinity ligand, but rather forms a heterodimer with other liganded family members (36). Heterodimeric complexes involving ErbB2 induce the strongest and most persistent signals of all the ErbB dimeric combinations (37). Heterodimerization between ErbB2 and EGFR may be particularly important in CRC since EGFR activity is important in tumor initiation and progression in both humans and mouse models of tumorigenesis (38–40). Signaling through ErbB2 heterodimers promotes cell proliferation and survival via the Ras-Raf-MAPK and PI3K-Akt-mTOR pathways (41). These signals contribute to β-catenin transcriptional activity by cooperating with other mitogenic signals to inactivate the β-catenin destruction complex, stabilizing β-catenin and promoting translocation of β-catenin from cytosol to nucleus where it activates transcriptional targets (27,42–44).
Upregulation of ErbB2-mediated RTK signaling is a feature of many cancers, including some CRC. At the level of the receptor, the molecular mechanisms include increased receptor expression at the genomic (gene amplification) or transcriptional level, activating mutations in the receptor, decreased inactivating receptor phosphatase activity, or escape from endocytic receptor downregulation (41,45,46). Our data show that ErB2 protein levels are increased in tumors from Apc min mice expressing Hsp70, but that transcription of ErbB2 is unaffected by Hsp70 expression. A previous report showed that ErbB2 interacts with the E3 ligase Cul5, which promotes its ubiquitination and degradation (47). Interestingly, the Hsp70 co-chaperone Hsp90 prevents ErbB2 association with Cul5 and Hsp90 inhibition leads to ErbB2 degradation in a proteasome-dependent manner (48,49). Our data also agree with a report from Meng et al., that the proliferative effects of ErbB2 overexpression are abrogated in the absence of Hsp70 in a mouse model of mammary tumor development (50). Therefore, Hsp70 likely stabilizes ErbB2 protein levels by preventing proteasome-dependent receptor degradation.
This study also identified a potential role for Hsp70 in signaling and β-catenin transcriptional activity downstream of RTK receptors since Hsp70 interacts with Akt, ERK and β-catenin in tumors from Apc min mice and Hsp70 deficient tumors have lower levels of all of these proteins. Though we mainly focused on the RTK signaling pathway, we also observed a decrease in the expression of axin2 mRNA, a direct WNT target in this model system, in the absence of Hsp70 (51). Thus, we speculate that Hsp70 supports β-catenin transcriptional activity through multiple upstream signals including RTK and Wnt pathways.
We have identified a novel and essential role for Hsp70 in intestinal tumorigenesis as a regulator of cell growth and proliferation that acts to support RTK and WNT pathway signaling. The end result of signaling through each of these pathways is upregulation of β-catenin transcriptional activity, a major contributor to cell growth and proliferation in many different types of cancer. Therefore, our findings have potentially broad implications for cancers with increased β-catenin transcriptional activity involving RTK signaling pathways, including notably ErbB2. They also suggest that inhibition of Hsp70 in cancer cells with high levels of RTK activity might synergize with other antineoplastic therapies to limit proliferation and accelerate cell death.
Supplementary material
Supplementary Figure 1 can be found at Supplementary Data
Funding
This study was supported by the following grants: DK47722 (E.B.C.), NIDDK P30 DK42086 (E.B.C.), CA164124 (M.B.), CCFA Senior Research Award #1836 (M.B.), American Cancer Society #215889 (K.H.G.), Gastrointestinal Research Foundation of Chicago (E.B.C./M.B.) and 1F32DK082104 (J.S.M.).
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance of Dr. Olufunmilayo Olopade who provided human colon cancer tissue arrays for these studies. Y.T. performed all experiments. J.S.M., K.H.G. and J.H. contributed to the analysis/review/interpretation of data and M.B. and E.B.C. conceived and were involved in all aspects of the study.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CRC
colorectal cancer
- Hsp70
heat shock protein 70
- MEF
mouse embryonic fibroblasts
- RTK
receptor tyrosine kinase
References
- 1. Siegel R., et al. (2014) Colorectal cancer statistics, 2014. CA. Cancer J. Clin., 64, 104–117. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A., et al. (2011) Global cancer statistics. CA. Cancer J. Clin., 61, 69–90. [DOI] [PubMed] [Google Scholar]
- 3. Haggar F.A., et al. (2009) Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg., 22, 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fearon E.R. (2011) Molecular genetics of colorectal cancer. Annu. Rev. Pathol., 6, 479–507. [DOI] [PubMed] [Google Scholar]
- 5. Calderwood S.K. (2013) Molecular cochaperones: tumor growth and cancer treatment. Scientifica (Cairo)., 2013, 217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lazaris A.C., et al. (1995) Heat shock protein 70 and HLA-DR molecules tissue expression. Prognostic implications in colorectal cancer. Dis. Colon Rectum, 38, 739–745. [DOI] [PubMed] [Google Scholar]
- 7. Tavaria M., et al. (1996) A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones, 1, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy M.E. (2013) The HSP70 family and cancer. Carcinogenesis, 34, 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zorzi E., et al. (2011) Inducible hsp70 in the regulation of cancer cell survival: analysis of chaperone induction, expression and activity. Cancers (Basel)., 3, 3921–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lanneau D., et al. (2010) Heat shock proteins: cell protection through protein triage. Scientific World J., 10, 1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nylandsted J., et al. (2000) Heat shock protein 70 is required for the survival of cancer cells. Ann. N. Y. Acad. Sci., 926, 122–125. [DOI] [PubMed] [Google Scholar]
- 12. Nylandsted J., et al. (2000) Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc. Natl. Acad. Sci. USA, 97, 7871–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tao Y., et al. (2009) Inducible heat shock protein 70 prevents multifocal flat dysplastic lesions and invasive tumors in an inflammatory model of colon cancer. Carcinogenesis, 30, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White B.D., et al. (2012) Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology, 142, 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fodde R., et al. (2001) APC, signal transduction and genetic instability in colorectal cancer. Nat. Rev. Cancer, 1, 55–67. [DOI] [PubMed] [Google Scholar]
- 16. Porter A.C., et al. (1998) Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene, 17, 1343–1352. [DOI] [PubMed] [Google Scholar]
- 17. Rasool S., et al. (2014) Genetic unraveling of colorectal cancer. Tumour Biol., 35, 5067–5082. [DOI] [PubMed] [Google Scholar]
- 18. Roskoski R., Jr (2014) The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol. Res., 79, 34–74. [DOI] [PubMed] [Google Scholar]
- 19. Najdi R., et al. (2011) Wnt signaling and colon carcinogenesis: beyond APC. J. Carcin., 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou P., et al. (2003) ErbB2 degradation mediated by the co-chaperone protein CHIP. J. Biol. Chem., 278, 13829–13837. [DOI] [PubMed] [Google Scholar]
- 21. Xu W., et al. (2001) Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J. Biol. Chem., 276, 3702–3708. [DOI] [PubMed] [Google Scholar]
- 22. Wong N.A., et al. (2002) Beta-catenin–a linchpin in colorectal carcinogenesis? Am. J. Pathol., 160, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang H., et al. (2013) Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One, 8, e56664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang D., et al. (2007) Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem., 282, 11221–11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ji H., et al. (2009) EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol. Cell, 36, 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng H., et al. (2013) Glycogen synthase kinase-3 beta regulates Snail and β-catenin expression during Fas-induced epithelial-mesenchymal transition in gastrointestinal cancer. Eur. J. Cancer, 49, 2734–2746. [DOI] [PubMed] [Google Scholar]
- 27. Krejci P., et al. (2012) Receptor tyrosine kinases activate canonical WNT/β-catenin signaling via MAP kinase/LRP6 pathway and direct β-catenin phosphorylation. PLoS One, 7, e35826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nandan M.O., et al. (2010) Genetic and chemical models of colorectal cancer in mice. Curr. Colorectal Cancer Rep., 6, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nandan M.O., et al. (2010) Genetic and chemical models of colorectal cancer in mice. Curr. Colorectal Cancer Rep., 6, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Goss K.H., et al. (2000) Biology of the adenomatous polyposis coli tumor suppressor. J. Clin. Oncol., 18, 1967–1979. [DOI] [PubMed] [Google Scholar]
- 31. Wu Z.Q., et al. (2012) Canonical Wnt suppressor, Axin2, promotes colon carcinoma oncogenic activity. Proc. Natl. Acad. Sci. USA, 109, 11312–11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pust S., et al. (2013) Flotillins as regulators of ErbB2 levels in breast cancer. Oncogene, 32, 3443–3451. [DOI] [PubMed] [Google Scholar]
- 33. Liu H., et al. (2009) Regulation of ErbB2 receptor status by the proteasomal DUB POH1. PLoS One, 4, e5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Radons J. (2016) The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mendelsohn J., et al. (2000) The EGF receptor family as targets for cancer therapy. Oncogene, 19, 6550–6565. [DOI] [PubMed] [Google Scholar]
- 36. Mendelsohn J., et al. (2000) The EGF receptor family as targets for cancer therapy. Oncogene, 19, 6550–6565. [DOI] [PubMed] [Google Scholar]
- 37. Rubin I., et al. (2001) The basic biology of HER2. Ann. Oncol., 12, S3–S8. [DOI] [PubMed] [Google Scholar]
- 38. Markman B., et al. (2010) EGFR and KRAS in colorectal cancer. Adv. Clin. Chem., 51, 71–119. [DOI] [PubMed] [Google Scholar]
- 39. Roberts R.B., et al. (2002) Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc. Natl. Acad. Sci. USA, 99, 1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moran A.E., et al. (2004) Apc deficiency is associated with increased Egfr activity in the intestinal enterocytes and adenomas of C57BL/6J-Min/+ mice. J. Biol. Chem., 279, 43261–43272. [DOI] [PubMed] [Google Scholar]
- 41. Herter-Sprie G.S., et al. (2013) Activating Mutations in ERBB2 and Their Impact on Diagnostics and Treatment. Front. Oncol., 3, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu Z., et al. (2003) Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell, 4, 499–515. [DOI] [PubMed] [Google Scholar]
- 43. Hu T., et al. (2010) Convergence between Wnt-β-catenin and EGFR signaling in cancer. Mol. Cancer, 9, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kaidanovich-Beilin O., et al. (2011) GSK-3: Functional Insights from Cell Biology and Animal Models. Front. Mol. Neurosci., 4, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roepstorff K., et al. (2008) Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem. Cell Biol., 129, 563–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ocaña A., et al. (2013) Targeting HER receptors in cancer. Curr. Pharm. Des., 19, 808–817. [DOI] [PubMed] [Google Scholar]
- 47. Ehrlich E.S., et al. (2009) Regulation of Hsp90 client proteins by a Cullin5-RING E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA, 106, 20330–20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marx C., et al. (2010) ErbB2 trafficking and degradation associated with K48 and K63 polyubiquitination. Cancer Res., 70, 3709–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jeong J.H., et al. (2008) Quercetin-induced ubiquitination and down-regulation of Her-2/neu. J. Cell. Biochem., 105, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meng L., et al. (2011) Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene, 30, 2836–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jho E.H., et al. (2002) Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol., 22, 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.