In a multicenter prospective study of 2016 adults hospitalized with radiographically confirmed community-acquired pneumonia, we found no evidence to suggest that statin use before and during hospitalization improved length of stay or in-hospital mortality.
Keywords: statins, hydroxymethylglutaryl-CoA reductase inhibitors, pneumonia, hospitalization
Abstract
Background. Prior retrospective studies suggest that statins may benefit patients with community-acquired pneumonia (CAP) due to antiinflammatory and immunomodulatory effects. However, prospective studies of the impact of statins on CAP outcomes are needed. We determined whether statin use was associated with improved outcomes in adults hospitalized with CAP.
Methods. Adults aged ≥18 years hospitalized with CAP were prospectively enrolled at 3 hospitals in Chicago, Illinois, and 2 hospitals in Nashville, Tennessee, from January 2010—June 2012. Adults receiving statins before and throughout hospitalization (statin users) were compared with those who did not receive statins (nonusers). Proportional subdistribution hazards models were used to examine the association between statin use and hospital length of stay (LOS). In-hospital mortality was a secondary outcome. We also compared groups matched on propensity score.
Results. Of 2016 adults enrolled, 483 (24%) were statin users; 1533 (76%) were nonusers. Statin users were significantly older, had more comorbidities, had more years of education, and were more likely to have health insurance than nonusers. Multivariable regression demonstrated that statin users and nonusers had similar LOS (adjusted hazard ratio [HR], 0.99; 95% confidence interval [CI], .88–1.12), as did those in the propensity-matched groups (HR, 1.03; 95% CI, .88–1.21). No significant associations were found between statin use and LOS or in-hospital mortality, even when stratified by pneumonia severity.
Conclusions. In a large prospective study of adults hospitalized with CAP, we found no evidence to suggest that statin use before and during hospitalization improved LOS or in-hospital mortality.
A dysregulated inflammatory response that leads to cellular damage is a major contributor to treatment failure and mortality among patients with severe community-acquired pneumonia (CAP) [1–4]. Therapies that modulate inflammation may improve clinical outcomes. Although best known for their benefits in cardiovascular disease, statins (3-hydroxy-3-methylgultaryl coenzyme A reductase inhibitors) have antiinflammatory and immunomodulatory effects [5–9], and several large observational studies suggest that statins may improve clinical outcomes in infectious processes [10–17]. Systematic reviews of mainly observational studies that examined the relationship between statins and various infections, including pneumonia mortality, reported significant protective effects [18–20]. In contrast, recent randomized controlled trials (RCTs) have not shown any benefit of statins for patients with acute respiratory distress syndrome (ARDS), including sepsis-associated ARDS, and ventilator-associated pneumonia [21–23].
No definitive RCTs have examined the effect of statins on CAP outcomes, including in hospitalized patients. Carefully done observational studies could fill this knowledge gap. Here, we determined whether statin use was associated with improved outcomes among adults hospitalized with CAP, including those with severe pneumonia, by comparing hospital length of stay (LOS) and in-hospital mortality between statin users and nonusers who were enrolled in the Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study [24]. LOS has been used as a surrogate for time to clinical stability [25], and shorter LOS is associated with decreased cost and risk of adverse nosocomial events [26]. We hypothesized that statin use would be associated with shorter hospital LOS and reduced mortality.
METHODS
Study Design and Study Population
We conducted a study nested within the EPIC study, a large, multicenter, prospective surveillance study that assessed incidence and etiology of CAP. Details of the EPIC study have been previously described [24]. Briefly, from 1 January 2010 to 30 June 2012, we enrolled individuals aged ≥18 years at 5 US hospitals in Nashville, Tennessee (2), and Chicago, Illinois (3). Adults admitted to study hospitals were eligible if they had CAP, defined as evidence of acute infection, acute respiratory illness, and radiographic findings consistent with pneumonia. Patients were excluded if they were recently hospitalized, severely immunocompromised, resided in a nursing home and unable to function independently, or had an alternative diagnosis. Informed consent was obtained prior to enrollment. The institutional review boards at each institution and the CDC approved the study protocol.
Demographic, epidemiological, and clinical information, including statin use, dosage, and timing, was systematically collected through patient interviews and medical chart abstraction. Since it is possible that any effect statins might have on CAP outcomes may only be observed if statins were administered early in the patient's clinical course, patients were excluded from analysis if the first dose of a statin was administered ≥48 hours after initial hospital arrival. Patients who were taking statins before hospitalization but did not continue during hospitalization were excluded; we performed a sensitivity analysis including these patients in the same group as those who did not use statins before or during hospitalization (results for these patients are in the Supplementary Materials). Patients who were not taking statins before hospitalization but were newly initiated on statins during hospitalization were also excluded (results for these patients are in the Supplementary Materials). Therefore, the final patient groups for comparison were adults using statins before admission who continued using them during hospitalization (statin users) and adults who never used statins prior to or during hospitalization (nonusers).
Study Endpoints
The primary outcome was hospital LOS, calculated as the hours between hospital arrival and discharge. In-hospital mortality was a secondary outcome.
Statistical Analyses
Multivariable Analysis of Statin Use and Length of Stay
Several analyses were conducted. First, we performed competing risk survival analysis using the Fine and Gray proportional subdistribution hazards model [27, 28] to examine the association between statin use and hospital LOS by modelling the hazard of discharge in all statin users and nonusers (full cohort). Given that those patients who die while in the hospital cannot experience the study outcome of being discharged alive, accounting for this competing risk is necessary. Unlike a traditional Cox proportional hazards model that would censor patients who die, our proportional subdistribution hazards model accounts for this issue by treating death as a competing event to the outcome of interest [29, 30]. We also used the same model to examine LOS among only those admitted to the intensive care unit (ICU) within 1 day of hospital admission (ICU cohort). A HR <1 indicates statin exposure was associated with decreased “risk” of discharge and therefore increased LOS. After bivariate analysis, we evaluated the demographic and clinical characteristics and comorbidities as potential confounders. We considered biological plausibility and used standard model fitting procedures such as Akaike information criterion [31] to select potential covariates for inclusion in multivariable regression models, excluding those who were missing complete covariate information. Age categories were defined as 18–34 years, 35–54 years, 55–64 years, and ≥65 years. Patients were considered insured if they had public or private insurance or both. Determination of comorbidities was based on chart abstraction and patient interview; classification of comorbidities has been previously defined [24]. In the final multivariable model for the full cohort, statin use, sex, and race/ethnicity (non-Hispanic white or not) were included in the model. The model also included age category, insurance status, education, home oxygen use, smoking status, as well as cardiovascular disease, chronic lung disease, and diabetes. We also examined in-hospital death as a secondary outcome using a multivariable logistic regression in both the full and ICU cohorts, adjusting for the same covariates as in the proportional subdistribution hazards model for LOS.
Stratification by Pneumonia Severity
Severity of pneumonia on hospital presentation was also categorized using the pneumonia severity index (PSI) [32]. Using a proportional subdistribution hazards model that included the same covariates as in the full cohort multivariable model, we examined the association between statin use and the primary outcome of LOS among patients stratified by PSI class (low risk [I, II, III], moderate risk [IV], and high risk [V]).
Propensity Score Analysis
In separate analyses, we accounted for confounding using propensity score matching [33, 34]. The propensity score calculated for each patient estimated the probability of statin use based on baseline covariates, regardless of actual statin use. The propensity score for each individual was calculated using a multivariable logistic regression model; the dependent variable was statin use. The propensity score model used for both the full and ICU cohorts included the following covariates: race/ethnicity; age category; sex; hospital site; history of cardiovascular, diabetes, and kidney and liver disease; home oxygen use; angiotensin-converting enzyme inhibitor use; insurance status; smoking status; and influenza vaccination. Influenza vaccination status during the most recent influenza season was based on patient interview.
Using the propensity score, we matched statin users with nonusers who had similar propensity scores using a 1:1 greedy matching algorithm [35]. Propensity-matched cohorts that had similar baseline characteristics except for statin use were created from both the full cohort and the ICU cohorts. Using these propensity-matched cohorts, LOS was analyzed using an unadjusted proportional subdistribution hazards model; models for in-hospital death used logistic regression. To account for residual confounding, sensitivity analyses that used the propensity-matched cohorts but that also adjusted for the same variables as in the main analysis were performed.
Sample size calculations are described in the Supplementary Materials. All statistical analyses were conducted using SAS (v. 9.3 and v. 9.4, SAS Institute, Cary, North Carolina) statistical software.
RESULTS
Study Population
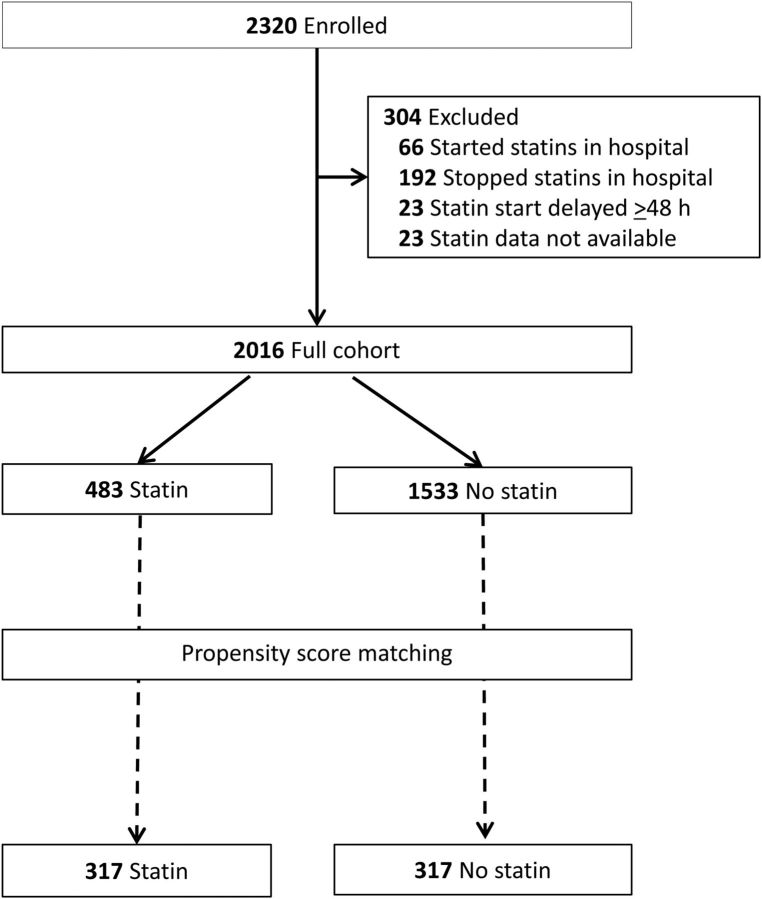
Among 2320 adults hospitalized with radiographically confirmed CAP enrolled in the EPIC study, 23 did not have complete statin data available. Sixty-six patients were initiated on statins in the hospital and 192 had been taking statins before hospitalization but were discontinued during hospitalization. Twenty-three statin users continued on statins during hospitalization but received their first dose ≥48 hours after the arrival time. All of these patients were excluded, leaving 2016 adults for analysis; 483 (24%) were statin users and 1533 (76%) nonusers (Figure 1). During hospitalization, simvastatin was administered most frequently (n = 251, 52%), followed by atorvastatin (n = 112, 23%), pravastatin (n = 56, 12%), rosuvastatin (n = 39, 8%), lovastatin (n = 24, 5%), and fluvastatin (n = 1, 0.2%). When compared with nonusers, statin users were significantly older (median 69 years vs 53 years), and more likely to be male and admitted to certain hospitals (Table 1). Patients in the statin group were significantly more likely to be obese, use oxygen at home, and have more comorbidities. Furthermore, they were significantly more likely to have health insurance, some college education, and to report receipt of an influenza vaccination than nonusers (Table 1). As in the full cohort, in the ICU cohort, statin users (n = 89) were significantly older and had more comorbidities when compared with nonusers (n = 227; Supplementary Table 1).
Figure 1.
Flow diagram of participants from the Etiology of Pneumonia in the Community study included in this analysis.
Table 1.
Characteristics of Adults Hospitalized With Community-Acquired Pneumonia, Among All Patients (Full Cohort) and in the Propensity-Matched Cohort
| Characteristic | Full Cohort (n = 2016) |
Propensity-Matched Cohorta (n
= 634) |
||||
|---|---|---|---|---|---|---|
| Statin Group No. (%) (n = 483) | Nonuser Group No. (%) (n = 1533) | P Value | Statin Group No. (%) (n = 317) | Nonuser Group No. (%) (n = 317) | P Value | |
| Age category, y | ||||||
| 18–34 | 3 (0.6) | 228 (15) | <.001 | 3 (1) | 1 (0.3) | .60 |
| 35–54 | 89 (18) | 619 (40) | 73 (23) | 66 (21) | ||
| 55–64 | 99 (21) | 305 (20) | 73 (22) | 70 (22) | ||
| ≥65 | 292 (61) | 381 (25) | 168 (53) | 180 (57) | ||
| Male | 257 (53) | 730 (47) | .03 | 156 (49) | 165 (52) | .47 |
| Non-Hispanic white | 277 (58) | 670 (44) | <.01 | 182 (57) | 181 (57) | .94 |
| Hospital | <.01 | |||||
| A | 287 (59) | 567 (37) | 176 (55) | 190 (52) | .89 | |
| B | 17 (4) | 191 (13) | 16 (5.0) | 15 (4.1) | ||
| C | 24 (5) | 251 (16) | 18 (5.7) | 18 (4.9) | ||
| D | 75 (16) | 215 (14) | 46 (15) | 69 (19) | ||
| E | 80 (17) | 309 (20) | 61 (19) | 77 (21) | ||
| Comorbidities | ||||||
| Chronic lung diseasea | 223 (46) | 603 (39) | .008 | 149 (47) | 153 (48) | .75 |
| Cardiovascular diseaseb | 330 (62) | 313 (20) | <.001 | 154 (49) | 155 (49) | .94 |
| Kidney disease | 117 (24) | 164 (11) | <.001 | 67 (21) | 63 (19) | .69 |
| Liver diseasec | 17 (4) | 97 (6) | .02 | 14 (4) | 12 (4) | .69 |
| Diabetes mellitus | 231 (48) | 217 (14) | <.001 | 120 (38) | 121 (38) | .94 |
| Neurologic diseased | 81 (17) | 119 (8) | <.001 | 43 (14) | 40 (13) | .72 |
| Immunocompromisede | 82 (17) | 239 (16) | .98 | 56 (18) | 60 (19) | .68 |
| Body mass index ≥30 kg/m2 | 205 (42) | 521 (34) | <.001 | 135 (43) | 122 (38) | .29 |
| Current smoker | 72 (15) | 475 (31) | <.001 | 58 (18) | 54 (17) | .68 |
| Former smoker | 242 (50) | 469 (31) | <.001 | 147 (46) | 150 (47) | .81 |
| Home oxygen use | 66 (14) | 132 (9) | <.001 | 40 (13) | 46 (15) | .49 |
| Angiotensin-converting enzyme inhibitor use | 188 (39) | 227 (15) | <.001 | 107 (34) | 107 (34) | 1.00 |
| Healthy user indicators | ||||||
| Influenza vaccination | 375 (78) | 905 (59) | <.001 | 240 (76) | 245 (77) | .64 |
| Has health insurance | 459 (95) | 1193 (79) | <.001 | 298 (94) | 298 (94) | 1.00 |
| Education level | ||||||
| Some college or more | 208 (45) | 517 (34) | <.001 | 137 (43) | 139 (44) | .87 |
The propensity-matched cohort included statin users and matched nonusers with a similar propensity for statin use prior to hospital admission. Propensity score variables included the following: race/ethnicity (white non-Hispanic vs non-white or Hispanic), age category, sex, site, history of cardiovascular, diabetes, kidney, and liver disease, home oxygen use, angiotensin-converting enzyme inhibitor use, insurance status (yes/no), influenza vaccination, and being a former smoker.
a Chronic lung disease included asthma and reactive airway disease, chronic obstructive pulmonary disease, and sleep apnea.
b Cardiovascular disease included history of coronary artery disease, congestive heart failure, myocardial infarction, percutaneous coronary intervention, coronary stent placement, or coronary artery bypass graft.
c Liver disease included cirrhosis, liver failure, and other liver disease.
d Neurologic conditions included stroke, seizures, dementia, and other neurologic conditions.
e Immunosuppression included human immunodeficiency virus, cancer, and immunosuppressive medications.
Hospital Length of Stay
Statin users had a longer crude median LOS compared with nonusers (92 hours, interquartile range [IQR] 56–154 vs 75 hours, IQR 48–127; P < .01). In a subdistribution hazard model for LOS in the full cohort, the unadjusted HR was 0.84 (95% confidence interval [CI], .77–.93). There were 1889 (94%) patients for whom complete covariate data were available, 36 of whom had death as a competing event. In the multivariable subdistribution hazard model, we found no difference in LOS between statin users and nonusers (adjusted HR [aHR]; 0.99, CI, .88–1.12; Table 2).
Table 2.
Proportional Subdistribution Hazards Model for Hospital Length of Stay in Statin Group Compared With Nonuser Group Among All Patients and Among Intensive Care Unit Patients
| Study Population | Sample Size | Hazard Ratio (95% Confidence Interval)a | P Value |
|---|---|---|---|
| Full cohortb | |||
| Multivariable model, full cohortc | 1889d | 0.99 (0.88–1.12) | .93 |
| Propensity-matched full cohorte | 634 | 1.03 (0.88–1.21) | .67 |
| ICU cohortf | |||
| Multivariable model, ICU cohortc | 295d | 1.16 (0.88–1.53) | .28 |
| Propensity-matched ICU cohorte | 114 | 1.38 (0.96–2.00) | .09 |
Abbreviation: ICU, intensive care unit.
a Hazard ratio <1 indicates longer length of stay for statin users. Death was considered a competing event in these models.
b The full cohort includes all adult patients hospitalized with community-acquired pneumonia.
c Multivariable models were adjusted for the following covariates: age, sex, race/ethnicity, insurance status, level of education, home oxygen use, smoking status, and history of the following comorbidities: cardiovascular disease, diabetes, and chronic lung disease.
d Patients were not included in the multivariable model if missing any of the model covariates.
e Patients were matched on propensity score, which included the following covariates: race/ethnicity; age category; sex; hospital site; history of cardiovascular disease, diabetes, and kidney and liver disease; home oxygen use; angiotensin-converting enzyme inhibitor use; insurance status; influenza vaccination; and being a former smoker.
f The ICU cohort includes all patients admitted to the ICU within 1 day of hospital arrival.
Statin users in the ICU cohort tended to have a longer crude median LOS compared with ICU nonusers, although this was not statistically significant (162 hours, IQR 95–258 vs 136 hours, IQR 87–233; P = .16). In a subdistribution hazard multivariable model adjusted for the same covariates as in the full cohort multivariable model, the aHR in the ICU cohort was 1.16 (CI, .88–1.53; Table 2). Results were unchanged when statin type, statin dosage, time to administration of first antibiotic, and antibiotic class were included in these models (data not shown).
When patients were stratified by PSI and a regression model adjusted for the same covariates as in the full cohort model, we found no difference in LOS between statin users and nonusers among those who had low risk (n = 1272; aHR, 0.98; CI, .84–1.14), moderate risk (n = 477; aHR, 0.96; CI, .81–1.23), or high risk (n = 140; aHR, 1.00; CI, .67–1.50) PSI.
After propensity score matching, there were 317 individuals in each of the statin user and nonuser groups in the propensity-matched full cohort, and 57 in each group in the propensity-matched ICU cohort. The Supplementary Figure shows the distribution of propensity scores before matching (full cohort) and after matching (propensity-matched full cohort). There were no significant differences in baseline characteristics between statin users and nonusers either in the propensity-matched full cohort (Table 1) or the propensity-matched ICU cohort (Supplementary Table 1).
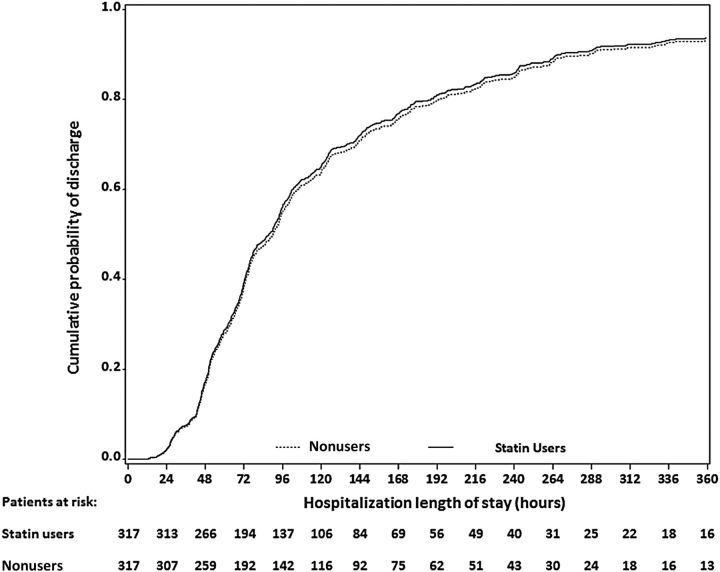
Among the 634 patients in the propensity-matched full cohort, statin users and nonusers had similar crude median LOS (86 hours, IQR 54–151 vs 88 hours, IQR 56–165). In the subdistribution hazard model for the propensity-matched full cohort, the HR for LOS in the statin group compared with the nonuser group was 1.03 (CI, .88–1.21; Figure 2, Table 2). Results were unchanged in the sensitivity analysis, which used the propensity-matched full cohort and adjusted for the same variables as in the main analysis (aHR, 1.00; CI, .86–1.18). In the propensity-matched ICU cohort (n = 114), statin users had a similar crude median LOS (150 hours, IQR 93–288) compared with nonusers (167 hours, IQR 94–262). In the subdistribution hazard model for the propensity-matched ICU cohort, there was no significant difference in LOS in the statin group compared with the nonuser group (aHR, 1.38; CI, .96–2.00).
Figure 2.
Cumulative incidence of hospital discharge by statin use category among patients in the propensity score-matched cohort. Statin users and nonusers were matched on propensity score, which included the following covariates: race/ethnicity; age category; sex; hospital site; history of cardiovascular, diabetes, and kidney and liver disease; home oxygen use; angiotensin-converting enzyme inhibitor use; insurance status; influenza vaccination; and being a former smoker.
Death
In the full cohort, 12/483 (3%) statin users and 30/1503 (2%) nonusers died. In the full multivariable model, the adjusted odds ratio (aOR) was 0.80 (CI, .35–1.82). In the ICU cohort, 3/89 (3%) statin users and 20/227 (9%) nonusers died; in the full multivariable model, we found no significant association between death and statin use (aOR, 0.29; CI, .07–1.15). For both the full and ICU cohorts, no statistically significant association between statin use and death was found on bivariate analysis, multivariable analysis using logistic regression, or in the propensity-matched cohorts (Supplementary Table 2).
DISCUSSION
Among a large prospectively enrolled cohort of adults hospitalized for CAP, we found no association between statin use—initiated before and continued during hospitalization—and CAP hospital LOS and in-hospital death, after adjusting for age, sex, comorbidities, and other variables such as education and insurance status. These results were consistent in multiple analyses that included all hospitalized CAP patients, matched statin users and nonusers on propensity for statin use, and stratified by severity, as determined either by ICU admission or PSI.
Statins have some antiinflammatory and immunomodulatory effects [5–9]. It is biologically plausible that they might benefit patients with pneumonia by modulating the inflammatory response that often accompanies severe infection. Animal models demonstrate improved survival with statin treatment prior to induction of sepsis [36, 37]. Systematic reviews including mostly nonrandomized studies examined the association between statins and infection and reported significant protective effects for various outcomes, including mortality and sepsis [18–20]. Multiple retrospective observational studies, most of which used large administrative datasets, have demonstrated a beneficial association of statins with several infectious processes [10–17]. However, adjusting for potential confounders is often challenging when comparing statin users to nonusers, and biases may be difficult to characterize. As we observed in this study, statin users typically have more comorbidities than nonusers [38, 39]. Furthermore, statin users—especially prevalent users (eg, those who have an indication for and adhere to long-term treatment)—also may have a constellation of healthier behaviors and characteristics associated with improved outcomes (the “healthy user effect”) [38–42]. These differences can potentially bias estimates. Observational studies using administrative data that lack detailed information on covariates such as smoking and vaccination status are unable to adequately adjust for these and other potential confounders.
Thus, careful consideration of the observational study design is needed for proper interpretation. In contrast to multiple retrospective studies [11–16], 2 prospective studies of adults hospitalized with CAP that, like our study, had detailed clinical information and used propensity scores to account for measured confounders, found no mortality benefit for statins [41, 42]. Misclassification of the timing of statin exposure could also introduce artifacts in statin effects [43], and many studies lack information on the timing of statin administration during hospitalization, as well as indicators of pneumonia severity at the time of hospitalization. Our study used prospectively collected, detailed clinical, laboratory, and demographic data from interview and medical record abstraction, as well a propensity score-matching strategy, to evaluate the effect of statins on LOS and death in patients with a range of CAP severity while adjusting for the potential confounders for which we had data.
An increasing body of evidence from RCTs also demonstrates a lack of association between statin use and the reduction of morbidity and mortality due to different infectious diseases in adults [21–23, 44–46]. However, most RCTS have been limited to severely ill patients and only 1 study, stopped prematurely given failure to achieve its recruitment targets, has specifically evaluated the effect of statins on clinical outcomes in patients hospitalized with CAP [47]. Two RCTs suggest that statins may decrease risk of progression to severe sepsis [48, 49], but a metaanalysis of 5 RCTs examining the effect of statins in septic patients showed no association between statin therapy and mortality [50], and several smaller RCTs failed to show significant clinical benefits [22, 45, 46, 51]. Two recent, large RCTs found no benefit associated with statin use in mechanically ventilated ARDS patients in the ICU, and 1 of those trials found that randomization to simvastatin may have had detrimental hepatic and renal effects [21, 23]. Importantly, RCTs of statins excluded prevalent or recent users of statins [21, 23, 47], whereas our study compared prevalent statin users to nonusers. Our findings suggest that the lack of benefit from statins previously demonstrated in ICU patients also extends to noncritically ill patients hospitalized with CAP. Results were consistent when we stratified by pneumonia severity, either by ICU admission occurring within 1 day of hospital arrival or by PSI.
This study has limitations. As in any observational study, residual confounding may be present. However, the wealth of data collected, including healthy user indicators such as education level and insurance status, and the consistency of findings in separate propensity score-matched analyses, should reduce this concern. In addition, we studied only patients hospitalized with CAP; we are thus unable to examine any possible effect of statin use on preventing hospitalization from CAP or on clinical outcomes in those CAP patients whose disease was not severe enough to warrant hospitalization. We also were dependent on self-report of statin use prior to hospitalization, which may have led to misclassification of statin use. We also lacked information on statin type, medication compliance, and duration of use prior to hospitalization. Overall, mortality in the EPIC study was low, thus we used LOS as the primary clinical outcome. While widely used, LOS may be affected by factors unrelated to clinical status. However, LOS does correlate with time to clinical stability [25], and shorter LOS is informative in that it is also associated with other factors, such as decreased cost and risk of adverse events associated with prolonged hospitalization [26]. In addition, inadequate sample size may have limited our ability to detect a significant association between LOS and statin use in the propensity-matched full cohort and in the ICU cohorts, and low numbers of in-hospital deaths likely limited our ability to detect a significant association between mortality and statin use.
In conclusion, after adjusting for potential confounding, we found no significant difference in LOS and in-hospital mortality between statin users and nonusers among adults hospitalized with CAP. Results were consistent when we examined all CAP patients, when CAP patients were stratified by ICU admission and PSI, and when CAP patients were matched on propensity for statin use prior to admission. Our study supports data from both RCTs and prospective observational studies that control for healthy user effects, which have not found associations between statin use and clinical outcomes in patients hospitalized with various infections.
Supplementary Material
Notes
Acknowledgments. We thank the participants who graciously consented to participate in this study.
Author contributions. F. H. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: F. H., S. J., L. F. Data acquisition, analysis, or interpretation: All authors. Drafting of the manuscript. F. H., S. J. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: F. H., S. J., A. M. B. Obtained funding: L. F., S. J., K. M. E., R. G. W. Administrative, technical, or material support: S. J., A. M. B. Study supervision: S. J., L. F.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention (CDC).
Financial support. This work was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the CDC through cooperative agreements with each study site.
Potential conflicts of interest. W. H. S. reports receiving fees for serving on advisory boards from BioFire Diagnostics and Venaxis Inc., and grant support through his institution from bioMerieux, Affinium Pharmaceuticals, Astute Medical, BRAHMS/ThermoFisher Scientific, Pfizer, Rapid Pathogen Screening, Venaxis, Sphingotec, and BioAegis. He also reports a pending patent related to a sterile blood culture collection system (13/632 874) and consulting fees from Abbott Point of Care. R. B. reports grant support and speaking honoraria from bioMerieux and fees for participation in the advisory boards of ThermoFisher Scientific and Roche Diagnostics. D. M. C. reports receiving fees through his institution from BRAHMS/Thermo Fisher Scientific to support sepsis research. E. J. A. reports receiving grant support through his institution from MedImmune, editorial support for an unrelated study from Roche, and consulting fees from AbbVie. C. G. G. reports receiving consulting fees from Pfizer. K. M. E. reports receiving fees for serving on a data safety monitoring board from Novartis, paid to her institution, as well as grant support from Novartis to conduct a vaccine study for group B strep in pregnant women. R. G. W. reports receiving consulting fees from bioMerieux, GenMark, Cepheid, Genentech/Roche, Rempex/The Medicines Company, Vical, Merck/Cubist Pharmaceuticals, Cempra, Cerexa, and Visterra. He has also received fees for participation in data safety monitoring boards for AstraZeneca, Vertex, Achaogen, and Pfizer. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Antunes G, Evans SA, Lordan JL, Frew AJ. Systemic cytokine levels in community-acquired pneumonia and their association with disease severity. Eur Respir J 2002; 20:990–5. [DOI] [PubMed] [Google Scholar]
- 2. Calbo E, Alsina M, Rodriguez-Carballeira M, Lite J, Garau J. Systemic expression of cytokine production in patients with severe pneumococcal pneumonia: effects of treatment with a beta-lactam versus a fluoroquinolone. Antimicrob Agents Chemother 2008; 52:2395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chalmers JD, Singanayagam A, Hill AT. C-reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med 2008; 121:219–25. [DOI] [PubMed] [Google Scholar]
- 4. Menendez R, Cavalcanti M, Reyes S et al. Markers of treatment failure in hospitalised community acquired pneumonia. Thorax 2008; 63:447–52. [DOI] [PubMed] [Google Scholar]
- 5. Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21:1712–9. [DOI] [PubMed] [Google Scholar]
- 6. Rosenson RS, Tangney CC, Casey LC. Inhibition of proinflammatory cytokine production by pravastatin. Lancet 1999; 353:983–4. [DOI] [PubMed] [Google Scholar]
- 7. Shi J, Wang J, Zheng H et al. Statins increase thrombomodulin expression and function in human endothelial cells by a nitric oxide-dependent mechanism and counteract tumor necrosis factor alpha-induced thrombomodulin downregulation. Blood Coagul Fibrinolysis 2003; 14:575–85. [DOI] [PubMed] [Google Scholar]
- 8. Terblanche M, Almog Y, Rosenson RS, Smith TS, Hackam DG. Statins and sepsis: multiple modifications at multiple levels. Lancet Infect Dis 2007; 7:358–68. [DOI] [PubMed] [Google Scholar]
- 9. Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov 2005; 4:977–87. [DOI] [PubMed] [Google Scholar]
- 10. Myles PR, Hubbard RB, Gibson JE, Pogson Z, Smith CJ, McKeever TM. The impact of statins, ACE inhibitors and gastric acid suppressants on pneumonia mortality in a UK general practice population cohort. Pharmacoepidemiol Drug Saf 2009; 18:697–703. [DOI] [PubMed] [Google Scholar]
- 11. Mortensen EM, Nakashima B, Cornell J et al. Population-based study of statins, angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors on pneumonia-related outcomes. Clin Infect Dis 2012; 55:1466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Douglas I, Evans S, Smeeth L. Effect of statin treatment on short term mortality after pneumonia episode: cohort study. BMJ 2011; 342:d1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nielsen AG, Nielsen RB, Riis AH, Johnsen SP, Sorensen HT, Thomsen RW. The impact of statin use on pneumonia risk and outcome: a combined population-based case-control and cohort study. Critical Care 2012; 16:R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rothberg MB, Bigelow C, Pekow PS, Lindenauer PK. Association between statins given in hospital and mortality in pneumonia patients. J Gen Intern Med 2012; 27:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vandermeer ML, Thomas AR, Kamimoto L et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis 2012; 205:13–9. [DOI] [PubMed] [Google Scholar]
- 16. Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest 2007; 131:1006–12. [DOI] [PubMed] [Google Scholar]
- 17. Mortensen EM, Pugh MJ, Copeland LA et al. Impact of statins and angiotensin-converting enzyme inhibitors on mortality of subjects hospitalised with pneumonia. Eur Respir J 2008; 31:611–7. [DOI] [PubMed] [Google Scholar]
- 18. Tleyjeh IM, Kashour T, Hakim FA et al. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med 2009; 169:1658–67. [DOI] [PubMed] [Google Scholar]
- 19. Janda S, Young A, Fitzgerald JM, Etminan M, Swiston J. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care 2010; 25:656.e7.–22. [DOI] [PubMed] [Google Scholar]
- 20. Chopra V, Rogers MA, Buist M et al. Is statin use associated with reduced mortality after pneumonia? A systematic review and meta-analysis. Am J Med 2012; 125:1111–23. [DOI] [PubMed] [Google Scholar]
- 21. The National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 2014; 370:2191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papazian L, Roch A, Charles PE et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: a randomized clinical trial. JAMA 2013; 310:1692–700. [DOI] [PubMed] [Google Scholar]
- 23. McAuley DF, Laffey JG, O'Kane CM et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med 2014; 371:1695–703. [DOI] [PubMed] [Google Scholar]
- 24. Jain S, Self WH, Wunderink RG et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halm EA, Fine MJ, Marrie TJ et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998; 279:1452–7. [DOI] [PubMed] [Google Scholar]
- 26. Harkanen M, Kervinen M, Ahonen J, Voutilainen A, Turunen H, Vehvilainen-Julkunen K. Patient-specific risk factors of adverse drug events in adult inpatients—evidence detected using the global trigger tool method. J Clin Nurs 2015; 24:582–91. [DOI] [PubMed] [Google Scholar]
- 27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509. [Google Scholar]
- 28. Gondara L. Competing risk survival analysis using SAS: When, why and how. In: SAS Global Forum 2015. Dallas, TX: SAS Institute Inc. 2015, 2015. [Google Scholar]
- 29. Resche-Rigon M, Azoulay E, Chevret S. Evaluating mortality in intensive care units: contribution of competing risks analyses. Critical Care 2006; 10:R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009; 170:244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. deLeeuw J. Introduction to Akaike (1973) information theory and an extension of the maximum likelihood principle. In: Breakthroughs in statistics 1992; 1:599–609. [Google Scholar]
- 32. Fine MJ, Auble TE, Yealy DM et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–50. [DOI] [PubMed] [Google Scholar]
- 33. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70:41–55. [Google Scholar]
- 34. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc 1984; 79:516–24. [Google Scholar]
- 35. Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy Matching techniques. Available at: http://www2.sas.com/proceedings/sugi26/p214-26.pdf Accessed 2014.
- 36. Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther 2000; 294:1043–6. [PubMed] [Google Scholar]
- 37. Merx MW, Liehn EA, Graf J et al. Statin treatment after onset of sepsis in a murine model improves survival. Circulation 2005; 112:117–24. [DOI] [PubMed] [Google Scholar]
- 38. Brookhart MA, Patrick AR, Dormuth C et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol 2007; 166:348–54. [DOI] [PubMed] [Google Scholar]
- 39. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med 2011; 26:546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Polgreen LA, Cook EA, Brooks JM, Tang Y, Polgreen PM. Increased statin prescribing does not lower pneumonia risk. Clin Infect Dis 2015; 60:1760–6. [DOI] [PubMed] [Google Scholar]
- 41. Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ 2006; 333:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yende S, Milbrandt EB, Kellum JA et al. Understanding the potential role of statins in pneumonia and sepsis. Crit Care Med 2011; 39:1871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grijalva CG, Arbogast PG, Griffin MR. Statins and influenza/COPD mortality. Chest 2007; 132:1407; author reply -8. [DOI] [PubMed] [Google Scholar]
- 44. Kruger P, Bailey M, Bellomo R et al. A multicentre randomised trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med 2013; 187:743–50. [DOI] [PubMed] [Google Scholar]
- 45. Kruger PS, Harward ML, Jones MA et al. Continuation of statin therapy in patients with presumed infection: a randomized controlled trial. Am J Respir Crit Care Med 2011; 183:774–81. [DOI] [PubMed] [Google Scholar]
- 46. Craig TR, Duffy MJ, Shyamsundar M et al. A randomized clinical trial of hydroxymethylglutaryl-coenzyme a reductase inhibition for acute lung injury (the HARP study). Am J Respir Crit Care Med 2011; 183:620–6. [DOI] [PubMed] [Google Scholar]
- 47. Viasus D, Garcia-Vidal C, Simonetti AF et al. The effect of simvastatin on inflammatory cytokines in community-acquired pneumonia: a randomised, double-blind, placebo-controlled trial. BMJ Open2015; 5:e006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patel JM, Snaith C, Thickett DR et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS trial). Critical Care 2012; 16:R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Novack V, Eisinger M, Frenkel A et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med 2009; 35:1255–60. [DOI] [PubMed] [Google Scholar]
- 50. Pasin L, Landoni G, Castro ML et al. The effect of statins on mortality in septic patients: a meta-analysis of randomized controlled trials. PloS One 2013; 8:e82775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kruger P, Bailey M, Bellomo R et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med 2013; 187:743–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.