Abstract
How do we understand and empathize with individuals whose bodies are drastically different from our own? We investigated the neural processes by which an individual with a radically different body, a congenital amputee who is born without limbs, engages her own sensory-motor representations as a means to understand other people’s body actions or emotional states. Our results support the prediction that when the goal of the action is possible for the observer, one’s own motor regions are involved in processing action observation, just as when individuals viewed those similar to themselves. However, when the observed actions are not possible, mentalizing mechanisms, relying on a different set of neural structures, are additionally recruited to process the actions. Furthermore, our results indicate that when individuals view others experiencing pain in body parts that they have, the insula and somatosensory cortices are activated, consistent with previous reports. However, when an individual views others experiencing pain in body parts that she does not have, the insula and secondary somatosensory cortices are still active, but the primary somatosensory cortices are not. These results provide a novel understanding for how we understand and empathize with individuals who drastically differ from the self.
Keywords: action observation, body image, empathy, fMRI, mentalizing, mirror neuron system, pain matrix
Introduction
Understanding other people’s actions and feelings is an essential component of successful social interactions, such as appreciating the meaning behind seeing someone open a bottle of champagne or empathizing when seeing someone slam a door on their finger. There are 2 predominant views on how one infers the mental states and emotions of others purely from observation. One view posits that shared circuits (SCs) involved in processing one’s own actions, sensations, and emotions are also involved in perceiving and understanding the actions, sensations, and emotions of others (Gallese and Goldman 1998; Gallese et al. 2004; Keysers and Gazzola 2009; Keysers et al. 2010). The other view concentrates on inferential processes (rationalization) as being essential for understanding other’s internal states (Fletcher et al. 1995; Saxe 2005; Amodio and Frith 2006), a process referred to as “mentalizing.”
Despite extreme stands on either end of the debate, many researchers have recently proposed hybrid models to bridge these 2 views and account for the dominance of SCs or mentalizing networks depending on the task or the context (Keysers and Gazzola 2007; Uddin et al. 2007; de Lange et al. 2008; Van Overwalle and Baetens 2009; Liew et al. 2010). In general, they posit that 2 different cognitive components in social cognition must be taken into account: intuitive (prereflective) processes and reflective processes. Intuitive processes might play a role when, for example, someone watches another pop open a bottle of champagne. Understanding this action may need to rely on brain regions such as the premotor cortices and the posterior parietal cortex that are related to processing actions (Gallese et al. 2004; Rizzolatti and Craighero 2004) and somatosensory cortices (SI, SII) for processing tactile sensations (Keysers et al. 2004), all of which form SCs that use our own sensory-motor representations to understand the bodily states of others (Keysers and Gazzola 2007; Keysers et al. 2010). These are automatic, intuitive, and empathic levels of representation and do not require reflective metacognition. Activity in these regions has been found to also correlate with higher scores on empathy scales (Gazzola et al. 2006; Jabbi et al. 2008; Aziz-Zadeh et al. 2010) and lower scores on autism symptom severity (Dapretto et al. 2006). By contrast, an example of a mentalizing task, involving more reflective, nonintuitive social cognition, might be to think about what a foreign visitor might want to eat for dinner. This process requires understanding and attributing mental states to another, and to answer the question one might use deductive reasoning, utilizing one’s knowledge and experience of the foreigner’s culture. This task may involve structures including the dorsolateral prefrontal cortex, the ventromedial prefrontal cortex, the cingulate cortex, the insula, the precuneus, and the temproparietal junction (TPJ); regions that have been found to be active in tasks requiring more reflective social cognition (Saxe 2005).
It is important to point out that while we used 2 extreme examples, a continuum of intuitive to reflective social contexts exists, and both SCs and mentalizing networks are probably involved, working side by side to varying degrees along this continuum. In fact, concurrent activity between these 2 networks has been suggested in recent studies predicting empathic accuracy (Zaki et al. 2009) and between-brain Granger causality when viewing 2 individuals playing charades (Schippers et al. 2009). In addition, recent work has shown that inferring someone’s intentions from their actions activates both mirror and mentalizing regions (Spunt et al. 2010). Interestingly, activity in mentalizing regions is parametrically increased as the task becomes more theoretical (e.g., “What is he doing?” vs. “Why is he doing it?”), while activity in SCs stay the same (Spunt et al. 2010). Thus, in the first example of observing someone opening a bottle of champagne, SCs may provide an intuitive understanding of the action while mentalizing regions will provide a more abstract understanding that the individual is celebrating an event. Still, there are many contexts that have been untested, creating gaps in our understanding of social cognition. One effective way to further explore the relations between these systems is to approach a dramatic case—that of individuals whose physical make up differs drastically from that of other people. How do individuals born without arms and legs, whose activity maps for limb actions cannot possibly be the same as that of the general population, understand other people’s arm and leg actions? How do they empathize when watching others experience pain in their arms or legs?
Given the likely distortion or even absence of equivalent sensory-motor representations, it may initially seem that simulating other people’s actions would be impossible for congenital amputees. That is, one might predict that it would not be possible to use SCs to process other people’s arm actions or empathize with pain in the arm if one doesn’t have arms, as matching cortical representations of the body are likely to be absent or distorted. Following this prediction, SCs for social cognition would not be utilized, and all processing would be completed by mentalizing mechanisms. However, research on action observation indicates that if the goal of the action is within the motor repertoire of the observer, then the means to achieve the goal are not as relevant; activity is still observed in one’s own motor representations (Gazzola, Rizzolatti, et al. 2007; Gazzola, van der Worp, et al. 2007). Thus, a previous study on individuals born without upper limbs has shown that observation of hand actions whose goals are possible for the amputee by using the mouth or foot (e.g., writing with a pencil using the foot rather than the hand) still activate motor-related cortices. These activations are in areas involved in the execution of foot and mouth actions and are within the range of normally developed subjects (Gazzola, van der Worp, et al. 2007). Thus, individuals recruit motor programs in their own repertoire for actions with corresponding goals despite the effector used to achieve the goal. Processing by SCs occurs even when movement kinematics fall outside the observer’s motor repertoire, as in the observation of reach-to-grasp actions made by an industrial robot (Gazzola, Rizzolatti, et al. 2007), or biting actions made by a monkey or dog (Buccino et al. 2004). However, it should be noted that in both studies, observation of human actions activated motor-related areas bilaterally, whereas observation of actions made by robots or nonconspecifics activated only the left motor-related areas. Again, the action goal (e.g., reach-to-grasp, bite) must fall within the repertoire of the observer, regardless of how the goal is accomplished (hand or mouth). Thus, if the action goal is within the repertoire of the observer, SCs should be involved in processing the observed action of even a dissimilar other.
By contrast, if the goal of the action is not within the repertoire of the observer, the sensory-motor systems fail to map observed actions. For example, in a functional magnetic resonance imaging study of humans observing speaking in humans, lip smacking in monkeys, and barking in dogs, only actions that were similar to the human’s motor repertoire (biting and speaking) activated SCs. Barking, however, was not within a human’s motor repertoire and did not activate SCs (Buccino et al. 2004).
Here, we report results of testing the dual neural routes by which we understand individuals who differ drastically from ourselves. We predict that for actions whose goals are within the motor repertoire of the congenital amputee, the SC system would be active (mirror neuron system [MNS]: inferior frontal gyrus, premotor cortex, and inferior parietal lobule [IPL]). Our study is a more extreme version of Gazzola et al. (2007), as we study a subject with neither arms nor legs as she observes arm, leg, and mouth actions. Furthermore, our study also investigates what occurs when the goal of the action is not possible for the observer. We predict that during observation of actions whose goals are not possible for the congenital amputee (e.g., using scissors, sewing, etc.), regions related to mentalizing processes, in particular the medial prefrontal cortices (mPFC), precuneus, and TPJ, will be active. This prediction is based on previous findings indicating that when the goal of an action falls outside of one’s motor repertoire, the MNS is not active (Buccino et al. 2004). Furthermore, previous data has shown that when one tries to understand actions at a more theoretical level or when viewing actions in implausible contexts, the mentalizing system is active (Saxe 2005; Brass et al. 2007; Schippers et al. 2009; Spunt et al. 2010).
A second component of the current study focuses on how we empathize with individuals who differ drastically from ourselves. Previous research indicates that we process and empathize with other people’s pain by activating the neural systems that process pain in our own bodies (SCs). This “pain matrix” includes the insula, the anterior and middle cingulate gyrus, the somatosensory cortices (SI and SII), and perhaps premotor areas as well (Singer et al. 2004, 2006; Jackson et al. 2006; Bufalari et al. 2007; Valeriani et al. 2008). However, it is thought that empathy is supported by 2 distinct cerebral processes, dividing the pain matrix into roughly 2 parts; one involving the cingulate cortex and insula; the other, the somatosensory cortices. These distinct processes enable us to empathize with others through internal psychological aspects (involving cingulate and insula) or external physical features (involving somatosensory cortices) (Avenanti et al. 2007; Valeriani et al. 2008). The latter type of empathic process would be automatic, appear earlier ontogenetically and phylogenetically, and involve SCs that would map sensory-motor characteristics in our own body (Preston and de Waal 2002; Avenanti et al. 2007).
Thus, empathy for pain would strongly involve simulation processes and SCs. Previous studies have found that sensorimotor or affective brain areas are modulated by observing dissimilar others, such as individuals from different racial groups (Xu et al. 2009; Avenanti et al. 2010; Liew et al. 2010). However, how is simulation possible when an individual does not have the observed body part? In line with our previous predictions, we hypothesize that when an individual without hands views someone receive an injection in the hand, they will not significantly activate the somatosensory cortices. They may show activity related to emotional aspects of pain empathy but not involve SCs that may allow localizing another person’s pain onto one’s own somatosensory body representations.
Materials and Methods
Subjects
All participants gave written informed consent and the study was approved by the Institutional Review Board at the University of Southern California.
Congenital Amputee (D.D.)
D.D., a 58-year-old female, was born without arms and legs. She has small upper arm stumps protruding a few inches past her shoulders and lower leg stumps that protrude a few inches past her pelvis. The etiology for her condition is unknown. While she used prostheses briefly as an adolescent, she has not used them for over the past 40 years. She holds a Master's degree in social work, and her cognitive abilities are within the normal to high range. According to a modified handedness questionnaire, she is “right-handed” (Oldfield 1971). She typically uses the right side of her body to perform actions, especially using her upper right stump (e.g., pushing objects, holding objects, using it in conjunction with her chin to move objects) and does not use her left upper stump for functional tasks.
Typically Developed Control Subjects (TD)
Thirteen female subjects matched in ethnicity (Caucasian), handedness (right), and education level (some college or higher) were recruited. Subjects were on average similar in age as D.D. (median: 52 years old; range: 36–68). Like D.D., all participants had normal vision and no history of neurological or psychiatric conditions.
Stimuli
Action Observation
In the action observation task, participants were presented with video clips depicting various actions performed by typically developed actresses. The video clips were 2 s in duration and depicted actions performed with the mouth, right hand, or right foot (TD actions). Action execution trials were also presented and were cued by a red box flashing briefly (500 ms) before a static image of a mouth, hand, or foot was presented (1500 ms). Static images depicting the mouth, hand, or foot (alone, as well as juxtaposed with an object next to the body part) were also presented as visual controls. The static images of the effector alone were used as controls for the action execution condition, and the static images of the effector juxtaposed with an object next to the body were used as controls to the action observation condition. Screenshots of various stimuli in each condition are presented in Figure 1. Video clips depicting D.D. performing different actions with her mouth and/or right upper stump were also presented, although the video clips of D.D.’s actions were used for a secondary study and will not be discussed further here.
Figure 1.
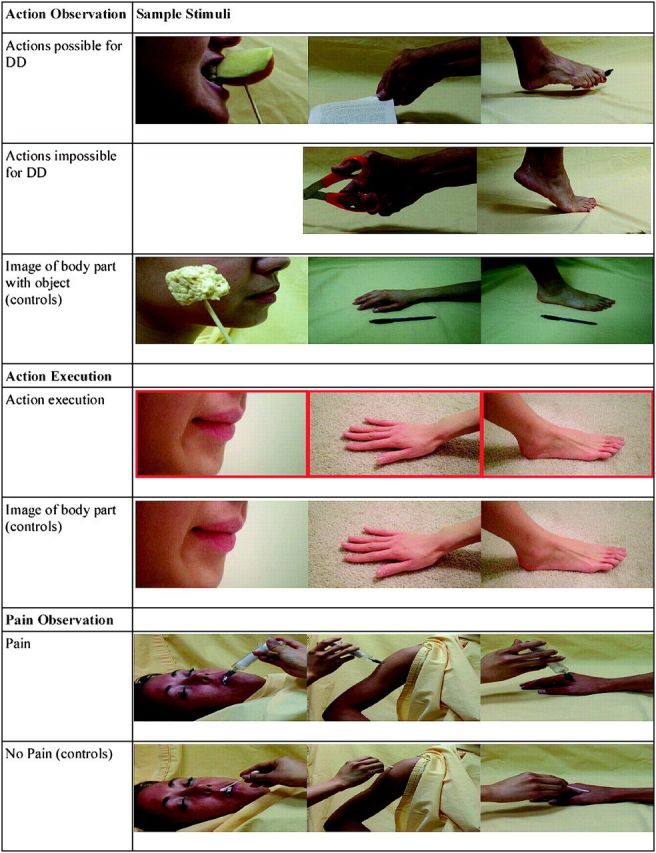
Examples of task stimuli. Sample visual stimuli used in the action observation, action execution, and pain observation tasks are presented.
Video clips of TD actions were categorized as 1) possible for D.D., with the same or a different effector and 2) impossible for D.D. regardless of the effectors involved. Possible/impossible criteria for actions were assessed with an open-ended questionnaire given to D.D. and by asking D.D. to perform the different actions. The possible videos depicted either the mouth, hand, or the foot, while impossible videos only depicted the hand or the foot, as all mouth actions are possible for D.D. (Fig. 1).
TD participants performed the same action observation task and viewed the same visual stimuli as D.D. However, the previously mentioned categorization criteria did not apply to TD participants as all depicted actions were possible for the TD group.
Pain Observation
In the pain observation task, participants were presented with 3s video clips depicting various body parts (lip, right shoulder, and right hand) either being injected with a syringe (pain) or touched with a q-tip (control).
Task
All subjects participated in 3 tasks: action observation, action execution, and pain observation. Action observation and action execution tasks were performed within the same runs, though, for the sake of clarity, they are described separately here. Pain observation was conducted in a separate run. For D.D., each action observation/execution run consisted of 116 trials and lasting 11:40 min. Stimuli were presented with an intertrial interval (ITI) that was jittered from 3 to 7 s, with a mean ITI of 4 s. A blank black screen was presented during the ITIs. For TD, each run consisted of 96 trials and lasted 9:38 min. Each TD participant performed 3 runs of action observation/execution, while D.D. performed 8 runs across 2 scanning sessions. Pain observation runs consisted of 60 trials with ITIs that were jittered from 3 to 7 s, with a mean ITI of 4 s. All subjects performed two 7:14 min runs of the pain observation task.
Action Observation (D.D.)
D.D. was familiarized to the stimuli and task prior to scanning. During action observation, D.D. was instructed to observe and attend to the actions presented. Forty-eight videos were shown in each run: 8 mouth actions which D.D. is able to do, 8 hand actions which D.D. is able to do with her mouth and upper stump, 8 hand actions which D.D. is able to do with her stump, 8 foot actions which D.D. is able to do with her stump, and 16 actions whose goal is impossible for D.D. (actions made by a foot or by a hand).
Action Execution (D.D.)
The action execution conditions were included to characterize the motor regions involved when D.D. performed actions. D.D. was instructed to perform the corresponding action when a body part cue appeared: 1) purse lips together as if giving a kiss (mouth cue); 2) reach with her right upper stump as if moving an object toward her (hand cue); 3) make a pressing motion with her right lower stump as if interacting with an object (foot cue). During visual control trials, D.D. was asked to simply view the stimuli.
Action Observation (TD)
Subjects were familiarized to the stimuli and task prior to scanning. During action observation, TD participants were instructed to observe and attend to the actions presented. All stimuli were the same as those presented to D.D., however, categorization of the stimuli differed due to the lack of distinction between possible versus impossible actions (as all actions were possible for TD participants). Thus, they were only categorized by effector: 1) mouth actions; 2) hand actions; 3) foot actions.
Action Execution (TD)
During action execution trials, participants were instructed to perform the corresponding action when a body part cue appeared: 1) purse lips together as if giving a kiss (mouth cue); 2) reach and grasp with their right hand as if reaching for a wine glass (hand cue); 3) move their right foot as if pressing down on the pedal of an automobile (foot cue). During visual control trials, they were instructed to simply view the stimuli.
Pain Observation
During pain observation/pain control trials, all participants were instructed to simply observe and attend to the stimuli. Each condition (lip, shoulder, hand; syringe and q-tip) was repeated 10 times per run.
Image Acquisition
All images were acquired using a Siemens MAGNETOM Trio 3 T magnetic resonance imaging scanner. A high-resolution T1-weighted anatomical volume was acquired from each participant (magnetization prepared rapid gradient echo; time repetition [TR] = 1950 ms, time echo [TE] = 2.56 ms, flip angle = 90°, 1 × 1 × 1 mm voxels, 208 coronal slices). Functional volumes were acquired while participants performed the action observation/execution and pain observation tasks (TR = 2000 ms, TE = 30 ms, flip angle = 90°, 3.5 × 3.5 × 3.5 mm voxels, 37 axial slices). Functional volumes were acquired using Siemens' prospective acquisition correction (PACE) technique for motion correction in which head movements were calculated by comparing successively acquired volumes and were corrected online (Thesen et al. 2000).
Data Processing and Analyses
Functional data were preprocessed and analyzed with SPM software (www.fil.ion.ucl.ac.uk/spm/; Wellcome Department of Imaging Neuroscience, London, UK). Functional images were slice timing corrected, realigned to the mean functional image, and normalized to the Montreal Neurological Institute (MNI) space. Normalization was done by first normalizing the high-resolution T1 structural scan to the T1 template and then applying those parameters to the realigned functional images that were in coregistration with the T1 structural scan. Images were then spatially smoothed using a 7.5-mm full-width at half-maximum Gaussian filter. First-level analyses were performed by convolving event onsets with the canonical hemodynamic response function and modeled with a general linear model. Group analyses were performed using random effects models with contrast estimates from individual subjects and were thresholded at P < 0.05 False Discovery Rate (FDR) corrected k > 5.
Action Observation
TD
To find regions that were active when TD participants observed actions, we conducted a whole-brain analysis, “all action observation—control.”
Regions of Interest Analyses
The aim of this study was to compare activation patterns in SC regions when D.D. viewed stimuli as compared to when the TD group viewed stimuli. To make this comparison, we identified regions involving the MNS and the pain matrix using data from the TD group. To identify brain regions involved in both the execution and observation of actions (MNS), a conjunction analysis was performed at the second level using the TD group-level contrasts “all execution—all controls” and “all observation—all controls,” each at a threshold of P < 0.05, FDR, k > 5 voxels. Significant clusters were found in bilateral ventral precentral gyri/inferior frontal cortices (consistent with what has previously been described as premotor cortices in action observation literature; pMC), bilateral IPLs, bilateral postcentral gyri, bilateral superior/middle temporal gyrus, left cuneus, left posterior insula, left cerebellum, and right lingual gyrus. Of these, we chose the regions of interest (ROIs) that have been consistently reported as being part of the putative human MNS: ventral precentral gyrus and pars opercularis and posterior pars triangularis of the inferior frontal gyrus (pMC), the IPL, and the posterior superior/middle temporal gyrus (Van Overwalle and Baetens 2009). While the STS is not commonly thought of as part of the MNS (as it is not a “motor” region), it is often active in conjunction with the MNS perhaps due to the visual components of the task (action observation as well the visual cue of a body part during the execution component) (Iacoboni et al. 2001). Thus, we have included it in this analysis. ROIs are depicted in Figure 2 and Table 1. An image of the ROIs mapped onto an anatomical image of D.D.’s brain can be found in Supplementary Figure S1. To determine whether D.D. recruited these regions when observing various actions being performed, we performed the contrast “all possible actions—all controls” for D.D.
Figure 2.
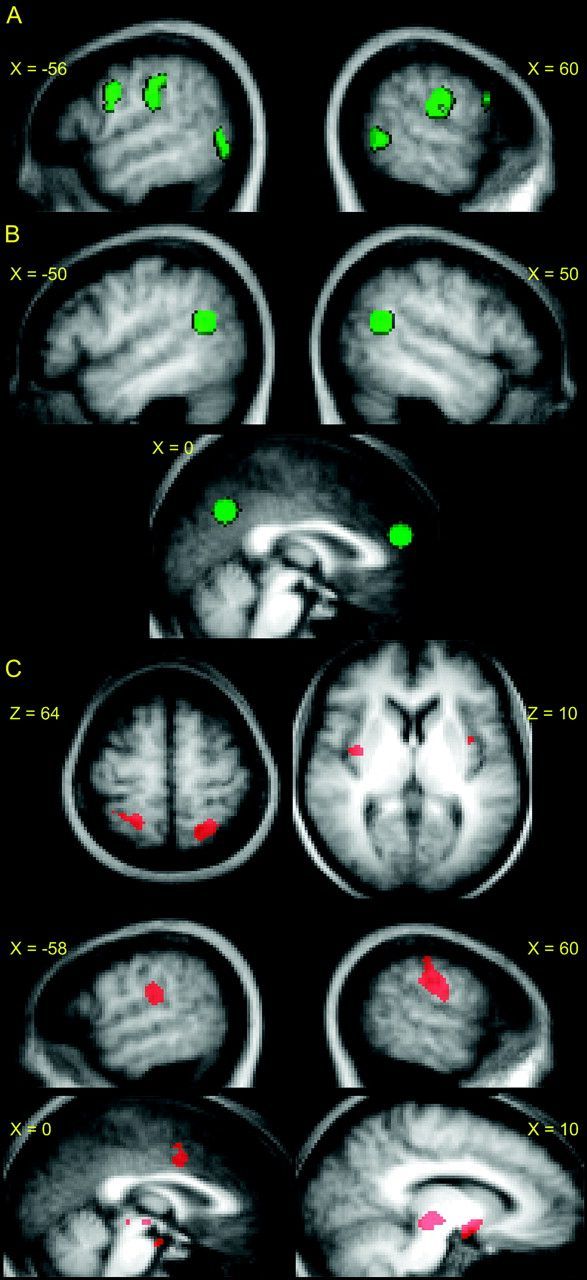
ROIs. (A) Mirror system ROIs (green) consisting of L/R precentral gyrus (premotor), L/R IPL, L/R posterior middle temporal gyrus/superior temporal sulcus (pSTS). (B) Mentalizing system ROIs (green) consisting of L/R TPJ, the mPFC, and medial precuneus. (C) ROIs for pain observation (red). These include bilateral postcentral gyri (SI), bilateral insula, bilateral inferior parietal/lateral postcentral gyri (SII), mid cingulate gyrus, and portions of the midbrain. All ROIs were overlaid onto a mean anatomical image constructed from the 13 TD participants’ structural scans (normalized to MNI space).
Table 1.
ROIs
| Brain region | MNI coordinates | Voxel T | Size (number of voxels) |
| Mirror system | |||
| L IPL | −54, −22, 32 | 5.17 | 338 |
| R IPL | 60, −22, 26 | 12.27 | 347 |
| L premotor cortex | −58, 2, 36 | 5.77 | 108 |
| R premotor cortex | 62, 12, 30 | 4.31 | 20 |
| L posterior MTG and STG | −50, −70, −4 | 6.27 | 298 |
| R posterior MTG | 58, −66, 8 | 6.97 | 208 |
| Mentalizing system | |||
| R/L mPFC | 0, 50, 24 | NA | 256 |
| Precuneus | 0, −64, 40 | NA | 256 |
| L TPJ | −52, −58, 24 | NA | 256 |
| R TPJ | 52, −58, 24 | NA | 256 |
| Observation of pain | |||
| L anterior SMG | −20, −58, 68 | 5.78 | 127 |
| R anterior SMG | 24, −62, 62 | 5.77 | 298 |
| L secondary sensory cortex | −58, −24, 24 | 6.49 | 401 |
| R secondary sensory cortex | 66, −18, 28 | 5.26 | 435 |
| L insula | −44, −4, 2 | 5.44 | 222 |
| R insula | 36, 4, 6 | 4.04 | 23 |
| Middle cingulate gyrus | −2, 2, 52 | 3.97 | 75 |
| Midbrain | 4, −12, −18 | 5.17 | 624 |
Note: As the mirror system ROIs were the result of conjunction analyses, they do not have peak voxels. Thus, MNI coordinates and voxel T values for the mirror system ROIs were based on the TD group-level peak voxel coordinates of the action observation—controls contrast. MNI coordinates for mentalizing system ROIs were converted from the Talairach coordinates presented in Van Overwalle and Baetens (2009).
We were furthermore interested in whether D.D. recruited brain regions associated with mentalizing activity when viewing actions that were not possible for her. As all actions were possible for TD subjects, it was not possible to create ROIs for mentalizing regions using activation patterns from the TD group. Thus, we defined ROIs for brain regions associated with mentalizing tasks based on previous reports (Brass et al. 2007; de Lange et al. 2008). Spherical ROIs with 8 mm radii centered around previously reported voxel coordinates (MNI coordinates, adapted from Van Overwalle and Baetens [2009]) were created for the mPFC (0, 50, 24), precuneus (0, −64, 40), and bilateral TPJ (±51, −58, 24) using MarsBar software (Brett et al. 2002). We investigated whether these regions were active when D.D. observed impossible actions (“all impossible actions—all controls,” “all possible actions—all impossible actions,” “all impossible actions—all possible actions”).
Pain Observation
TD
To find regions that were active when TD participants observed others in pain, we conducted a whole-brain analysis, “all pain observation—all no pain.” We also compared activations for observing pain in individual body parts, using the following contrasts: “mouth pain—mouth no pain,” “hand pain—hand no pain,” “mouth pain—hand pain,” and “hand pain—mouth pain.”
ROIs Analyses
Similar to the action observation analysis, we were interested in comparing activation patterns from the TD group to activation patterns in D.D. We made this comparison by identifying ROIs in the TD group for the pain matrix and then investigating whether D.D. also recruited these regions when observing others in pain. Thus, second-level analyses were performed for the 13 TD participants. To identify regions involved in the observation of painful stimuli, we performed the contrast “all pain - all no pain.” Regions in the bilateral SI and SII, bilateral inferior frontal gyri (opercularis bilaterally; triangularis in RH), bilateral inferior temporal/middle occipital gyri, bilateral midbrain (extending into the periaqueductal gray), bilateral insular cortices, bilateral orbitofrontal cortices, middle cingulate gyrus, and left anterior superior temporal gyrus were active (P < 0.05, FDR, k > 5). As we did for the observation of actions, a subset of these regions were defined as ROIs (middle cingulate gyrus, bilateral insula, bilateral SI and SII, midbrain); the regions chosen were those typically reported as part of the pain matrix (Peyron et al. 2000). ROIs are depicted in Figure 2 and Table 1.
To investigate whether D.D. also recruited these regions when observing painful stimuli, we performed ROI analyses. To determine whether these brain regions were active when viewing painful stimuli in general, we used the contrast “all pain—all no pain.” To determine whether the ROIs were active when D.D. watched others experience pain in a body part that she has, we used the contrast “mouth pain—mouth no pain” and “mouth pain—hand pain”. Similarly, to determine whether the ROIs were active when D.D. watched others experience pain in a body part she lacks, we used the contrast “hand pain—hand no pain” and “hand pain—mouth pain”.
Results
Action Observation
T.D.
The “all action observation—controls” contrast in TD participants revealed activations in the bilateral precentral gyri/posterior inferior frontal gyri, bilateral superior parietal cortices, bilateral IPLs, left anterior superior temporal gyrus, bilateral posterior middle temporal gyri (extending posteriorly into temporal-occipital junction and lateral occipital gyri and inferiorly into inferior temporal gyri), left middle insula, and right anterior parahippocampal gyrus (P < 0.05, FDR, k > 5; Supplementary Fig. S3).
D.D.
Observation of possible actions.
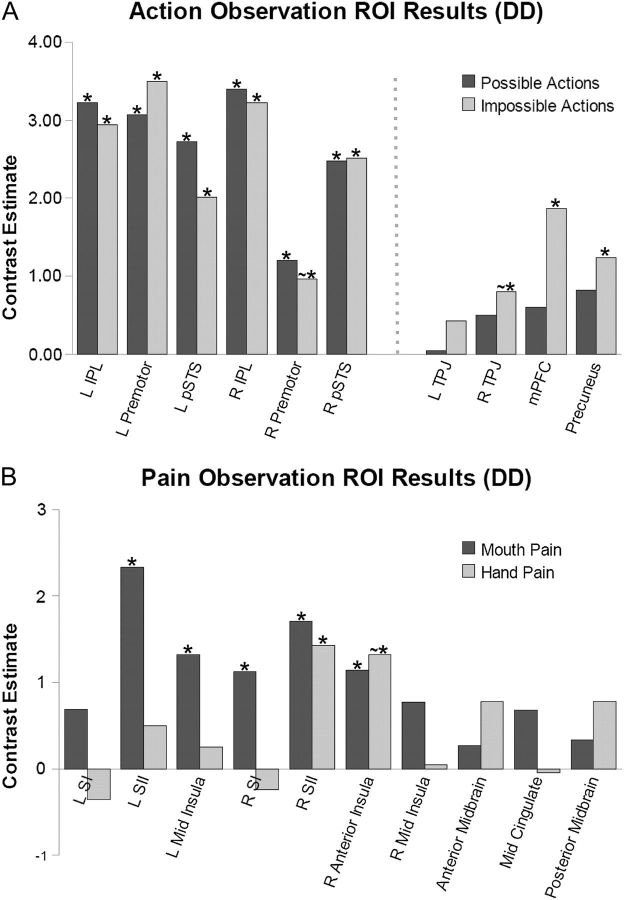
In D.D., all mirror system ROIs were significantly active (L and R pMC, L and R IPL, L and R pSTS; P < 0.05) for the contrast “all possible actions—all controls”. As expected, none of the mentalizing ROIs were found to be significantly active (Fig. 3).
Figure 3.
Action observation and pain observation ROI results for D.D. (A) Contrast estimates in mirror system (left) and mentalizing (right) ROIs for D.D. during observation of possible actions and impossible actions (each contrasted against controls). (B) Contrast estimates in pain observation ROIs for D.D. during observation of mouth pain and hand pain (contrasted with mouth no pain, hand no pain, respectively). * indicates P < 0.05; ∼* indicates P < 0.1.
Observation of impossible actions.
In the contrast “all impossible actions—all controls”, regions associated with mentalizing activity (the mPFC and precuneus) were significantly active (P < 0.05), while the R TPJ was nearly significant (P = 0.093). In line with what we found earlier with the possible actions, all the mirror system ROIs except R pMC (L pMC, L and R IPL, L and R pSTS) were also significantly active (P < 0.05). In the direct comparison “all impossible actions—all possible actions”, no ROIs were active, though the mPFC showed a trend (P = 0.054).
Pain Observation
TD
To identify regions involved in the observation of painful stimuli, we performed the contrast “all pain—all no pain”. Regions with significant activation include the inferior frontal gyri (opercularis bilaterally; triangularis in RH), bilateral SI and SII, bilateral inferior temporal/middle occipital gyri, bilateral midbrain (extending into the periaqueductal gray), bilateral insular cortices, bilateral orbitofrontal cortices, middle cingulate gyrus, and left anterior superior temporal gyrus (P < 0.05, FDR, k > 5). For the contrasts “mouth pain—mouth no pain”, “mouth pain—hand pain”, “hand pain—hand no pain”, and “hand pain—mouth pain”, no significant activations were found at P < 0.05, FDR (k > 5).
D.D.
To investigate whether the pain observation ROIs were active when D.D viewed someone experiencing pain, we performed the contrast “all pain—all no pain”. SII was significantly active bilaterally (P < 0.05), while a marginal effect was found in the right and left middle insula (P = 0.07 and P = 0.09, respectively). The latter analysis conflated observation of body parts D.D. has (e.g., mouth), with body parts D.D. does not have (e.g., hand). To investigate whether the pain observation ROIs were active when D.D. viewed someone experiencing pain in a body part that she has, we used the contrast “mouth pain—mouth no pain” and found the left middle insula, right anterior insula, right SI, and bilateral SII to be active (P < 0.05). To investigate whether the pain observation ROIs were active when D.D. viewed someone experiencing pain in a body part that she doesn’t have, we used the contrast “hand pain—hand no pain” and found the right SII to be active (P < 0.05) and a marginal effect in right anterior insula (P = 0.07). In the direct comparison, “mouth pain—hand pain”, we found the L SII and R SI ROIs to be active (P < 0.05), while we found L SI to be nearly significant (P = 0.08). No ROIs were active in the opposite contrast, “hand pain—mouth pain”.
Post hoc whole-brain analyses
Given D.D.’s atypical physical body, it may be that her motor and sensory cortices are developed differently from TD individuals. For this reason, as well as to observe D.D.'s brain activity beyond the a priori ROIs, we also conducted whole-brain analyses. The results support the ROI analyses reported here and are discussed in full detail in the supplementary materials (Figs. S2–S4).
Discussion
Action Observation
As expected, when viewing other people complete actions that are possible for D.D., TD control subjects and D.D. showed activation in mirror regions, including the premotor cortex and the IPL (Fig. 3 and Supplementary Fig. S3). Thus, even when D.D. does not have the effector used to complete the observed action, she activates her own motor representations provided the action goal is possible for her. These results are consistent with previous reports indicating that mirror regions are sensitive to the goals of the action, even when the body part used to achieve the goal differ (Gazzola, van der Worp, et al. 2007). Furthermore, we find stronger activity in the left premotor cortex, a finding that is consistent with previous reports of observation of human actions activating motor-related areas bilaterally, whereas observation of actions made by robots or nonconspecifics would activate only the left motor-related areas (Buccino et al. 2004; Gazzola, Rizzolatti, et al. 2007). Of further note is that neither TD nor D.D. showed activity in regions associated with mentalizing, indicating that only sensory-motor representations are involved when asked to simply observe others make actions.
However, when D.D. observed others complete actions that were impossible for her to perform, she still exhibited activity in mirror regions but additionally recruited brain regions associated with mentalizing processes (precuneus, right TPJ, mPFC). These results suggest that when observing actions that are impossible for her, D.D. utilizes her own body maps to process certain aspects of actions but needs to engage more inferential processing to imagine others' states and intentions. Thus, both simulation and mentalizing processes seem to be working in conjunction to help represent and possibly understand actions that are impossible for her to perform.
While this is the first time these data are seen in an individual with a drastically different body than those that she observes, these results are, in part, consistent with previous studies. A recent meta-analysis (Van Overwalle and Baetens 2009) indicated that MNS regions tend to be active for observations of biological movement, with stronger activity when observing actions for which one has an existing motor pattern (Calvo-Merino et al. 2005; Cross et al. 2006). In contrast, regions associated with mentalizing (TPJ, precuneus, mPFC) are more active in the absence of existing motor patterns, such as observation of actions that are out of context or nonstereotypic implausible actions (Brass et al. 2007; Kilner and Frith 2008; Liepelt et al. 2008).
Of further note is that both the pMC and parietal regions of the MNS are active when D.D. views actions that are impossible for her. The pMC is thought to be involved with processing the goal of the observed action, while the posterior regions are thought to be involved in processing the affordances used in the action (Oztop and Arbib 2002; Iacoboni et al. 2005). Thus, it appears that even when D.D. cannot make the action herself, she nevertheless uses not only her own sensory-motor representations to process the action goal but, interestingly, also uses her posterior motor regions to model the action affordances. This is consistent with a previous study that showed that biomechanically impossible actions still activate components of the MNS (Costantini et al. 2005). However, in that study, the observed actions were intransitive movements without a goal whereas here we demonstrate activity in the MNS even when the goal of the action is impossible for the observer.
It may be surprising that D.D. activates the MNS even when observing actions that are impossible for her. Indeed, a few previous studies indicate a lack of MNS activation when the observed action is not within the motor repertoire of the observer, such as observing a dog bark (Buccino et al. 2004). However, this may be due to the fact that while the observed actions are not ones for which D.D. has motor patterns, they are actions for which she has extensive visual and conceptual knowledge of and are part of the normal human repertoire of actions. Indeed, while D.D. cannot sew or use scissors, she has a lifetime of observing others sew, use scissors, and so forth. This is in contrast to the previous example of watching a dog bark, where the action is outside of the human repertoire. In fact, Cross et al. (2006) and Ferrari et al. (2005) have indicated that observational learning of actions can increase activity in the MNS, similar to what happens with actual motor learning. Thus, the current data may demonstrate the strong modulatory effects of observational learning on the MNS, even when the actions are impossible for the observer.
As our study focuses on the most dramatic case—an individual born without both upper and lower extremities—future studies may explore similar patterns in individuals born without only the upper or lower extremities to see if extremity location influences these data. Furthermore, given D.D.’s atypical physical body, it may be a concern that her motor and sensory cortices are developed differently from TD individuals. For this reason, as well as to observe D.D.'s brain activity beyond the a priori ROIs, we also conducted whole-brain analyses. These methods and results are described in the supplementary materials and are consistent with the ROI results reported here. We also note that this is a case study and further work with larger sample sizes is needed to make strong claims. The results from D.D., however, tentatively suggest a model where the MNS and mentalizing systems serve complementary roles, with MNS regions being recruited when observing actions for which one has an existing motor pattern and mentalizing regions being recruited when one has to process actions that are not within one’s motor repertoire. However, more power would be needed to make this a significant given that post hoc analyses indicate that D.D. falls within the distribution of normals (see Supplementary Materials).
Pain Observation
As expected, when viewing other people experiencing painful situations, TD control subjects showed activation in the pain matrix, including regions such as the cingulate, insula, and somatosensory cortices (Fig. S4). These results are consistent with previous reports indicating that we utilize common regions for both experiencing pain and observing or thinking about another person in pain (Hein and Singer 2008). In TD participants, these results were found when comparing all the pain observation trials to control trials, and no significant activations were found when looking at pain in a specific body part, which may be due to the smaller number of trials for those contrasts.
Like the TD group, when D.D. observes other people experience pain in a body part that she has (mouth), she activates the left middle insula, right anterior insula, right SI, and bilateral SII. Furthermore, in the whole-brain analysis of D.D., the cingulate is also found to be active, like the TD group. However, when D.D. views others experience pain in the hand (a body part she lacks), the insula is still active, but the primary somatosensory cortices are not. This result suggests that she understands and is able to empathize with the “suffering” of another person, but she does not activate her own primary sensing cortices.
Previous studies have shown that activity in the primary somatosensory cortex may be related to observing another person’s pain and may indicate the mapping of sensory attributes of observed pain on to one’s own somatosensory representations (Avenanti et al. 2005, 2006; Bufalari et al. 2007). In particular, one study suggested that when observing another person in pain, focusing on the intensity of the pain enhanced activity in SI. By contrast, focusing on the unpleasantness of the pain was not correlated with activity in the SI (Lamm et al. 2007). When viewing pain in body parts she does not have, D.D. still reports feeling empathy for the person in pain as expressed in a postscan questionnaire, and indeed, brain regions associated with more emotional aspects of empathy for pain are activated (e.g., insula). However, the primary somatosensory cortex is only active when she observes pain inflicted on body parts that she has (mouth) and not when viewing painful stimulation on body parts that she does not have (hand). This result is consistent with a previous study investigating pain empathy in individuals with congenital insensitivity to pain, which reported activity in the insula and cingulate cortices but not in somatosensory regions (Danziger et al. 2009). Furthermore, similar to our results, a recent study found that observation of others in pain only activated SI when both observers and actors felt a stimulus to be painful (Lamm et al. 2010). Taken together with the current results, it appears that primary somatosensory activation during pain observation may be contingent on having the existing sensory representations to process the pain in a localized bodily region. Thus, the current finding of a lack of somatosensory activation when viewing pain in body parts that D.D. does not have may indicate that, while she empathizes with the other person’s suffering, she may not be able to map the person’s pain onto her own somatosensory body representations. Once more, we note that further work with larger sample sizes is needed to make strong claims. While this result is suggestive that not having a body part leads to a lack of somatosensory activation when viewing that body part in pain, more power is needed to make this a significant effect, given that post hoc analyses indicate that D.D. falls within the distribution of normal individuals for the somatosensory ROI analyses (see Supplementary materials). Thus, future work with increased sample sizes is vital to making strong claims from this data.
Conclusions
To date there has been a great deal of research indicating that SCs (the mirror system, the pain matrix) may be involved in understanding and empathizing with other people whose bodies are similar to ours. The present study suggests that viewing someone with a body greatly different from one’s own results in a slightly different pattern of activity. We found that when viewing people with bodies different from her own, D.D. engages her own motor and sensory representations. However, when the observed action is impossible for her, additional regions that have been associated with inferential and mentalizing processes seem to be necessary to fully process the visual stimuli. Similarly, when observing someone experience pain in a body part that she does not have, she still empathizes with the other’s suffering and activates the structures involved in the emotional pain processing (insula) but cannot represent the bodily site of pain, failing to activate brain regions involved in the primary sensing of pain (somatosensory cortices). While these results should be tested in larger populations, they provide a novel approach to understanding the neural processes used in observing and empathizing with individuals who physically differ from ourselves.
Funding
The Brain and Creativity Institute; The Division of Occupational Science and Occupational Therapy at USC; National Science Foundation Graduate Research Fellowship; USC Provost's PhD Fellowship.
Supplementary Material
Acknowledgments
We thank D.D. and all of our subjects for participating in this study. We also thank Henryk Bukowski, Gelya Frank, and Antonio Damasio for their assistance with this study. Conflict of Interest: None declared.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bolognini N, Maravita A, Aglioti SM. Somatic and motor components of action simulation. Curr Biol. 2007;17:2129–2135. doi: 10.1016/j.cub.2007.11.045. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci. 2005;8:955–960. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Paluello IM, Bufalari I, Aglioti SM. Stimulus-driven modulation of motor-evoked potentials during observation of others’ pain. Neuroimage. 2006;32:316–324. doi: 10.1016/j.neuroimage.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Sirigu A, Aglioti SM. Racial bias reduces empathic sensorimotor resonance with other-race pain. Curr Biol. 2010;20(11):1018–1022. doi: 10.1016/j.cub.2010.03.071. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Sheng T, Gheytanchi A. Common premotor regions for the perception and production of prosody and correlations with empathy and prosodic ability. PLoS One. 2010;5:e8759. doi: 10.1371/journal.pone.0008759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Schmitt RM, Spengler S, Gergely G. Investigating action understanding: inferential processes versus action simulation. Curr Biol. 2007;17:2117–2121. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an SPM toolbox [abstract] 2002 Presented at the 8th International Conference on Functional Mapping of the Human Brain; 2002 Jun 2–6; Sendai (Japan). Available on CD-ROM in NeuroImage, Vol 16, No 2, abstract 497. [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro CA, Rizzolatti G. Neural circuits involved in the recognition of actions performed by nonconspecifics: an FMRI study. J Cogn Neurosci. 2004;16:114–126. doi: 10.1162/089892904322755601. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex. 2007;17:2553–2561. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P. Action observation and acquired motor skills: an FMRI study with expert dancers. Cereb Cortex. 2005;15:1243–1249. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Costantini M, Galati G, Ferretti A, Caulo M, Tartaro A, Romani GL, Aglioti SM. Neural systems underlying observation of humanly impossible movements: an FMRI study. Cereb Cortex. 2005;15(11):1761–1767. doi: 10.1093/cercor/bhi053. [DOI] [PubMed] [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST. Building a motor simulation de novo: observation of dance by dancers. Neuroimage. 2006;31:1257–1267. doi: 10.1016/j.neuroimage.2006.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger N, Faillenot I, Peyron R. Can we share a pain we never felt? Neural correlates of empathy in patients with congenital insensitivity to pain. Neuron. 2009;61:203–212. doi: 10.1016/j.neuron.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Curr Biol. 2008;18:454–457. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Ferrarri PF, Maiolini C, Addessi E, Fogassi L, Visalberghi E. The observation and hearing of eating actions activates motor programs related to eating in macaque monkeys. Behav Brain Res. 2005;161(1):95–101. doi: 10.1016/j.bbr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 2006;16:1824–1829. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Rizzolatti G, Wicker B, Keysers C. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage. 2007;35:1674–1684. doi: 10.1016/j.neuroimage.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gazzola V, van der Worp H, Mulder T, Wicker B, Rizzolatti G, Keysers C. Aplasics born without hands mirror the goal of hand actions with their feet. Curr Biol. 2007;17:1235–1240. doi: 10.1016/j.cub.2007.06.045. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobiol. 2008;18:153–158. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, Mazziotta JC, Rizzolatti G. Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci U S A. 2001;98(24):13995–13999. doi: 10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Bastiaansen J, Keysers C. A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS One. 2008;3:e2939. doi: 10.1371/journal.pone.0002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn Sci. 2007;11:194–196. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol. 2009;19:666–671. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Keysers C, Wicker B, Gazzola V, Anton JL, Fogassi L, Gallese V. A touching sight: SII/PV activation during the observation and experience of touch. Neuron. 2004;42:335–346. doi: 10.1016/s0896-6273(04)00156-4. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Frith CD. Action observation: inferring intentions without mirror neurons. Curr Biol. 2008;18:R32–R33. doi: 10.1016/j.cub.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. J Cogn Neurosci. 2010;22:362–376. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- Liepelt R, Von Cramon DY, Brass M. How do we infer others’ goals from non-stereotypic actions? The outcome of context-sensitive inferential processing in right inferior parietal and posterior temporal cortex. Neuroimage. 2008;43:784–792. doi: 10.1016/j.neuroimage.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Liew SL, Han S, Aziz-Zadeh L. Familiarity modulates mirror neuron and mentalizing regions during intention understanding. Human Brain Mapping. 2011 doi: 10.1002/hbm.21164. 32. doi: 10.1002/hbm.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oztop E, Arbib MA. Schema design and implementation of the grasp-related mirror neuron system. Biol Cybern. 2002;87:116–140. doi: 10.1007/s00422-002-0318-1. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion: 20–71. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Saxe R. Against simulation: the argument from error. Trends Cogn Sci. 2005;9:174–179. doi: 10.1016/j.tics.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Schippers MB, Gazzola V, Goebel R, Keysers C. Playing charades in the fMRI: are mirror and/or mentalizing areas involved in gestural communication? PLoS One. 2009;4:e6801. doi: 10.1371/journal.pone.0006801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Satpute AB, Lieberman MD. Identifying the what, why, and how of an observed action: an fMRI study of mentalizing and mechanizing during action observation. J Cogn Neurosci. 2010;23:63–74. doi: 10.1162/jocn.2010.21446. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Betti V, Le Pera D, De Armas L, Miliucci R, Restuccia D, Avenanti A, Aglioti SM. Seeing the pain of others while being in pain: a laser-evoked potentials study. Neuroimage. 2008;40:1419–1428. doi: 10.1016/j.neuroimage.2007.12.056. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. J Neurosci. 2009;29(26):8525–8529. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proc Natl Acad Sci U S A. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.