Abstract
Since the inception of the British Neuroscience Association, there have been major advances in our knowledge of the mechanistic basis for stroke-induced brain damage. Identification of the ischaemic cascade led to the development of hundreds of new drugs, many showing efficacy in preclinical (animal-based) studies. None of these drugs has yet translated to a successful stroke treatment, current therapy being limited to thrombolysis/thrombectomy. However, this translational failure has led to significant improvements in the quality of animal-based stroke research, with the refinement of rodent models, introduction of new technologies (e.g. transgenics, in vivo brain imaging) and improvements in study design (e.g. STAIR, ARRIVE and IMPROVE guidelines). This has run in parallel with advances in clinical diagnostic imaging for detection of ischaemic versus haemorrhagic stroke, differentiating penumbra from ischaemic core, and improved clinical trial design. These preclinical and clinical advances represent the foundation for successful translation from the bench to the bedside in the near future.
Keywords: Stroke, cerebral ischaemia, preclinical, brain injury
Clinical diagnosis of stroke: a transformational story
Significant advances in brain imaging mean that stroke patients can now expect an acute diagnostic scan to differentiate between haemorrhagic, ischaemic and stroke mimics when admitted to hospital (Heit and Wintermark, 2017). If the stroke is ischaemic, the occluded vessel can be identified and time from stroke onset used to determine whether thrombolysis, to break down an occluding embolus/thrombus, or endovascular embolus/thrombus extraction (thrombectomy) is warranted. Thrombolytic treatment with intravenous recombinant tissue plasminogen activator (IV rtPA), given up to 4.5 h from stroke onset, significantly increases the prospect of a good recovery, with the greatest benefit seen with the shortest onset-to-treatment times (Röther et al., 2013).
In patients where a large proximal occlusion is identified, endovascular thrombectomy within 6 h of stroke onset improves the chance of a good outcome (Powers et al., 2015). However, less than 10% of acute ischaemic stroke patients receive intravenous thrombolysis in most centres and only 7%–15% are expected to qualify for acute endovascular intervention (Henninger and Fisher, 2016). Therefore, there is significant scope for stroke researchers (clinical and preclinical) to develop strategies for more patients to benefit from thrombolysis/thrombectomy and test new therapies as adjunct or standalone treatments for patients where thrombolysis/thrombectomy is contraindicated.
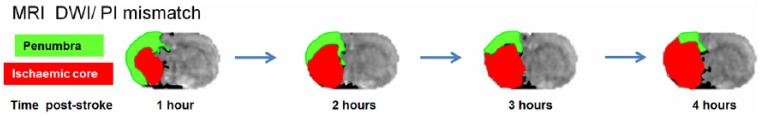
Ischaemic stroke patients being considered for recruitment into a clinical trial may also have a computed tomography (CT) or magnetic resonance imaging (MRI) ‘penumbra’ scan to identify how much tissue affected by the stroke is still capable of recovery (Warach et al., 2016). Since penumbral tissue has a limited lifespan of hours and available tissue for salvage varies significantly in the patient population, penumbral imaging also ensures that only patients with remaining biological target tissue are recruited into acute stroke therapeutic trials (Muir and Macrae, 2016). The lack of penumbral imaging in the early, unsuccessful clinical neuroprotection trials, some of which recruited patients at time points beyond the likely survival of penumbra, is likely to have resulted in recruitment of stroke patients with no remaining target tissue for salvage, thereby diluting the power to detect therapies with potential efficacy. MRI penumbral imaging is now available for longitudinal rodent stroke studies where loss of penumbra over time can be tracked and the consequences of therapeutic intervention determined (Figure 1), thereby providing more informative data on the potential to successfully translate findings to the clinic (Muir and Macrae, 2016).
Figure 1.
Loss of penumbral tissue over time in a rat permanent middle cerebral artery occlusion model. By superimposing perfusion images (PIs) of cerebral blood flow, which reveal areas of hypoperfusion, onto apparent diffusion coefficient maps derived from diffusion-weighted images (DWIs) and revealing early ischaemic injury (ischaemic core, red), ‘penumbra’ (green) is identified as the area of hypoperfused tissue which is not yet showing signs of ischaemic injury. Without intervention or restoration of blood flow through the occluded artery, penumbral tissue gradually deteriorates over a number of hours and becomes incorporated into the irreversibly damaged ischaemic core.
Modelling stroke: steady development over the years
Rodent models of focal cerebral ischaemia were first described in the early 1980s and have been in common use ever since (Albanese et al., 1980; Tamura et al., 1981). Most in vivo research has been conducted using models of permanent and transient middle cerebral artery occlusion (MCAO) and embolic stroke (Macrae, 2011). Rodent stroke models have enabled stroke researchers to (a) understand the complex biochemical and molecular mechanisms that comprise the ‘ischaemic cascade’ and initiate cell death in the first minutes, hours and days following stroke (Moskowitz et al., 2010); (b) determine the cerebral blood flow (CBF) thresholds at which irreversible cell death, electrical failure, inhibition of protein synthesis and energy depletion occur and thereby establish the CBF thresholds that define potentially salvageable penumbra (i.e. hypoperfused tissue which is still metabolising oxygen and glucose; Astrup et al., 1981); and (c) develop and test new therapies to limit acute ischaemic injury, salvage available penumbra and promote long-term repair and recovery (Dirnagl and Endres, 2014). Published studies in experimental stroke have therefore been mechanistic, increasing knowledge of pathophysiology and repair mechanisms, or exploratory and confirmatory, confirming mechanisms of action and testing the efficacy of new therapies. Outcome measures in drug efficacy studies include assessment of infarct size and neurological/sensorimotor/behavioural deficits (Macrae, 2011). Most in vivo stroke studies still use young, adult male rodents, but the importance of studies using both sexes, aged animals and incorporating other risk factors and comorbidities (e.g. hypertension, diabetes, obesity, infection, etc.) is recognised (Fisher et al., 2009) and becoming more common in the literature (Haley and Lawrence, 2016).
No animal model is a perfect reflection of the disease under study and each MCAO model has strengths and limitations when trying to model the complex heterogeneous nature of stroke in humans (Fluri et al., 2015). Therefore, it is important that a new drug or therapy should demonstrate efficacy in a range of models reflecting the patient population before being considered for clinical trials. This is not really achievable within a single lab or centre where output is generally single proof-of-principle, dose–response or time window–type efficacy studies. However, with strategic planning, there can be more collaboration across groups in preclinical stroke research (Dirnagl et al., 2013) and ultimately follow the clinical model of international multicentre trials (Dirnagl and Fisher, 2012). The first multicentre stroke studies have recently been published (Llovera et al., 2015; Maysami et al., 2016) and the Multi-PART (Multicentre Preclinical Animal Research Team) consortium, an European Union (EU)-funded international network of preclinical stroke researchers, have set up a platform to conduct high-quality multicentre preclinical studies (http://www.dcn.ed.ac.uk/multipart/).
Understanding stroke: mechanistic insights using new technologies
While early preclinical stroke research focussed largely on approaches to identify the ischaemic core and penumbra and critical thresholds of CBF for neuronal survival, subsequent studies made use of increasingly sophisticated tools to further delineate the ischaemic cascade. The use of transgenic animals, gene silencing techniques and advanced in vivo imaging approaches (e.g. molecular imaging, intravital and two-photon microscopy) has revealed ever more details of the cellular and molecular mechanisms involved in ischaemic stroke. These approaches have identified inflammation as a key contributor to ischaemic injury with early expression of cytokines and chemokines after MCAO leading to upregulation of adhesion molecules and subsequent infiltration of leukocytes, which release matrix metalloproteinases, resulting in disruption of the blood–brain barrier (Anrather and Iadecola, 2016). Alongside excitotoxicity and oxidative stress, inflammation has therefore been one of the most targeted mechanisms in acute neuroprotectant studies (Chamorro et al., 2016). Early use of gene-deficient mice confirmed the importance of vascular adhesion molecule, namely, Intercellular Adhesion Molecule 1 (ICAM-1), in stroke, though subsequent clinical trials of an anti-ICAM antibody were unsuccessful. However, other therapies targeting immune processes are still ongoing (Fu et al., 2015). One of the most promising of these potential stroke treatments is interleukin-1 (IL-1) receptor antagonist, a competitive inhibitor of the pro-inflammatory cytokine IL-1 (Sobowale et al., 2016). Whereas it is widely accepted that neutrophils have an early detrimental role in stroke, the contribution of microglial and peripheral macrophages/monocytes has been much more hotly contested. Earlier studies largely reported that microglial activation after MCAO was detrimental, whereas recent work, using bone marrow chimeras and other means to deplete or modify microglia and/or monocytes, reveals a complex role for these cells in the ischaemic brain that still remains to be fully understood (Szalay et al., 2016; Wattananit et al., 2016). Current consensus is that post-stroke inflammation is extremely dynamic in nature and that microglia and monocytes undoubtedly have different and potentially opposing roles, at various stages post stroke (Anttila et al., 2017; Ma et al., 2016). The use of two-photon imaging is greatly enhancing this research, allowing the dynamic nature of microglia and other immune-related cells to be observed in real time (Scheller et al., 2014), albeit still with the confounding factors of general anaesthesia and surgery. The development of molecular imaging tools to allow in vivo visualisation of cerebrovascular (Gauberti et al., 2013) and microglial (Boutin et al., 2015) activation has also greatly advanced the field, with some ligands (e.g. PK11195) licensed for clinical use (Thiel et al., 2010), allowing both forward and backward translation. The ability to identify pathophysiological changes clinically in stroke is essential for the development of better preclinical models that are more representative of the clinical situation and this is becoming increasingly doable as imaging techniques advance.
Treating stroke: failure reveals the importance of experimental design
Despite the advances in our understanding of stroke pathophysiology, the identification of hundreds of targets and the testing of many drugs in animal stroke models, by the late 1990s the failure to translate any new treatment to the clinic undoubtedly hampered stroke research. Many large pharmaceutical companies, having spent millions on failed clinical trials, pulled out of stroke research and the validity of animal stroke models was questioned. Flaws in both preclinical (e.g. failure to control potential bias, underpowered) and clinical trials (e.g. no penumbral imaging and patient recruitment beyond the point of likely penumbra survival) could potentially explain this ‘Translational Roadblock’ (Dirnagl and Macleod, 2009; Howells et al., 2014). Consequently, in 1999, a group of clinical and preclinical stroke experts met to investigate and address this translational failure (Stroke Therapy Academic Industry Roundtable (STAIR), 1999). STAIR have since published a series of guidelines for the improvement of both preclinical studies and clinical trials (Fisher et al., 2005, 2009; Saver et al., 2009). Recommendations for preclinical neuroprotection studies included incorporation of randomisation, blinding and sample size calculations into study design, use of comorbid animal strains, both male and female animals, investigation of appropriate dose–response relationships and testing drugs in at least two independent laboratories. The National Centre for the Replacement Refinement and Reduction of Animals in Research (NC3Rs)-led ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (Kilkenny et al., 2010) and National Institutes of Health (NIH)-led RIGOR guidelines (Lapchak et al., 2012) have supported further improvements in study design, data analysis and reporting. Preclinical stroke research has undoubtedly improved as a consequence, providing the framework for improvements in studying other neurodegenerative diseases, where similar problems exist (Avey et al., 2016; Bespalov et al., 2016). With the recent positive clinical trials of endovascular thrombectomy in acute ischaemic stroke (Muir and Macrae, 2016), the stroke community has been reinvigorated, opening up the potential for future translation of adjunctive treatments that can be given alongside thrombectomy/thrombolysis.
Stroke research in the future
With increased collaboration, identification of the need for rigorous experimental design and closer interactions between clinical and preclinical researchers, there is a fresh wave of optimism in the stroke field that new and improved treatments will be found to benefit patients. This is perhaps best illustrated by the excitement and reinvigoration of the field brought about by the recent successes of endovascular therapy, mentioned earlier. Recent publication of guidelines (Ischaemia Models: Procedural Refinements Of in Vivo Experiments (IMPROVE)) developed by an NC3Rs-led working group that aim to improve preclinical models of stroke provide further evidence of the progress being made to address the translational roadblock (Percie du Sert et al., 2017).
With increasing numbers of patients surviving stroke, there is also a need to focus more on post-stroke complications that affect quality of life. These include not only motor and speech impairments, but also depression, dementia, epilepsy and anxiety, among other things. One of the major causes of mortality in stroke is infection, as a result of stroke-induced immunosuppression, yet mechanisms remain relatively poorly understood (Haeusler et al., 2008). A greater appreciation of systemic immune changes post stroke, helped by increased collaborations with immunologists, is therefore needed. Interestingly, recent evidence suggests the loss of B cells in the spleen as a potential mechanism underlying increased risk of post-stroke infection (McCulloch et al., 2017). Such studies were extremely rare in the stroke research field in recent years (Liesz et al., 2009), with the central nervous system typically being the focus, yet peripheral changes are potentially more druggable and future research in this area may therefore have significant impact in improving outcomes after stroke.
The focus of preclinical stroke research over the last 50 years has been largely focussed on finding acute neuroprotective drugs and now the field needs to turn its attention to understanding these post-stroke complications and ways to reduce their impact. Early phase clinical trials of stem cells show promise (Kalladka et al., 2016), while there are various non-invasive paradigms (Faralli et al., 2013), including brain stimulation, that might also have potential benefit in promoting post-stroke repair and recovery. The challenge now for stroke researchers is to identify mechanisms by which such therapies are producing benefit as well as continuing to find new ways to limit the initial injury. There is also a need for more focus on haemorrhagic stroke, which is relatively understudied when compared to acute ischaemic stroke, despite having similar impact in terms of mortality and morbidity, despite a much lower incidence.
Whatever the focus, any future research in the stroke field should aim to confirm efficacy across multiple labs and for the most advanced therapies to conduct multicentre preclinical trials, which provide confidence in moving forward to subsequent large and expensive clinical evaluations.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors acknowledge the financial support of the Medical Research Council, Wellcome Trust, British Heart Foundation and Stroke Association for their research.
References
- Albanese V, Tommasino C, Spadaro A, et al. (1980) A transbasisphenoidal approach for selective occlusion of the middle cerebral artery in rats. Experientia 36(11): 1302–1304. [DOI] [PubMed] [Google Scholar]
- Anrather J, Iadecola C. (2016) Inflammation and stroke: An overview. Neurotherapeutics 13(4): 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila JE, Whitaker KW, Wires ES, et al. (2017) Role of microglia in ischemic focal stroke and recovery: Focus on Toll-like receptors. Progress in Neuro-psychopharmacology & Biological Psychiatry 79(Pt A): 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup J, Siesjö BK, Symon L. (1981) Thresholds in cerebral ischemia – The ischemic penumbra. Stroke 12(6): 723–725. [DOI] [PubMed] [Google Scholar]
- Avey MT, Moher D, Sullivan KJ, et al. (2016) The devil is in the details: Incomplete reporting in preclinical animal research. PLoS ONE 11(11): e0166733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov A, Steckler T, Altevogt B, et al. (2016) Failed trials for central nervous system disorders do not necessarily invalidate preclinical models and drug targets. Nature Reviews Drug Discovery 15(7): 516. [DOI] [PubMed] [Google Scholar]
- Boutin H, Murray K, Pradillo J, et al. (2015) 18F-GE-180: A novel TSPO radiotracer compared to 11C-R-PK11195 in a preclinical model of stroke. European Journal of Nuclear Medicine and Molecular Imaging 42(3): 503–511. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Dirnagl U, Urra X, et al. (2016) Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. The Lancet Neurology 15(8): 869–881. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Endres M. (2014) Found in translation: Preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke 45(5): 1510–1518. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Fisher M. (2012) International, multicenter randomized preclinical trials in translational stroke research: It’s time to act. Journal of Cerebral Blood Flow and Metabolism 32(6): 933–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Macleod MR. (2009) Stroke research at a road block: The streets from adversity should be paved with meta-analysis and good laboratory practice. British Journal of Pharmacology 157(7): 1154–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Hakim A, Macleod M, et al. (2013) A concerted appeal for international cooperation in preclinical stroke research. Stroke 44(6): 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli A, Bigoni M, Mauro A, et al. (2013) Noninvasive strategies to promote functional recovery after stroke. Neural Plasticity 2013: 854597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Albers GW, Donnan GA, et al. (2005) Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry Roundtable. Stroke 36(8): 1808–1813. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, et al. (2009) Update of the Stroke Therapy Academic Industry Roundtable preclinical recommendations. Stroke 40(6): 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluri F, Schuhmann MK, Kleinschnitz C. (2015) Animal models of ischemic stroke and their application in clinical research. Drug Design, Development and Therapy 9: 3445–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Liu Q, Anrather J, et al. (2015) Immune interventions in stroke. Nature Reviews Neurology 11(9): 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauberti M, Montagne A, Marcos-Contreras OA, et al. (2013) Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes. Stroke 44(7): 1988–1996. [DOI] [PubMed] [Google Scholar]
- Haeusler KG, Schmidt WUH, Föhring F, et al. (2008) Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovascular Diseases 25(1–2): 50–58. [DOI] [PubMed] [Google Scholar]
- Haley MJ, Lawrence CB. (2016) Obesity and stroke: Can we translate from rodents to patients? Journal of Cerebral Blood Flow and Metabolism 36(12): 2007–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit JJ, Wintermark M. (2017) New developments in clinical ischemic stroke prevention and treatment and their imaging implications. Journal of Cerebral Blood Flow and Metabolism 38(9): 1533–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger N, Fisher M. (2016) Extending the time window for endovascular and pharmacological reperfusion. Translational Stroke Research 7(4): 284–293. [DOI] [PubMed] [Google Scholar]
- Howells DW, Sena ES, Macleod MR. (2014) Bringing rigour to translational medicine. Nature Reviews Neurology 10(1): 37–43. [DOI] [PubMed] [Google Scholar]
- Kalladka D, Sinden J, Pollock K, et al. (2016) Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 388(10046): 787–796. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, et al. (2010) Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biology 8(6): e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Zhang JH, Noble-Haeusslein LJ. (2012) RIGOR guidelines: Escalating STAIR and STEPS for effective translational research. Translational Stroke Research 4(3): 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Hagmann S, Zschoche C, et al. (2009) The spectrum of systemic immune alterations after murine focal ischemia: Immunodepression versus immunomodulation. Stroke 40(8): 2849–2858. [DOI] [PubMed] [Google Scholar]
- Llovera G, Hofmann K, Roth S, et al. (2015) Results of a preclinical randomized controlled multicenter trial (pRCT): Anti-CD49d treatment for acute brain ischemia. Science Translational Medicine 7(299): 299ra121. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wang J, Wang Y, et al. (2016) The biphasic function of microglia in ischemic stroke. Progress in Neurobiology 157: 247–272. [DOI] [PubMed] [Google Scholar]
- McCulloch L, Smith CJ, McColl BW. (2017) Adrenergic-mediated loss of splenic marginal zone B cells contributes to infection susceptibility after stroke. Nature Communications 8: 15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae IM. (2011) Preclinical stroke research – Advantages and disadvantages of the most common rodent models of focal ischaemia. British Journal of Pharmacology 164(4): 1062–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maysami S, Wong R, Pradillo JM, et al. (2016) A cross-laboratory preclinical study on the effectiveness of interleukin-1 receptor antagonist in stroke. Journal of Cerebral Blood Flow and Metabolism 36(3): 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. (2010) The science of stroke: Mechanisms in search of treatments. Neuron 67(2): 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir KW, Macrae IM. (2016) Neuroimaging as a selection tool and endpoint in clinical and pre-clinical trials. Translational Stroke Research 7(5): 368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du, Sert N, Alfieri A, Allan SM, et al. (2017) The IMPROVE guidelines (Ischaemia Models: Procedural Refinements Of in Vivo Experiments). Journal of Cerebral Blood Flow and Metabolism 37(11): 3488–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Derdeyn CP, Biller J, et al. (2015) 2015. American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46(10): 3020–3025. [DOI] [PubMed] [Google Scholar]
- Röther J, Ford GA, Thijs VNS. (2013) Thrombolytics in acute ischaemic stroke: Historical perspective and future opportunities. Cerebrovascular Diseases 35(4): 313–319. [DOI] [PubMed] [Google Scholar]
- Saver JL, Albers GW, Dunn B, et al. (2009) Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke 40(7): 2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller A, Vivien D, Kirchhoff F, et al. (2014) Imaging neuroinflammation after brain injuries by ultrasensitive MRI and two-photon laser-scanning microscopy. Romanian Journal of Morphology and Embryology 55(3): 735–743. [PubMed] [Google Scholar]
- Sobowale OA, Parry-Jones AR, Smith CJ, et al. (2016) Interleukin-1 in stroke: From bench to bedside. Stroke 47(8): 2160–2167. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) (1999) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30(12): 2752–2758. [DOI] [PubMed] [Google Scholar]
- Szalay G, Martinecz B, Lénárt N, et al. (2016) Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nature Communications 7: 11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, et al. (1981) Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. Journal of Cerebral Blood Flow and Metabolism 1(1): 53–60. [DOI] [PubMed] [Google Scholar]
- Thiel A, Radlinska BA, Paquette C, et al. (2010) The temporal dynamics of poststroke neuroinflammation: A longitudinal diffusion tensor imaging-guided PET study with 11C-PK11195 in acute subcortical stroke. Journal of Nuclear Medicine 51(9): 1404–1412. [DOI] [PubMed] [Google Scholar]
- Warach SJ, Luby M, Albers GW, et al. (2016) Acute stroke imaging research roadmap III imaging selection and outcomes in acute stroke reperfusion clinical trials: Consensus recommendations and further research priorities. Stroke 47(5): 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattananit S, Tornero D, Graubardt N, et al. (2016) Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. Journal of Neuroscience 36(15): 4182–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]