Abstract
Mucociliary clearance is a crucial component of innate defense of the lung. In respiratory diseases, such as asthma, chronic obstructive pulmonary disease, and cystic fibrosis, mucus with abnormal properties contributes to obstruction of the airways. The failure in function of mucus in airway clearance and pathogen protection leads to chronic infection and risk of death. Polymeric mucins (MUC5AC and MUC5B) provide the structural framework of the airway mucus gel. The intracellular synthesis and assembly of these enormous, polymeric O-linked glycoproteins is a complex, multistage process involving intra- and intermolecular disulfide bond formation and extensive addition of O-glycan chains. The fully formed polymers are packaged in a highly organized and condensed form within secretory granules inside specialized secretory cells, and after the appropriate stimulus, mucins are released and expand to form mucus. This short article brings together the current knowledge on the different steps in the production of mucin polymers and the molecular mechanisms that condense them into a packaged form in secretory granules. It is by unraveling the molecular mechanisms that control intracellular mucin supramolecular structure that we might gain new insight into what determines mucus gel properties in health and disease.
Keywords: mucus, mucin, MUC5B, MUC5AC
The mucus barrier provides a dynamic interface between epithelial tissues and the environment. This barrier is a critical element of innate defense of the respiratory tract, and provides the first line of protection against infections and environmental pollutants. The components of mucus, including water, salts, secreted glycoproteins (polymeric mucins), and proteins (at least 250 distinct molecular species), come together to form a physical and antimicrobial barrier (1). Transport of flowing mucus out of the airways by the action of cilia (mucociliary clearance) and cough is critical for health, and accumulation of mucus, with nonoptimal transport properties, is a pathologic feature of cystic fibrosis, chronic obstructive pulmonary disease, and asthma. Increased mucus concentration (% solids) and mucin concentration contribute to aberrant mucus transport properties, impacting on mucociliary clearance by “osmotically compressing” the periciliary layer and collapsing the cilia (2, 3). Dysfunctional mucociliary clearance results in the accumulation of mucus in the airway lumen and mucus plugging, increasing the risk of infection and chronic inflammation, both key aspects of morbidity and mortality in obstructive lung disease (4, 5).
Polymeric mucins are important contributors to the functional and structural characteristics of mucus gels. These multidomain, high–molecular weight (2–50 mD) O-linked glycoproteins (see Figure 1 for more detail) provide the molecular framework of epithelial mucus gels, and mucin polymers are fully assembled intracellularly and stored in secretory granules before secretion to form mucus (6). The respiratory tract mucus gel in humans is comprised of a heterogeneous mixture of two polymeric mucins, MUC5AC and MUC5B (7). Arranged in a network-like formation, these glycoproteins confer viscoelastic properties onto airway secretions. However, the mucins alone do not dictate the complete viscoelastic properties of airway mucus (8), which also depends on the dynamic interactions between mucins and other components of the secretion, for example, with calcium (9) and globular proteins (10), to form functional mucus with the correct transport and protective properties. Deletion of Muc5ac and Muc5b in mice has given some insight into the function of these two mucins. Mice deficient in Muc5b have shown that this mucin is indispensable for the mucociliary clearance that controls infection and has an important role in immune homeostasis in the lung (5, 11, 12). In contrast, Muc5ac is not required for mucociliary clearance, but is upregulated in allergic inflammation (13), where it plays an important role in mucus plugging of the airways (14). Importantly, MUC5AC tethering to the epithelium has been implicated in mucociliary dysfunction and mucus plugging in asthma (15). In human obstructive airway disease, mucin concentration is increased, with MUC5B being the predominant polymeric mucin in cystic fibrosis and chronic obstructive pulmonary disease sputum (3, 6, 16–18) and increased amounts of MUC5AC have been measured in asthmatic sputum (19, 20). Importantly, different studies have reported an association of MUC5B with disease severity and airway obstruction (17, 18, 21). In particular, MUC5B isolated from an asthmatic gel plug was shown to have a highly cross-linked morphology (21), in marked contrast to the linear mucin chains identified in induced sputum from healthy respiratory airways (22). Although the molecular basis for this abnormal mucin form has yet to be determined, recent studies have implicated thiol cross-linking between mucin chains, catalyzed by reactive oxygen species in the airway lumen, as a potential mechanism for mucus plug formation (23, 24). However, alterations in polymeric mucin intracellular assembly could explain the altered mucin macromolecular form, which might then contribute to the aberrant properties of mucus in disease. This short article summarizes the current state of knowledge on airway mucin intracellular assembly and packaging within secretory granules, and highlights future directions for research in this area.
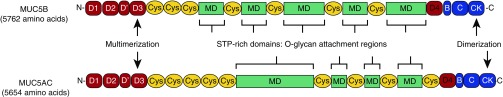
Figure 1.
Schematic diagram of the major airways polymeric mucins, MUC5AC and MUC5B. The multidomain structure of the MUC5B and MUC5AC polypeptides shows the central mucin domains (MDs) that are enriched in serine, threonine, and proline (STP) residues (STP-rich regions) and are the sites of O-glycan attachment. The MD of MUC5B differs in size and sequence to the MD of MUC5AC. The MDs are interrupted and flanked by Cys domains (Cys) that are folded and stabilized by intramolecular disulfide linkages. The N-terminal (D1, D2, D′, D3) and C-terminal (D4, B, C, CK) protein domains share high sequence similarity with related domains in von Willebrand factor (vWF), and in both vWF and mucins these domains are stabilized by intramolecular disulfide bonds and are important for polymer formation. For vWF, a detailed analysis of these domains and their known and predicted disulfide-bond connectivity has been reported by Zhou and colleagues (44). Intermolecular disulfide linkages between CK domains are responsible for dimerization, whereas intermolecular disulfide linkages between D3 domains are responsible for multimerization of the CK-mediated mucin dimers. This assembly process (see Figure 2) produces linear mucin chains.
Polymeric Mucin Assembly and Packaging
Mucin polymers undergo a complex, multistage intracellular assembly and packaging process within granules inside specialized secretory cells in the surface epithelium and submucosal glands, and they are secreted in response to a variety of stimuli (25, 26). The current knowledge on the covalent, disulfide-bond–mediated assembly of mucin polymers and the noncovalent cross-links within or between mucin chains that might be active during packaging are summarized in Figure 2. In this section, we draw together the information on the timescale and cellular location of mucin polymer synthesis, and then what is known about their intragranular packaging.
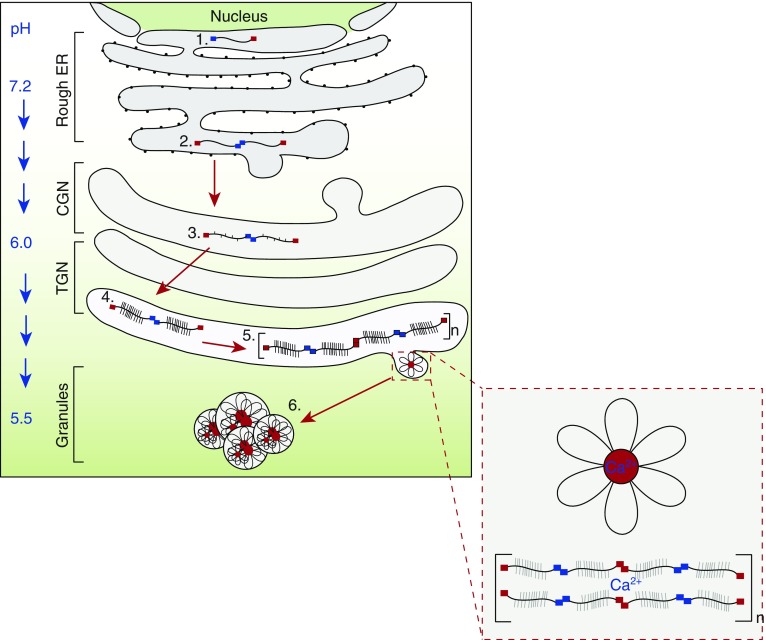
Figure 2.
Overview of polymeric mucin intracellular assembly and intragranular packaging. Polymeric mucin synthesis is a multistep process. 1) The N- and C-terminal domains and central Cys domains of the mucin polypeptide are folded in the endoplasmic reticulum (ER) via the formation of multiple intramolecular disulfide bonds. 2) The polypeptide then undergoes dimerization through disulfide linkage between C-terminal CK domains (blue squares). 3) After transit to the cis-Golgi network (CGN), O-glycan addition is initiated by the addition of N-acetylgalactosamine (GalNAc) to serine and threonine residues in the central mucin domains via the action of polypeptide-GalNAc transferases. 4) As the polypeptide dimer transits through the Golgi to the trans-Golgi network (TGN), O-glycan chains are elaborated via the action of multiple glycosyltransferases. 5) The O-glycosylated dimers multimerize by intermolecular disulfide linkages between N-terminal D3 domains (red squares). 6) Multimers are then packaged into secretory granules via noncovalent calcium-dependent interactions between N-terminal protein domains. These reversible interactions between mucins are most active at the lower pH of the secretory granule, and are suggested to organize the polymers within the granule. The structure highlighted (in the red dashed-line box) depicts the putative granular form of MUC5B (looped strands emanating from central nodes) observed by electron microscopy (35). Moreover, Kesimer and colleagues (35) localized the N-termini of MUC5B to the central nodes (red circles) of these structures by immuno-electron microscopy. The full mechanistic details of packaging of the large mucin polymers have not yet been elucidated. The charge shielding of the negatively charged mucin glycans by Ca2+ ions (37) is not shown.
Mucin Polymer Synthesis
Radiolabel pulse-chase experiments, performed in mucin-secreting cells in culture (both transformed and primary cells) and tissue explants (27–29), have delineated the major steps in the biosynthetic pathway of the two airways mucins, MUC5AC and MUC5B (Figure 2). Under baseline conditions, the O-glycosylated mucin polymers are assembled within 2–4 hours; however, mucin polymers can be stored within the cells for up to 72 hours, with the majority released after 48 hours (29). These studies have also revealed that non–O-glycosylated precursor forms of MUC5B, monomers and C-terminal dimers mediated by disulfide linkages between CK domains, are synthesized in the endoplasmic reticulum (ER) within 20 minutes.
In addition to the intermolecular disulfide linkage that forms the mucin dimers, multiple intramolecular disulphide bonds are formed in the ER that facilitate folding of the mucin N and C termini and the Cys domains (30). Moreover, the Cys domains of MUC5AC and MUC5B have been shown to be C-mannosylated on WXXW motifs, and this post-translational modification has been suggested to play a role in folding of the Cys domains and in the export of the mucin dimers from the ER (31).
The mucin dimers are transported to the Golgi apparatus, where they are fully glycosylated before multimerization (28); the latter is mediated by disulfide linkage between N-terminal D3 domains (32). Although the enzymes (glycosyltransferases) that add mucin glycans are known, information on the precise details of glycosylation is still incomplete (33). A mucin polymer may be substituted with thousands of O-glycan chains, attached to serine and threonine residues predominantly in the central mucin domains. The polypeptide sequences of the mucin domains can be repeated multiple times, and similar sequences in other heavily glycosylated molecules (e.g., aggrecan) have been demonstrated to confer an extended conformation to the unglycosylated polypeptide (34). This extended conformation may be important for the optimal presentation of the large number of potential glycosylation sites on a mucin to the glycosylation machinery.
Mucin Polymer Intragranular Packaging
The final step in polymeric mucin synthesis is its highly organized packaging within the secretory granule (Figure 2; step 6); this organization is dramatically changed as the mucin is secreted and hydrates, and expands in the extracellular environment on the epithelial surface (35). The mechanisms behind mucin packaging into secretory granules (and unpackaging after secretion) are still not completely understood, but noncovalent cross-links between mucin polymers play a key role in both processes. Before secretion, the assembled linear, disulfide-linked mucin polymers are packaged in a condensed and dehydrated state inside granules within the secretory cells in which they are produced. There is increasing evidence that calcium-mediated changes to mucin supramolecular topology have an important role in packaging (and unpackaging) (9, 32, 36); however, the precise mechanism that underlies packaging requires further research to gain a more detailed molecular understanding of the process. It is well established that Ca2+ ions charge-shield the negative charges on sialic acid and sulfate groups on the mucin O-glycans to help attain a condensed state (37). It is becoming increasingly clear that calcium-dependent cross-links between mucins, through protein sites at the N-terminal D domains on the polypeptide (32, 36), are active in this packaging process.
By using recombinant mucin N-terminal protein domains (the entire N terminus D1D2D′D3 and subdomains D1, D1–D2, D2–D′–D3, and D3), we have shown that calcium-mediated interactions between disulfide-linked dimers of the entire N-terminal structure, but not subdomains, results in noncovalent cross-linking of the MUC5B protein (32). More recently, it has been demonstrated that this calcium-dependent interaction results in the formation of noncovalent tetramers (dimer of dimers) where the D1–D2 domains are hooked into each other (38). Calcium-mediated protein cross-links have been reported for the ordered packaging of the related glycoprotein von Willebrand factor (vWF) within the secretory granules (Weibel Palade bodies) of endothelial cells (39). Like polymeric mucins, N-terminal D domains have a crucial role in this process; however, in vWF, proteolytic processing by furin that separates D1D2 from D3 is a feature of the granular organization of the vWF polymer (39, 40). In contrast, intracellular proteolytic processing of MUC5B does not occur (29). This is not surprising, as the furin cleavage site in vWF is absent from MUC5B and MUC5AC, suggesting that MUC5C does not undergo proteolytic processing during synthesis.
The calcium-dependent organization of the MUC5B protein is most active at low pH (pH 5–6, the pH encountered in the secretory granules), and there is growing evidence that the N-terminal protein domains promote mucin condensation via the formation of noncovalently cross-linked polymers, and this appears to be part of the mechanism involved in mucin packaging in secretory granules (32, 36, 38). It remains unclear how calcium and pH are coordinated to create the condensed mucin polymers found in secretory granules, and how this changes to enable the rapid and effective expansion of mucin polymers after secretion. What also remain uncharacterized are the detailed molecular transitions that enable the mucin polymer network to become a barrier for lung protection, which is viscoelastic and yet is able to flow when driven by cilia, and how this is compromised in obstructive lung disease.
Future Perspectives
Although our understanding of mucin packaging within secretory granules has increased (32, 35, 36, 41–43), there are still major gaps in our mechanistic understanding of this process. Importantly, we have yet to establish if the other mucin-protein folded domains (C-terminal and Cys domains; see Figure 1) have a role in forming the highly condensed intragranular form of polymeric mucins. Moreover, how the large, heavily glycosylated central mucin domains influence the organization of the mucins within the granule is unknown. Whether these domains constrain potential interactions between recombinant mucin protein domains produced in their absence needs to be addressed. The large size and polydispersity of native polymeric mucins makes this difficult to study. Recombinant “mini-mucins” containing the mucin-protein folded domains, together with a smaller, defined-size mucin domain, may help unravel the role of the glycosylated central domains in mucin packaging. Ultimately, defining the molecular mechanisms that control intragranular mucin supramolecular structure may provide insight into what determines aberrant mucus gel production, and identify novel ways to combat the accumulation of aberrant mucus in diseased airways.
Supplementary Material
Footnotes
Supported by Medical Research Council grant MR/R002800/1 (C.R.) and previously by Cystic Fibrosis Foundation Therapeutics grant THORNT07XXX0 (C.R.), and by a Biotechnology and Biological Sciences Research Council Doctoral Training Partnership studentship (C.S.); the Wellcome Trust Centre for Cell-Matrix Research, University of Manchester, is supported by Wellcome Trust core funding grant 203128/Z/16/Z.
Author Contributions: D.J.T. and C.R. wrote the first draft. D.J.T., C.S., and C.R. edited the manuscript. C.S. provided the figures.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, et al. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296:L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Button B, Cai L-H, Ehre C, Kesimer M, Hill DB, Sheehan JK, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson AG, Ehre C, Button B, Abdullah LH, Cai L-H, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livraghi-Butrico A, Grubb BR, Wilkinson KJ, Volmer AS, Burns KA, Evans CM, et al. Contribution of mucus concentration and secreted mucins Muc5ac and Muc5b to the pathogenesis of muco-obstructive lung disease. Mucosal Immunol. 2017;10:395–407. doi: 10.1038/mi.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 7.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raynal BD, Hardingham TE, Thornton DJ, Sheehan JK. Concentrated solutions of salivary MUC5B mucin do not replicate the gel-forming properties of saliva. Biochem J. 2002;362:289–296. doi: 10.1042/0264-6021:3620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raynal BD, Hardingham TE, Sheehan JK, Thornton DJ. Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J Biol Chem. 2003;278:28703–28710. doi: 10.1074/jbc.M304632200. [DOI] [PubMed] [Google Scholar]
- 10.Radicioni G, Cao R, Carpenter J, Ford AA, Wang T, Li L, et al. The innate immune properties of airway mucosal surfaces are regulated by dynamic interactions between mucins and interacting proteins: the mucin interactome. Mucosal Immunol. 2016;9:1442–1454. doi: 10.1038/mi.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grubb BR, Livraghi-Butrico A, Rogers TD, Yin W, Button B, Ostrowski LE. Reduced mucociliary clearance in old mice is associated with a decrease in Muc5b mucin. Am J Physiol Lung Cell Mol Physiol. 2016;310:L860–L867. doi: 10.1152/ajplung.00015.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young HW, Williams OW, Chandra D, Bellinghausen LK, Pérez G, Suárez A, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans CM, Raclawska DS, Ttofali F, Liptzin DR, Fletcher AA, Harper DN, et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nat Commun. 2015;6:6281. doi: 10.1038/ncomms7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonser LR, Zlock L, Finkbeiner W, Erle DJ. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest. 2016;126:2367–2371. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horsley A, Rousseau K, Ridley C, Flight W, Jones A, Waigh TA, et al. Reassessment of the importance of mucins in determining sputum properties in cystic fibrosis. J Cyst Fibros. 2014;13:260–266. doi: 10.1016/j.jcf.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkham S, Kolsum U, Rousseau K, Singh D, Vestbo J, Thornton DJ. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:1033–1039. doi: 10.1164/rccm.200803-391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesimer M, Ford AA, Ceppe A, Radicioni G, Cao R, Davis CW, et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377:911–922. doi: 10.1056/NEJMoa1701632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachowicz-Scroggins ME, Yuan S, Kerr SC, Dunican EM, Yu M, Carrington SD, et al. Abnormalities in MUC5AC and MUC5B protein in airway mucus in asthma. Am J Respir Crit Care Med. 2016;194:1296–1299. doi: 10.1164/rccm.201603-0526LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh KG, Rousseau K, Fisher G, Bonser LR, Bradding P, Brightling CE, et al. MUC5AC and a glycosylated variant of MUC5B alter mucin composition in children with acute asthma. Chest. 2017;152:771–779. doi: 10.1016/j.chest.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehan JK, Howard M, Richardson PS, Longwill T, Thornton DJ. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem J. 1999;338:507–513. [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton DJ, Davies JR, Kraayenbrink M, Richardson PS, Sheehan JK, Carlstedt I. Mucus glycoproteins from ‘normal’ human tracheobronchial secretion. Biochem J. 1990;265:179–186. doi: 10.1042/bj2650179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci Transl Med. 2015;7:276ra27. doi: 10.1126/scitranslmed.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128:997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonser LR, Erle DJ. Airway mucus and asthma: the role of MUC5AC and MUC5B. J Clin Med. 2017;6:E112. doi: 10.3390/jcm6120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 27.van Klinken BJ, Dekker J, van Gool SA, van Marle J, Büller HA, Einerhand AW. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am J Physiol. 1998;274:G871–G878. doi: 10.1152/ajpgi.1998.274.5.G871. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan JK, Kirkham S, Howard M, Woodman P, Kutay S, Brazeau C, et al. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J Biol Chem. 2004;279:15698–15705. doi: 10.1074/jbc.M313241200. [DOI] [PubMed] [Google Scholar]
- 29.Ridley C, Kirkham S, Williamson SJ, Davis CW, Woodman P, Thornton DJ. Biosynthesis of the polymeric gel-forming mucin MUC5B. Am J Physiol Lung Cell Mol Physiol. 2016;310:L993–L1002. doi: 10.1152/ajplung.00046.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem. 1999;274:31751–31754. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Vilar J, Randell SH, Boucher RC. C-mannosylation of MUC5AC and MUC5B Cys subdomains. Glycobiology. 2004;14:325–337. doi: 10.1093/glycob/cwh041. [DOI] [PubMed] [Google Scholar]
- 32.Ridley C, Kouvatsos N, Raynal BD, Howard M, Collins RF, Desseyn JL, et al. Assembly of the respiratory mucin MUC5B: a new model for a gel-forming mucin. J Biol Chem. 2014;289:16409–16420. doi: 10.1074/jbc.M114.566679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jowitt TA, Murdoch AD, Baldock C, Berry R, Day JM, Hardingham TE. Order within disorder: aggrecan chondroitin sulphate-attachment region provides new structural insights into protein sequences classified as disordered. Proteins. 2010;78:3317–3327. doi: 10.1002/prot.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol. 2010;298:L15–L22. doi: 10.1152/ajplung.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ambort D, Johansson ME, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verdugo P, Deyrup-Olsen I, Aitken M, Villalon M, Johnson D. Molecular mechanism of mucin secretion: I. The role of intragranular charge shielding. J Dent Res. 1987;66:506–508. doi: 10.1177/00220345870660022001. [DOI] [PubMed] [Google Scholar]
- 38.Trillo-Muyo S, Nilsson HE, Recktenwald CV, Ermund A, Ridley C, Meiss LN, et al. Granule-stored MUC5B mucins are packed by the non-covalent formation of N-terminal head-to-head tetramers. J Biol Chem. 2018;293:5746–5754. doi: 10.1074/jbc.RA117.001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadler JE. von Willebrand factor assembly and secretion. J Thromb Haemost. 2009;7:24–27. doi: 10.1111/j.1538-7836.2009.03375.x. [DOI] [PubMed] [Google Scholar]
- 40.Huang RH, Wang Y, Roth R, Yu X, Purvis AR, Heuser JE, et al. Assembly of Weibel-Palade body–like tubules from N-terminal domains of von Willebrand factor. Proc Natl Acad Sci USA. 2008;105:482–487. doi: 10.1073/pnas.0710079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Walker NM, Ootani A, Strubberg AM, Clarke LL. Defective goblet cell exocytosis contributes to murine cystic fibrosis–associated intestinal disease. J Clin Invest. 2015;125:1056–1068. doi: 10.1172/JCI73193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L542–L549. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou YF, Eng ET, Zhu J, Lu C, Walz T, Springer TA. Sequence and structure relationships within von Willebrand factor. Blood. 2012;120:449–458. doi: 10.1182/blood-2012-01-405134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.