Fighting the ageing process is a long held human desire. For example, the idea of the Fountain of Youth, a legendary spring that restores the youthful appearance of anyone who drinks or bathes in its waters, first appeared as early as the fifth century B.C. in the writings of Herodotus. While this quest might appear driven by vanity, it has been recognised that ageing itself is the major risk factor for many of the diseases that plague modern society. Coupled with a revolution in our understanding of the regulation of longevity, largely driven by genetic studies in worms, flies and mice, the idea of intervening in the ageing process has become a reality. However, the molecular basis for organismal ageing has remained elusive which has hindered therapeutic interventions to increase healthspan. A connection between cellular senescence and ageing was suggested as a potential explanation more than 50 years ago1 but this link remained unproven. Baker et al. now demonstrate that blocking senescence delays ageing and increases healthspan providing broad-spectrum protection against pathology thereby potentially opening up a new therapeutic window in this area.
Senescence is a stress programme that results in an irreversible growth arrest. The proliferative arrest is implemented by the induction of the p53 and p16Ink4a/Rb tumour suppressor pathways. Senescent cells undergo other changes including chromatin remodelling and metabolic reprogramming2. Senescent cells also secrete a complex cocktail of factors that includes pro-inflammatory cytokines and matrix metalloproteinases and is termed the senescence-associated secretory phenotype (SASP). Interestingly, senescent cells accumulate during ageing and are present in a broad range of pathologies including neoplasias, diabetes, sarcopenia, atherosclerosis and renal disease3.
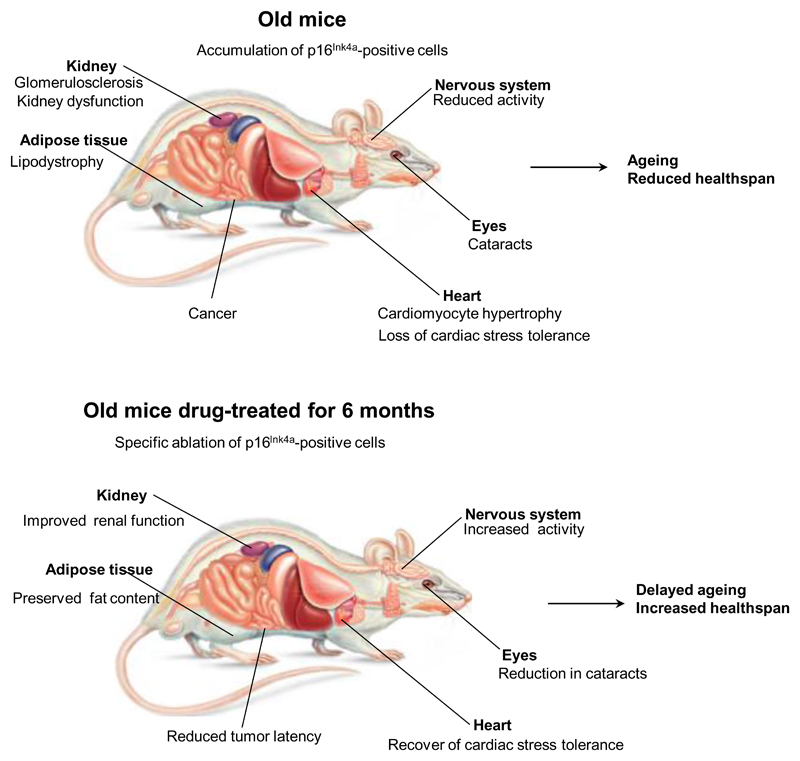
However, it is still unclear whether the accumulation of senescent cells plays a causative role in ageing and age-related pathology. While the authors of the current study previously revealed that clearing senescent cells from a mouse model of accelerated ageing delayed a range of pathologies4, the question remained as to the relevance of these observations to the normal ageing process. In the current study, Baker et al. directly examined this using their INK-ATTAC transgenic model that incorporates a drug-inducible caspase under the control of a portion of the p16Ink4a promoter to eliminate senescent cells. Expression of the ATTAC transgene correlates with that of endogenous p16Ink4a and other senescence markers, including SASP components such as IL6, PAI or MMP1. Critically, induction of the transgene and elimination of p16Ink4a positive cells resulted in increased lifespan regardless of sex and genetic background. In addition, ablating senescent cells ameliorated a range of age-dependent pathologies. These included lipodystrophy, renal dysfunction due to glomerulosclerosis, and cardiac abnormalities such as cardiomyocyte hypertrophy. Improvements were also seen in spontaneous activity, exploratory behaviour and the incidence of cataracts although this was confounded by genetic background. Elimination also significantly delayed the onset of cancer without affecting the spectrum of observed tumour types. Together, these findings suggest that the accumulation of p16Ink4a positive cells during normal ageing shortens healthy lifespan.
The INK-ATTAC is a powerful model to investigate the physiological relevance of senescence but is not devoid of limitations. A premise of the model is its selective elimination of senescent cells. Although not all p16Ink4a positive cells are necessarily senescent, the ATTAC transgene seems to be expressed only in senescent p16Ink4a positive cells as a result of the partial promoter used. However, it is unclear whether only a subset of ‘late senescence’ cells, expressing high levels of p16Ink4a and ATTAC5, are being removed or the elimination is more general. Moreover, drug treatment fails to kill senescent lymphocytes or cells in the liver or colon, limiting the reach of this model. It is clear that improved characterization of the nature of the (senescent) cells eliminated is necessary to fully understand the cellular basis of the altered pathologies.
Another caveat of the current findings is that the inducible elimination of senescent cells requires twice weekly long-term intraperitoneal injections. Indeed the control males display shorter than typical lifespan profiles perhaps as a consequence of subjecting the mice to this treatment regime. New models allowing for the selective ablation of senescent cells without the requirement of repeated injections, or indeed specifically within different tissues at different times will refine and extend the current findings.
It remains unclear why and how the ablation of senescent cells ameliorates some age-related pathologies but not others. While this could reflect limitations of the INK-ATTAC model, it could also suggest that senescent cells only contribute to certain diseases. The kidney data might help to explain why a small percentage of senescent cells can be so disruptive during ageing. In the aged kidney senescence is mostly observed in tubular brush-border epithelial cells, although the main pathology occurs in glomeruli suggesting that SASP components secreted by the epithelial cells could be responsible.
To translate this study to the clinic we will need compounds that can selectively eliminate senescent cells. The search for such compounds (termed senolytics) has already started6. Other alternatives to harness senescence for therapeutic benefit could aim to repress the SASP. Indeed, inhibition of JAK to reduce the SASP has been recently shown to alleviate frailty in old mice7. Intriguingly, rapamycin, an mTOR inhibitor in clinical use as an immunosuppressant, robustly extends mouse lifespan8 and also regulates the SASP9,10 potentially suggesting common therapeutic mechanisms may be at work. It will be essential to limit any negative effects of such strategies, since senescence is a protective response that limits fibrosis and cancer and cells expressing senescence makers are involved in wound healing. Critically, the current study suggests that ablation of senescent cells has limited negative effects including no evidence for increased fibrosis or cancer development.
In summary, Baker et al. provide compelling evidence that senescent cells have a negative impact on normal healthspan and lifespan and suggests that the selective elimination of ‘bad’ senescent cells without eroding the protective effects of senescence will have benefits for the diseases of human ageing.
Figure 1. Improving healthspan.
As mice age p16Ink4a positive senescent cells accumulate in various organs causing impaired cellular and tissue function leading to age-related pathology. Selective ablation of the accumulating senescent cells using a transgenic mouse with a drug-inducible caspase under the control of a portion of the p16Ink4a promoter results in increased lifespan and healthspan with reduction in a range of age-related pathologies.
References
- 1.Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 2.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 4.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu M, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112:E6301–6310. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herranz N, et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat Cell Biol. 2015;17:1205–1217. doi: 10.1038/ncb3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laberge RM, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]