Abstract
We have developed a method for measuring fluxes of PCBs from natural waters using air and water passive samplers deployed simultaneously in the Indiana Harbor and Ship Canal (IHSC). Net volatilization of ƩPCBs was determined for 2017, and ranged from 1.4 to 2.8 μg m−2 d−1, with a median of 2.0 μg m−2 d−1. We confirm earlier findings that the IHSC experiences constant release of gas-phase PCBs. Gas-phase and freely-dissolved water ƩPCB samples median were 4.0 ng m−3 and 14 ng L −1, both exhibiting increasing concentrations over the year of study, and with a strong positive correlation between them (R2 = 0.93 for ƩPCBs). The relative concentrations of individual PCB congeners were very similar between air and water samples, and resemble Aroclor 1248, a mixture previously reported to contaminate the IHSC sediments. Monthly variability of the volatilization fluxes was primarily driven by the freely-dissolved water concentration changes (R2 = 0.87). Although different sampling methods were performed to estimate air-water fluxes between the month of August of 2006 and 2017, ƩPCB net fluxes have decreased by more than 60%, suggesting that either dredging at IHSC from 2012 to 2017 or reduction of upstream sources have decreased the freely-dissolved water concentrations of PCBs, thus reducing the air-water net volatilization in IHSC. Finally, we have shown that this passive sampling approach represents a simple and cost-effective method to assess the air-water exchange of PCBs, increase analytical sensitivity, enable measurements over time, and reduce uncertainties related to unexpected episodic events.
Keywords: PCB, Air-water flux, IHSC, Volatilization, Passive sampler
1. Introduction
Contaminated waterways are important sources of airborne semi-volatile organic compounds (SVOCs). For example, net volatilization fluxes of polychlorinated biphenyls (PCBs) at the Hudson River Estuary were ~40 times higher than atmospheric deposition, with an annual loss through volatilization of 28 kg yr −1, clearly showing the role of the water as an airborne PCB source (Totten et al., 2001; Yan et al., 2008). Similar results were found for the Delaware River, where net volatilization fluxes ranged from 360 to 3000 ng m −2 d −1 for ƩPCBs (Rowe et al., 2007). Likewise, the contaminated waters from New Bedford Harbor are responsible for most of the airborne PCB concentrations in the surrounding area (Martinez et al., 2017). The air-water exchange process is a crucial component for determining the fate of water-borne SVOCs and airborne exposures to communities living near contaminated waterways. Both spatial and temporal resolution is required to better understand the dynamics of the system.
Passive sampling of SVOCs in air and water is a promising approach to better collect spatial and temporal data (Greenwood et al., 2007). Furthermore, due to longer deployments, which increase the amount of chemical capture, passive sampling improves the sensitivity and accuracy of the analytical methods (Bohlin et al., 2014; Mills et al., 2014).
Increasingly, passive sampling techniques are used to estimate the air-water exchange of SVOCs. For example, air-water exchange of PCBs and PAHs have been determined in the Lower Great Lakes (Liu et al., 2016), the Lower Duwamish waterway, Washington (Apell and Gschwend, 2017), the Gulf of Mexico (Tidwell et al., 2016), and the Willamette River, Oregon (Tidwell et al., 2017). These studies found that a passive sampling approach improves the spatial resolution, as well as allows direct determination of the freely-dissolved phase of the compounds both in the air and water compartments.
Here we report a new passive sampling design to determine the direction and magnitude of PCB air-water fluxes. We developed a dual-sampling system of polyurethane foam passive air (PUF-PAS) and low-density polyethylene (LDPE) water samplers that was deployed in the Indiana Harbor and Ship Canal (IHSC), IN, USA. IHSC is located on the southern shore of Lake Michigan, and it is contaminated with PCBs, PAHs and heavy metals from more than 70 years of industrial operations. We previously reported an annual release from the water to the air of about 7 kg of PCBs, and almost 45 kg were exported to Lake Michigan in 2006 (Martinez et al., 2010b). In 2012, a navigational dredging operation began, and it was not clear if dredging had reduced or increased the fluxes of PCBs. Using a comparable modeling approach to interpret air-water fluxes, we are now able to evaluate the monthly changes in PCB fluxes over the course of 2017 and compare to findings more than a decade earlier.
2. Materials and methods
2.1. Passive sampling device
The passive sampling device consisted of a PUF-PAS set approximately 1 m above the water (see grafical abstract) and 2.5 cm × 30 cm LDPE strips threaded onto solvent-cleaned stainless steel metals rings attached to a long, steel chain with a weight at the bottom. Four depths were measured for the first two deployments (~0.3, 1.5, 3.5 and 5.3 m from the water surface, total depth 5.5 m), but due to little vertical variability found in the freely-dissolved concentration, only the top and bottom depths were measured for the rest of the deployments. The passive sampling device was deployed at IHSC, adjacent to a USGS streamflow-gaging and water monitoring station (USGS 04092750). The device was deployed for ten consecutive periods, from November 2016 to October 2017, ranging from 21 to 62 days of deployment times, with an average of 35 days.
2.2. Gas-phase PCB measurements
PUF-PAS was used to measure gas-phase PCB concentrations as previously described for other studies (Ampleman et al., 2015; Herkert et al., 2016; Martinez et al., 2017). Prior to deployment, PUF disks were cleaned in an ASE-350 (Dionex™ Accelerated Solvent Extraction), with a mixture of 1:1 (v/v) acetone:hexane solution. PUFs were dried for at least 1 h in a ventilated fume hood, wrapped in combusted aluminum foil, placed individually in plastic Ziploc bags, and stored at 4 °C until deployment. Prior to deployment, PUF disks were spiked with 1000 ng of a mixture of depuration compounds (DCs) or performance reference compounds of PCB9-d5 (2,5-dichlorobiphenyl-2′,3′,4′,5′,6′-d5, deuterated), PCB22-d4 (2,3,4′-trichlorobiphenyl-2′,3′,5′,6′-d4, deuterated), PCB52-d3 (2,2′,5,5′-tetrachlorobiphenyl-3,4,6-d3, deuterated), PCB116-d5 (2,3,4,5,6-pentachlorobiphenyl-2′,3′,4′,5′,6′-d5, deuterated) and PCB156-d3 (2,3,3′,4,4′,5-hexachlorobiphenyl-2′,6,6′-d3, deuterated), from CDN Isotopes Inc., Canada. We used a previously described method (Persoon et al., 2010).
Concentrations of gas-phase PCB congeners were determined by dividing the mass of each congener collected in the PUF disk by the congener-specific effective sampling volume, as described in equation (1):
| (1) |
where CPCBi a is the gas-phase concentration for the ith PCB (ng m−3), mPUF PCBi is the mass of the ith PCB on the PUF (ng) and Veff PCBi is the effective sampling volume for the ith PCB (m3). The effective sampling volumes were obtained from modeled and calculated sampling rates from local meteorology (Herkert et al., 2018) and the spiked depuration compounds. Sampling rates ranged from 2.9 to 8.4 m3 d −1, whereas congener specific sampling volumes ranged from 26 to 400 m3.
2.3. Freely-dissolved PCB water measurements
Freely-dissolved PCB water measurements were carried out using LDPE (25 μm, Menards®, Iowa City, IA, USA), as others have used (Burgess et al., 2015; Liu et al., 2016). Prior to deployment, the LDPE strips were cleaned with a series of solvent soaks and rinses of dichloromethane, methanol and deionized water, for 24 h each. Clean LDPE strips were stored in deionized water until deployment. To account for non-equilibrium during the field deployment, the same DCs used for the PUF disks were loaded into the LDPE strips following the procedure described by Booij et al. (2002) and others (USEPA/SERDP/ESTCP, 2017). Briefly, 1000 ng of DCs in hexane solution were spiked into a clean 1000 mL Erlenmeyer flask and the hexane was allowed to evaporate in a fume hood for ~20 min. Methanol:deionized water mixture of 80:20 (v/v) was added to the flask, air bubbles were removed to the best of our ability, and the mixture was left to equilibrate for 7 days. Next, LDPE strips were soaked with deionized water for 24 h and subsequently rinsed with deionized water. Loaded LDPE strips were stored in sealed clean glass containers with deionized water, ready to be deployed in the field.
Freely-dissolved PCB water concentrations were calculated using equation (2):
| (2) |
where is the freely-dissolved water concentration for the ith PCB (ng L −1), CLDPE–PCBi is the concentration collected in the LDPE strip for the ith PCB, KLDPE–wi is the congener specific LDPE-water partition coefficient, and feqi is the fraction of equilibrium of the specific congener between the LDPE and the water. The congener specific fraction of equilibrium for native PCBs was computed using a graphical user interface (GUI) for water column samples developed by Apell et al. (2016), Tcaciuc et al. (2015) and Thompson et al. (2015), and implemented by US EPA (USEPA/SERDP/ESTCP, 2017), with the calculated fraction of equilibrium from the depuration compounds as input. Calculated fractions of equilibrium for the native PCB congeners ranged from 0.002 for the highly chlorinated congeners (i.e., octa-to decachlorobiphenyl) to 1.0 for the low chlorinated congeners (mono-to trichlorobiphenyls). Congener specific LDPE-water partition coefficients and water diffusivity were corrected by water temperature from each deployment period (see supplementary data).
2.4. Fugacity ratio calculation
The water-air fugacity ratio (fw/fa) was used to assess the air-water exchange direction of the individual PCB congeners, following eq. (3):
| (3) |
where KPCBi a/w is the equilibrium air-water partition constant (nondimensional Henry’s Law Constant) for the ith PCB (Dunnivant et al., 1992) corrected by air and water temperatures (Goss, 2006). A ratio equal to 1 represents equilibrium, a ratio >1 volatilization and a ratio <1 adsorption.
2.5. PCB flux calculations
Individual PCB congener fluxes were calculated as the air-water mass transfer coefficient multiplied by the water-air concentration gradient [eq. (4)] (Martinez et al., 2017; Martinez et al., 2010b; Schwarzenbach et al., 2003; Zhang et al., 1999).
| (4) |
where FPCBi a/w is the flux between the air and the water for the ith PCB (ngm−2 d−1) and VPCBi a/w is the overall air-water mass transfer coefficient for the ith PCB (m d−1). We calculated the net, gross volatilization (i.e., CPCBi a = 0) and gross absorption (i.e., CPCBi w = 0) fluxes using eq. (4). The mass transfer coefficient was calculated following the Whitman two-film model, where individual velocities across air and water films were calculated to compute the overall air-water mass transfer coefficient [eq. (5)]:
| (5) |
where VPCBi w is the water transfer velocity for the ith PCB (m d−1) and VPCBi a is the air transfer velocity for the ith PCB (m d−1). All physical and chemical properties of the individual PCB congeners used to calculate these mass transfer coefficients included adjustments for environmental and meteorological conditions including air and water temperatures, wind speed, and water flow. Details of the calculations are shown in the supplementary data.
2.6. Analytical methods
Gas-phase PCBs collected via PUF-PAS were extracted and cleaned as previously described (Ampleman et al., 2015; Marek et al., 2017; Martinez et al., 2017). Briefly, PUF disks were spiked with 50 ng of surrogate standards: PCB14 (3,5-dichlorobiphenyl), PCB65-d5 (2,3,5,6-tetrachlorabiphenyl-d5, deuterated) and PCB166 (2,3,4,4′,5,6-hexachlorobiphenyl), and extracted utilizing an ASE-350 with equal parts of acetone and hexane. The extracts were cleaned through a Pasteur pipette filled with 0.1 g of combusted silica gel and 1 g of acidified silica gel (2:1 silica gel:sulfuric acid w/w) and eluted with hexane. The eluates were concentrated to ~0.5 mL and 25 ng of internal standard [PCB30-d5 (2,4,6-trichlorobiphenyl-2‘,3‘,4‘,5‘,6‘-d5, deuterated) and PCB204 (2,2‘,3,4,4‘,5,6,6‘-octachlorobiphenyl)] was added.
PCBs collected using LDPE were extracted and cleaned similarly to previously reported methods (Apell and Gschwend, 2017; Fernandez et al., 2014; Fernandez et al., 2012). LDPE strips were rinsed with deionized water, dried with laboratory tissue, and spiked with 50 ng of surrogate standards: PCB14 (3,5-dichlorobiphenyl), PCB65-d5 (2,3,5,6-tetrachlorabiphenyl-d5, deuterated) and PCB166 (2,3,4,4′,5,6-hexachlorobiphenyl). The LDPE strips were then transferred to a sealed glass vial with hexane. After equilibration for 24 h, the hexane volumes were reduced to ~0.5 mL and the concentrates were cleaned through Pasteur pi-pettes as described above for the PUF. The eluates were concentrated to ~0.5 mL and 25 ng of internal standard [PCB30-d5 (2,4,6trichlorobiphenyl-2‘,3‘,4‘,5‘,6‘-d5, deuterated) and PCB204 (2,2‘,3,4,4‘,5,6,6‘-octachlorobiphenyl)] was added.
PCBs were detected and quantified as previously described (Martinez et al., 2017). Tandem Mass Spectrometry GC-MS/MS (Agilent 7000) in multiple reaction monitoring (MRM) mode was used to quantify all 209 congeners in 171 individual or coeluting congener peaks. The GC was equipped with a Supelco SBP-Octyl capillary column (30m × 0.25mm ID, 0.25mm film thicknesses) with helium as the carrier gas.
2.7. Quality assurance & quality control (QA/QC)
Surrogate standards, as well as laboratory and field blanks were used to evaluate the quality of our methods. The percentage recoveries of surrogate standards for the air samples were 79 ± 9, 80 ± 11 and 85 ± 12 for PCB14, PCB65-d5, and PCB166, respectively. The percentage recoveries of surrogate standards for the water samples were 85 ± 19, 85 ± 18, and 99 ± 22 for PCB14, PCB65-d5, and PCB166, respectively. Recovery correction was performed on all samples to account for any losses during laboratory processes; PCB1 to 39, PCB40 to 127, and PCB128 to 209 were corrected with recoveries from PCB14, PCB65-d5, and PCB166, respectively. The average ΣPCB mass measured in the PUF-PAS field blanks was 5.6 ± 1.1 ng sample−1 (n = 10) and 8.8 ± 6.3 ng gLDPE−1 for water samples (n = 6). Subsequently, a congener specific limit of quantification (LOQ) was applied to all samples; calculated as the upper limit of the 95% confidence interval of the blanks. Congener masses below the LOQ were assigned a mass of zero. The congener specific LOQ for the air samples ranged from 0.002 to 0.3 ng sample−1 with an average of 0.04 ng sample−1. For the water samples, the congener specific LOQ ranged from 0.003 to 1.2 ng gLDPE−1 with an average of 0.1 ng gLDPE−1.
2.8. Monte Carlo simulations
We used Monte Carlo simulations to assess the uncertainty of the calculated fugacity ratios and air-water fluxes (Martinez et al., 2010b). We considered the distribution for each parameter instead of single variables (see supplementary data). These frequency distributions were obtained of the standard deviations from reported values or calculated from available data of the parameters. For example, standard deviations of environmental conditions such as air and water temperatures were computed from the collected data. Henry’s Law Constant error was obtained from its publication (Dunnivant et al., 1992). We assumed normal distribution of all errors and that uncertainty in the chemical measurements was primarily due to chemical analysis (20%). We used RStudio (Version 1.1.442) to perform the simulations, which were repeated 1000 times and provided frequency distribution of the fugacity ratios and fluxes. The RStudio codes are provided in the supplementary data.
2.9. Meteorological data
Meteorological data were used to calculate air and water PCB concentrations, fugacity ratios and fluxes. Air and water temperatures, and water flows were obtained from the USGS monitoring station next to our passive sampling device. Wind speed and atmospheric pressure were reported by the Gary/Chicago International Airport weather station.
All the data generated in this research, i.e., individual congener specific masses captured by the PUFs and LDPEs, LOQ for both PUF and LDPE, effective sampling volumes, fraction of equilibrium, LDPE-water partition coefficient, water-air fugacity ratios, fluxes (net, volatilization and absorption), and the meteorological data, are available at https://doi.org/10.1594/PANGAEA.894908 (Martinez et al., 2018).
3. Results and discussion
3.1. Gas-phase PCB measurements
Median concentration of gas-phase ƩPCB was 4.0 ng m−3, with an interquartile range (IQR) of 1.6–5.9 ng m−3. On average, 125 peaks representing individual and coeluting congeners were measured in our samples. Individual congener concentrations are presented in Table S1. Even though the samples were collected at the same location, a factor of eight between the highest and the lowest measurements shows an interesting increasing concentration with time that is well correlated with the freely-dissolved water concentration changes (Fig. 1). More than 90% of the gas-phase concentration variability of ƩPCB was explained by the freely-dissolved water concentration changes (R2 = 0.93) (Fig. 2). This was also true for individual congeners, where 47% of the congeners showed significant correlation (p < 0.05) (Fig. 2). This gas-phase PCB trend is consistent with reported airborne PCB concentrations measured by the Army Corps of Engineers at five locations nearby our sampling location (Fig. 3). The five congeners measured by the Army Corps (i.e., PCBs8, 15, 18, 28 and 31) showed a similar temporal trend of increasing concentrations throughout the year.
Fig. 1.
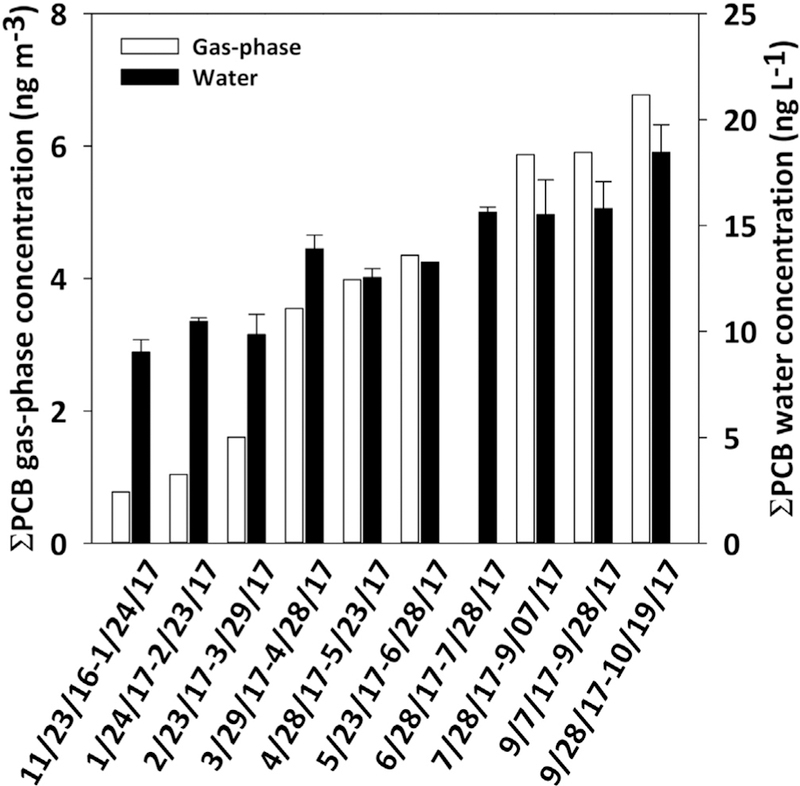
Concentrations of gas-phase (white bars) and mean freely-dissolved water (black bars) of ƩPCBs at IHSC. X-axis indicates the deployment time. Errors bars from the freely-dissolved water bar plots represent the standard deviation of at least two samples collected at the same time. PUF-PAS from 6/28/17e7/28/17 was lost.
Fig. 2.
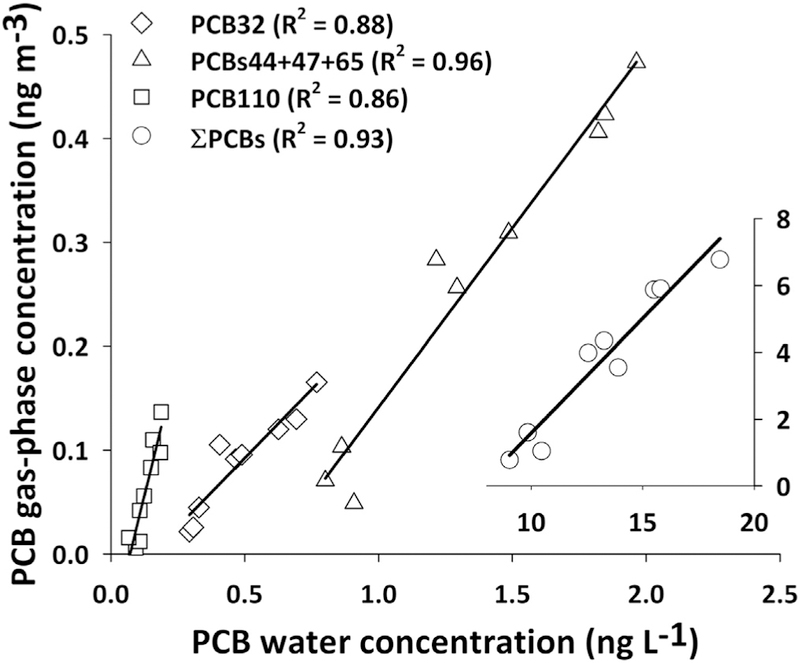
Gas-phase and freely-dissolved water concentrations for ƩPCBs and selected congeners. Insert plot represents ƩPCBs. Data are from the nine deployments. All correlations are statistically significant (p < 0.05).
Fig. 3.
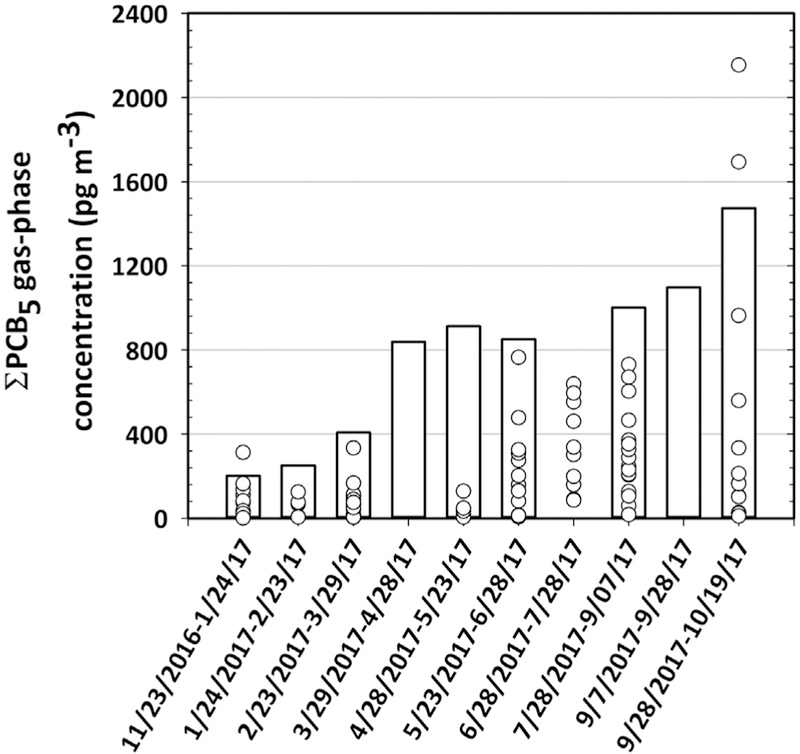
Concentrations of gas-phase of ƩPCB5 (i.e., PCBs8, 15, 18, 28 and 31) from our PUF-PAS samples (cyan bars) and active sampling from the Army Corps of Engineers (void circles). The active samplers were obtained from five locations nearby our sampling location. Source: http://web.ead.anl.gov/inharbor/data/analysis/publicTables/index.cfm.
Our measured concentrations of gas-phase ƩPCB agree with measurements carried out at IHSC above the water using active sampling in August 2006 (1.9–8.9 ng m−3) (Martinez et al., 2010b). Using the same passive sampling approach, our values are slightly higher than values reported around 2000 m from our sampling location in East Chicago outdoor schools (0.03–3 ng m−3) (Marek et al., 2017) and Chicago and Cleveland (0.3–4 ng m−3) (Persoon et al., 2010).
3.2. Freely-dissolved PCB measurements
Over the course of the study, freely-dissolved ƩPCB water concentrations increased from 9 to 18 ng L−1, with a median of 14 ng L−1 and an IQR of 11–16 ng L−1 (Fig. 1). On average, 140 peaks representing individual and coeluting congeners were measured in our samples. Individual congener concentrations are presented in Table S1. LDPE placed simultaneously at four different depths for the first two deployments showed that the system is well mixed at the sampling location (relative standard deviation (RSD) of freely-dissolved ƩPCB concentration < 7%).
We know of two possible explanations for the increasing trend in the freely-dissolved water concentrations: (i) change in equilibrium and biological processes driven by an increase in water temperature; and (ii) effluent discharge from an upstream confined disposal facility (CDF). We considered the effect of temperature because we recorded a steady increase in the monthly average water temperature over our study period (from 8 to 21°C). Warmer water enhances sediment-water exchange of SVOCs such as PCBs and DDTs through increasing growth and activity of benthic organisms, diagenesis, gas ebullition and bioturbation (Connolly et al., 2000; Erickson et al., 2005; Sethajintanin and Anderson, 2006). Two studies found a significant positive correlation between chemical water concentration and water temperature. Bremle and Larsson concluded that increasing temperature promoted desorption of PCBs from sediment (Bremle and Larsson, 1997). Sthajintanin and Anderson suggested that increasing water temperature spurred biological activity and subsequent PCB release at the sediment-water interface (Sethajintanin and Anderson, 2006). Our measurements show the same outcome: concentrations of freely-dissolved PCBs (R2 = 0.71) and 44% of individual congeners exhibit significant correlation with water temperature (p < 0.05). The majority of these PCB congeners (80%) were also significantly correlated between the gas-phase and the freely-dissolved concentrations. Additionally, treated effluent from the CDF was discharged from March to September of 2017 (Thai, 2018), after which we observed an increasing trend in our concentrations of freely-dissolved PCBs (Fig. 1). Dredged sediment from the IHSC is disposed in the CDF next to the Lake George Branch, located up-stream of our sampling location. It is possible that both water temperature increase and the CDF discharges explain the trend in freely-dissolved water concentration. However, during our last deployment, we observed the highest measurement of freely-dissolved water concentration (Fig. 1), which is coincident with nearby dredging operations at the end of September of 2017 (Thai, 2018). Indeed, the concentration of freely-dissolved PCBs measured in the last deployment is 30% more than predicted from the temperature correlation of the remaining measurements. Although we cannot definitively conclude if the increasing trend of freely-dissolved PCBs is due to natural processes or activities associated with the navigational dredging, this trend drives an increase in gas-phase concentrations from the system over the course of the study year (Figs. 1 and 2).
Our 2006 measurements of the dissolved water phase of ƩPCB using active sampling (i.e., collected from pumping filtered water through XAD) were higher than our 2017 water measurements, ranging from 21 to 70 ng L−1 (Martinez et al., 2010b). It is likely that the two sampling methods are not directly comparable because our passive sampling method using LDPE does not capture PCBs bound to DOC, whereas water samples collected from active sampling do. To account for this difference, we calculated the freely-dissolved phase obtained from the active sampling via XAD-2 resin from 2006 by subtracting the amount of PCBs bound to DOC, using a two-phase partitioning model [eq. (6)]:
| (6) |
where is the dissolved-phase concentration for the ith PCB (ng L−1) measured using the active sampling (Martinez et al., 2010b), [DOC] is the water concentration of DOC (3.9 ± 1.0mg L−1) from Risch (2006), and KPCBi DOC is the partition coefficient between dissolved organic carbon and the freely-dissolved phase for the ith PCB (KPCBi DOC = 0.06 x octanol-water partition coefficient for the ith PCB corrected by water temperature) from Burkhard (2006). Details of the calculations are presented in the supplementary data. We found a 20% reduction in the water measurements from the active samples from August 2006 when PCBs bound to DOC were removed, i.e., from 40 to 34 ng L−1. We conclude that the difference is not an artifact of our sampling methods: The dissolved-phase concentrations are nearly 50% lower in 2017 than in 2006. This reduction suggests that sediment dredging from 2012 to 2017, control of upstream sources (Martinez et al., 2010b), or a combination of the two has effectively reduced the freely-dissolved water concentrations of PCBs in IHSC.
3.3. PCB congener profiles
As previously reported, the PCB congener distribution present in IHSC resembles the commercial mixture Aroclor 1248 (Martinez and Hornbuckle, 2011; Martinez et al., 2010a; Martinez et al., 2010b). The former refinery Atlantic Richfield CO, located next to the canal, discharged at least 1000 kg of Aroclor 1248 into the sewer system and directly into the environment in the early 1970s (Waller, 1971). More than 40 years later, our 2017 air and water samples still capture the Aroclor 1248 signature, with very small variability between individual air and water samples, and also high similarity between matrices (Fig. 4). To quantitatively estimate similarities between PCB congener profiles, we used cos θ. This metric uses the cosine of the angle between two multivariable vectors (the profiles) where a value of 1.0 describes two identical vectors and 0.0 describes two completely different (Magar et al., 2005). The average cos θ between all the gas-phase and freely-dissolved samples yielded 0.9 ± 0.04, whereas all the samples versus Aroclor 1248 yielded a cos θ of 0.8 ± 0.05. These results show the similarities between the sample profiles and Aroclor 1248.
Fig. 4.
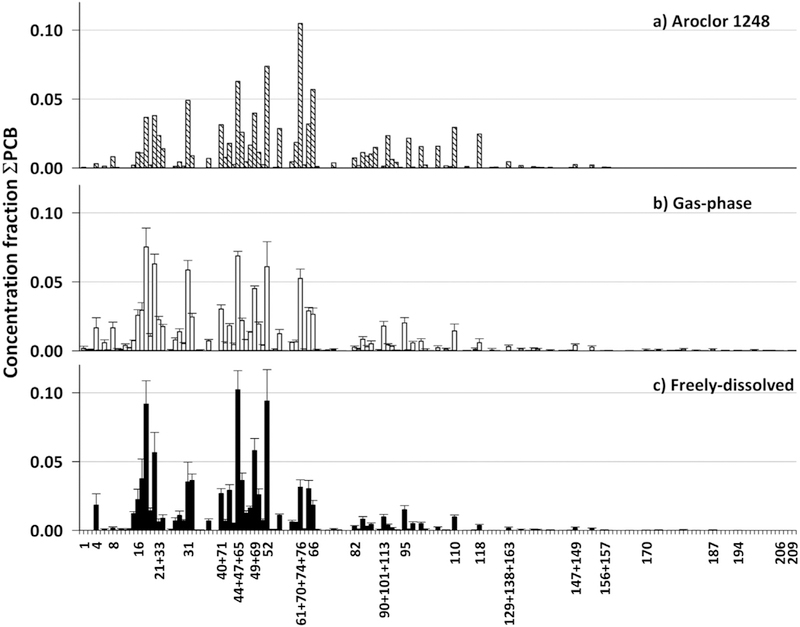
Mean individual PCB congener profile of (a) Aroclor 1248, (b) gas-phase (n = 9) and (c) freely-dissolved samples (n = 23). Each congener was normalized to the total concentration of PCBs in the sample. The error bars represent one standard deviation above the mean.
3.4. PCB fugacity ratios
Individual PCB congener fugacity ratios indicate a tendency of the PCB congeners to volatilize from the water. The ratios (fw/fa) ranged from 0.8 to 850 for all the deployment times, with 99.9% of the (fw/fa) > 1. However, if the uncertainty of the air and water temperatures, Henry’s Law Constant and analytical error are included into the Monte Carlo simulation, the outcome is different. Here, the fugacity ratio was >1 within a 95% confidence interval (95% CI) only 33% of the time, and 67% of the time it was not possible to determine if the (fw/fa) were above, below or equal to 1 with a 95% CI. For example, within the 95% CI, PCB18 always showed (fw/fa) > 1 for all the deployment times, PCB84 resulted in six deployments > 1, PCB144 had only two deployments > 1 and PCB203 never showed values > 1 (Fig. S2). Since both gas-phase and freely-dissolved concentrations exhibited a similar trend (Fig. 2), the fugacity ratios were generally constant and thus, no temporal trend was found for these ratios (Fig. S2).
3.5. PCB fluxes
Gross volatilization fluxes were at least 10 times higher than gross absorption fluxes of ƩPCBs, resulting in net volatilization fluxes of PCBs from IHSC (Fig. 5). Total PCB net fluxes ranged from 1.4 ± 0.2 to 2.8 ± 0.3 mgm−2 d−1, with a median of 2.0 μg m−2 d−1 and an IQR of 1.5–2.1 μgm−2 d−1 (Fig. 5). Further, net volatilization fluxes doubled over the course of our study. This increase in fluxes was mostly due to the trend in the freely-dissolved water concentration (Fig. 1), which explained 87% of the variability of ƩPCB fluxes (p < 0.0003) (Fig. S3). Gas-phase and air and water temperatures also showed significant correlations with the net fluxes, but to a lesser extent (Fig. S3).
Fig. 5.
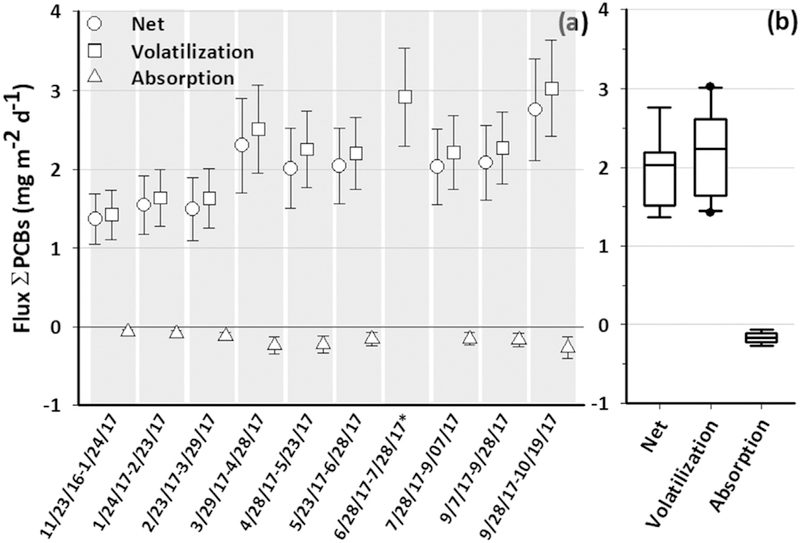
Average net fluxes (grey circles), volatilization (cyan circles) and absorption (blue circles) of ΣPCBs in μg m2 d−1 for the sampling periods (a). Error bars in plot (a) represent the 2.5th and 97.5th percentiles calculated from the Monte Carlo simulations. Net and absorption from 6/28/17–7/28/17* were not calculated due to loss of air sample. Plot (b) summarizes the average net, volatilization and absorption fluxes for the ten sampling periods via box plots, describing from top to bottom the maximum, 75th percentile, median, 25th percentile and the minimum value.
Individual congeners showed the same tendency towards volatilization as ƩPCBs, 90% of the time yielding net volatilization (>0) and only 10% of the time yielding net absorption (<0). Individual PCB congener net fluxes ranged from –9.2 × 10−5 ± 7.9 × 10−5 to 0.2 ± 0.1 μgm−2 d−1 for PCB3 and PCBs18 + 30, respectively. On average, 30 individual PCB congeners contributed to more than 90% of the fluxes, mostly tri-, tetra- and pentachlorobiphenyls (Fig. S4, b). Within a 95% CI, PCB congener fluxes yielded net volatilization 44% of the time, net absorption only 3% of the time, and 53% of the time it was not possible to determine the direction of the flux with certainty, i.e., the 2.5th and the 97.5th percentiles had a different sign for the same congener. As expected, these results are similar to what the fugacity ratios yielded. For example, PCBs18 + 30 always exhibited a net volatilization within a 95% CI, whereas PCB84 exhibited volatilization 56% of the time, but it was not possible to determine the direction of the fluxes 44% of the time. PCB144 yielded net volatilization flux only 11% of the time, but it was not possible to determine the direction of the fluxes 89% of the time. For PCB203, it was never possible to determine the direction of the fluxes (Fig. 6). Further, individual congener net fluxes yielded different temporal results. For example, PCBs18 + 30 and PCB144 did not show any temporal net flux trends, whereas PCB84 showed an increase in the fluxes and PCB203 a slight decrease with time (Fig. 6).
Fig. 6.
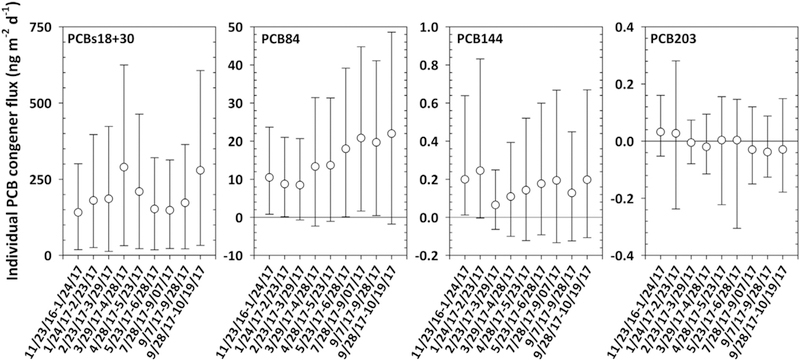
Selected PCB congeners net fluxes (PCBs18 + 30, 84, 144 and 203) for the nine deployments. Void circles represent the measure values and the error bars represent the 2.5th and 97.5th percentiles (i.e., 95% confidence interval). Monte Carlo simulations were run 1000 times. Net flux from deployment 6/28/17–7/28/17 was not calculated due to loss of PUF sample. Please note the difference in the y-axis scales.
PCB net air-water fluxes from IHSC show a decrease since 2006. Net fluxes were recalculated using air and water samples collected in August 2006 nearby our 2017 sampling location (Martinez et al., 2010b). Because the 2006 water samples were collected via active sampling, a DOC correction to water samples was performed for a direct comparison with this study (see eq. (6)]. Further, and as performed with our 2017 estimations, a Monte Carlo simulation was used to recalculate the net flux from August 2006. We found a reduction of ~60% of net ƩPCB fluxes from 5.0 ± 0.6 to 2.0 ± 0.3 μg m−2 d−1 between August 2006 and 2017, which is in agreement with the calculated reduction in the freely-dissolved water concentration of PCBs from 2006 to 2017. As observed with the water concentration reduction, the sediment dredging from 2012 to 2017, the control of upstream sources (Martinez et al., 2010b), or a combination of the two has effectively reduced the net air-water PCB fluxes. We noticed that air and water temperatures and wind speed were very similar in August of 2006 and 2017, reducing the effect of these environmental conditions in the air-water exchange calculations and comparison.
Although the fluxes have decreased in the last decade, the area-normalized release of PCBs from the IHSC is still higher than has been reported elsewhere. For example, net fluxes from the Lower Duwamish Waterway, Washington, ranged from 0.06 to 0.09 μg m−2 d−1 (Ʃ27PCBs) (Apell and Gschwend, 2017) and open waters from Lake Erie and Lake Ontario ranged from 0.002 to 0.009 mg m−2 d−1 (Ʃ29PCBs) (Liu et al., 2016). Freely-dissolved ƩPCBs are lower in these systems than in the IHSC, and hence experience lower air to water fluxes.
4. Implications
Advantages of using a passive sampling approach compared to active sampling include: (i) increased mass collected to improve detection; (ii) reduced extraction and cleanup analytical steps; (iii) direct measurement of the freely-dissolved water concentrations, i.e., no correction of PCBs bound to DOC is needed; (iv) longer temporal measurements, which reduce the risk of missing episodic events and eliminate the need to extrapolate concentrations to estimate longer time period fluxes (Martinez et al., 2010b; Yan et al., 2008); and (v) eliminate the need for electrical power and specialized personnel to perform the sampling. However, prudence should be taken when environmental concentrations are calculated from the mass of the chemical collected from passive sampler devices. Fortunately, existing web-based and GUI mathematical models are available to back-calculate environmental concentrations from masses collected from passive sampling devices (Apell et al., 2016; Herkert et al., 2018; Tcaciuc et al., 2015; Thompson et al., 2015). Currently, water passive sampling media such as LDPE still needs to be spiked with DCs; for PUF-PAS, if the sampling location is not common, e.g., above water or the location presents unusual high wind speed (>10 m s−1), DCs should also be spiked to verify/calculate sampling rates.
Although we did not set up our sampling campaign to evaluate air-water PCB fluxes changes due to sediment dredging and related activities, our results suggest a possible effect on the fluxes due to these activities. Therefore, more monitoring is needed, and a passive sampling approach is the most appropriate and efficient option.
Supplementary Material
Acknowledgments
This work was funded by the Iowa Superfund Research Program, National Institute of Environmental Health Sciences Grant P42ES013661. The authors thank Cheryl Silcox from the USGS for help setting up our samplers and Le Thai from the U.S. Army Corps of Engineers, Chicago District for sharing information related to the dredging and air PCB data. We also thank Dr. Loretta Fernandez, Northeastern University, for her helpful discussion regarding the use of LDPE. Brandon Barquist of the IIHR-Hydroscience and Engineering Services provided leadership in the design of the samplers. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
This paper has been recommended for acceptance by Eddy Y. Zeng.
References
- Ampleman MD, Martinez A, DeWall J, Rawn DFK, Hornbuckle KC, Thorne PS, 2015. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ. Sci. Technol 49, 1156–1164. http://10.1021/es5048039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apell JN, Gschwend PM, 2017. The atmosphere as a source/sink of poly-chlorinated biphenyls to/from the Lower Duwamish Waterway Superfund site. Environ. Pollut 227, 263–270. http://10.1016/j.envpol.2017.04.070. [DOI] [PubMed] [Google Scholar]
- Apell JN, Tcaciuc AP, Gschwend PM, 2016. Understanding the rates of nonpolar organic chemical accumulation into passive samplers deployed in the environment: guidance for passive sampler deployments. Integr. Environ. Assess. Manag 12, 486–492. http://10.1002/ieam.1697. [DOI] [PubMed] [Google Scholar]
- Bohlin P, Audy O, Skrdlikova L, Kukucka P, Pribylova P, Prokes R, Vojta S, Klanova J, 2014. Outdoor passive air monitoring of semi volatile organic compounds (SVOCs): a critical evaluation of performance and limitations of polyurethane foam (PUF) disks. Environ. Sci. Process Impacts 16, 433–444. http://10.1039/c3em00644a. [DOI] [PubMed] [Google Scholar]
- Booij K, Smedes F, van Weerlee EM, 2002. Spiking of performance reference compounds in low density polyethylene and silicone passive water samplers. Chemosphere 46, 1157–1161. http://10.1016/s0045-6535(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Bremle G, Larsson P, 1997. Long term variations of PCB in the water of a river in relation to precipitation and internal sources. Environ. Sci. Technol 31, 3232–3237. http://10.1021/es9702172. [Google Scholar]
- Burgess RM, Lohmann R, Schubauer-Berigan JP, Reitsma P, Perron MM, Lefkovitz L, Cantwell MG, 2015. Application of passive sampling for measuring dissolved concentrations of organic contaminants in the water column at three marine superfund sites. Environ. Toxicol. Chem 34, 1720–1733. http://10.1002/etc.2995. [DOI] [PubMed] [Google Scholar]
- Burkhard LP, 2006. Estimating dissolved organic carbon partition coefficients for nonionic organic chemicals. Environ. Sci. Technol 34, 4663–4668. http://10.1021/es001269I. [Google Scholar]
- Connolly JP, Zahakos HA, Benaman J, Ziegler CK, Rhea JR, Russell K, 2000. A model of PCB fate in the upper Hudson River. Environ. Sci. Technol 34, 4076–4087. http://10.1021/es001046v. [Google Scholar]
- Dunnivant FM, Elzerman AW, Jurs PC, Hasan MN, 1992. Quantitative structure-property relationships for aqueous solubilities and Henry’s law constants of polychlorinated biphenyls. Environ. Sci. Technol 26, 1567–1573. http://DOI:10.1021/es00032a012. [Google Scholar]
- Erickson MJ, Turner CL, Thibodeaux LJ, 2005. Field observation and modeling of dissolved fraction sediment-water exchange coefficients for PCBs in the Hudson River. Environ. Sci. Technol 39, 549–556. http://10.1021/es034520g. [DOI] [PubMed] [Google Scholar]
- Fernandez LA, Lao WJ, Maruya KA, Burgess RM, 2014. Calculating the diffusive flux of persistent organic pollutants between sediments and the water column on the palos verdes shelf superfund site using polymeric passive samplers. Environ. Sci. Technol 48, 3925–3934. http://10.1021/es404475c. [DOI] [PubMed] [Google Scholar]
- Fernandez LA, Lao WJ, Maruya KA, White C, Burgess RM, 2012. Passive sampling to measure baseline dissolved persistent organic pollutant concentrations in the water column of the palos verdes shelf superfund site. Environ. Sci. Technol 46, 11937–11947. http://10.1021/es302139y. [DOI] [PubMed] [Google Scholar]
- Goss K-U, 2006. Prediction of the temperature dependency of Henry’s law constant using poly-parameter linear free energy relationships. Chemosphere 64, 1369–1374. http://DOI:10.1016/j.chemosphere.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Greenwood R, Mills G, Vrana B, 2007. Comprehensive Analytical Chemistry: Passive Sampling Techniques in Environmental Monitoring Elsevier Publishing Company. [Google Scholar]
- Herkert NJ, Martinez A, Hornbuckle KC, 2016. A model using local weather data to determine the effective sampling volume for PCB congeners collected on passive air samplers. Environ. Sci. Technol 50, 6690–6697. http://10.1021/acs.est.6b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkert NJ, Spak SN, Smith A, Schuster JK, Harner T, Martinez A, Hornbuckle KC, 2018. Calibration and evaluation of PUF-pas sampling rates across the global atmospheric passive sampling (GAPS) network. Environ. Sci. Process Impacts 20, 210–219. http://10.1039/c7em00360a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang SY, McDonough CA, Khairy M, Muir DCG, Helm PA, Lohmann R, 2016. Gaseous and freely-dissolved PCBs in the Lower Great Lakes based on passive sampling: spatial trends and air-water exchange. Environ. Sci. Technol 50, 4932–4939. http://10.1021/acs.est.5b04586. [DOI] [PubMed] [Google Scholar]
- Magar VS, Johnson GW, Brenner RC, Quensen JF, Foote EA, Durell G, Ickes JA, Peven-McCarthy C, 2005. Long-term recovery of PCB-contaminated sediments at the Lake Hartwell superfund site: PCB dechlorination. 1. End-member characterization. Environ. Sci. Technol 39, 3538–3547. [DOI] [PubMed] [Google Scholar]
- Marek RF, Thome PS, Herkert NJ, Awad AM, Hornbuckle KC, 2017. Airborne PCBs and OH-PCBs inside and outside urban and rural US schools. Environ. Sci. Technol 51, 7853–7860. http://10.1021/acs.est.7b01910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Awad AM, Herkert NJ, Hornbuckle KC, 2018. PCB congener data of gas-phase, freely-dissolved water, air-water fugacity ratios and air-water fluxes in Indiana Harbor and Ship Canal. PANGAEA, USA https://doi.pangaea.de/10.1594/PANGAEA.894908.
- Martinez A, Hadnott BN, Awad AM, Herkert NJ, Tomsho K, Basra K, Scammell MK, Heiger-Bernays W, Hornbuckle KC, 2017. Release of airborne polychlorinated biphenyls from new Bedford harbor results in elevated concentrations in the surrounding air. Environ. Sci. Technol. Lett 4, 127–131. http://10.1021/acs.estlett.7b00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Hornbuckle KC, 2011. Record of PCB congeners, sorbents and potential toxicity in core samples in Indiana Harbor and Ship Canal. Chemosphere 85, 542–547. http://10.1016/j.chemosphere.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Norstrom K, Wang K, Hornbuckle KC, 2010a. Polychlorinated biphenyls in the surficial sediment of Indiana harbor and Ship canal, lake Michigan. Environ. Int 36, 849–854. http://10.1016/j.envint.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Wang K, Hornbuckle KC, 2010b. Fate of PCB congeners in an industrial harbor of Lake Michigan. Environ. Sci. Technol 44, 2803–2808. http://10.1021/es902911a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills GA, Gravell A, Vrana B, Harman C, Budzinski H, Mazzella N, Ocelka T, 2014. Measurement of environmental pollutants using passive sampling devices - an updated commentary on the current state of the art. Environ. Sci. Process Impacts 16, 369–373. http://10.1039/c3em00585b. [DOI] [PubMed] [Google Scholar]
- Persoon C, Peters TM, Kumar N, Hornbuckle KC, 2010. Spatial distribution of airborne polychlorinated biphenyls in Cleveland, Ohio and Chicago, Illinois. Environ. Sci. Technol 44, 2797–2802. http://10.1021/es901691s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch MR, 2006. Mercury in the Grand Calumet River/Indiana Harbor Canal and Lake Michigan, Lake County, Indiana, August 2001 and May 2002 US Department of the Interior, US Geological Survey, p. 55. [Google Scholar]
- Rowe AA, Totten LA, Xie M, Fikslin TJ, Eisenreich SJ, 2007. Air-water exchange of polychlorinated biphenyls in the Delaware river. Environ. Sci. Technol 41, 1152–1158. http://doi:10.1021/es061797i. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach RP, Gschwend PM, Imboden DM, 2003. Environmental Organic Chemistry, second ed. John Wiley & Sons Inc., New York. [Google Scholar]
- Sethajintanin D, Anderson KA, 2006. Temporal bioavailability of organochlorine pesticides and PCBs. Environ. Sci. Technol 40, 3689–3695. http://10.1021/es052427h. [DOI] [PubMed] [Google Scholar]
- Tcaciuc AP, Apell JN, Gschwend PM, 2015. Modeling the transport of organic chemicals between polyethylene passive samplers and water in finite and infinite bath conditions. Environ. Toxicol. Chem 34, 2739–2749. http://10.1002/etc.3128. [DOI] [PubMed] [Google Scholar]
- Thai L, 2018. U.S. Army Corps of Engineers, Chicago District. Personal communication
- Thompson JM, Hsieh CH, Luthy RG, 2015. Modeling uptake of hydrophobic organic contaminants into polyethylene passive samplers. Environ. Sci. Technol 49, 2270–2277. http://10.1021/es504442s. [DOI] [PubMed] [Google Scholar]
- Tidwell LG, Allan SE, O’Connell SG, Hobbie KA, Smith BW, Anderson KA, 2016. PAH and OPAH flux during the deepwater horizon incident. Environ. Sci. Technol 50, 7489–7497. http://10.1021/acs.est.6b02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell LG, Paulik LB, Anderson KA, 2017. Air-water exchange of PAHs and OPAHs at a superfund mega-site. Environ. Sci. Technol 603, 676–686. http://10.1016/j.scitotenv.2017.01.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten LA, Brunciak PA, Gigliotti CL, Dachs J, Glenn TR, Nelson ED, Eisenreich SJ, 2001. Dynamic air-water exchange of polychlorinated biphenyls in the New York - New Jersey Harbor Estuary. Environ. Sci. Technol 35, 3834–3840. http://10.1021/es010791k. [DOI] [PubMed] [Google Scholar]
- USEPA/SERDP/ESTCP, 2017. Laboratory, Field, and Analytical Procedures for Using Passive Sampling in the Evaluation of Contaminated Sediments: User’s Manual
- Waller MG, 1971. Respond to Questionnare on Use of PCB’s from Atlantic Richfield CO for the East Chicago Refinery to Division of Water Pollution Central Indiana State Board of Health. [Google Scholar]
- Yan S, Rodenburg LA, Dachs J, Eisenreich SJ, 2008. Seasonal air-water exchange fluxes of polychlorinated biphenyls in the Hudson River Estuary. Environ. Pollut 152, 443–451. http://10.1016/j.envpol.2007.06.074. [DOI] [PubMed] [Google Scholar]
- Zhang H, Eisenreich SJ, Franz TR, Baker JE, Offenberg JH, 1999. Evidence for increased gaseous PCB fluxes to Lake Michigan from Chicago. Environ. Sci. Technol 33, 2129–2137. http://doi:10.1021/es981073+. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


