Abstract
MicroRNAs are a class of small non-coding RNA that regulate gene expression at a post-transcriptional level. MicroRNAs have been identified in various body fluids under normal conditions and their stability as well as their dysregulation in disease has led to ongoing interest in their diagnostic and prognostic potential. Circulating microRNAs may be valuable predictors of early-life complications such as birth asphyxia or neonatal seizures but there are relatively few data on microRNA content in plasma from healthy babies. Here we performed small RNA-sequencing analysis of plasma processed from umbilical cord blood in a set of healthy newborns. MicroRNA levels in umbilical cord plasma of four male and four female healthy babies, from two different centres were profiled. A total of 1,004 individual microRNAs were identified, which ranged from 426 to 659 per sample, of which 269 microRNAs were common to all eight samples. Many of these microRNAs are highly expressed and consistent with previous studies using other high throughput platforms. While overall microRNA expression did not differ between male and female cord blood plasma, we did detect differentially edited microRNAs in female plasma compared to male. Of note, and consistent with other studies of this type, adenylation and uridylation were the two most prominent forms of editing. Six microRNAs, miR-128-3p, miR-29a-3p, miR-9-5p, miR-218-5p, 204-5p and miR-132-3p were consistently both uridylated and adenylated in female cord blood plasma. These results provide a benchmark for microRNA profiling and biomarker discovery using umbilical cord plasma and can be used as comparative data for future biomarker profiles from complicated births or those with early-life developmental disorders.
Introduction
Complications during childbirth and pre-term births can lead to developmental and neurological dysfunction in later life in a subset of children [1–4]. There remains a major unmet need for molecular biomarkers of maternal and neonatal complications such as hypoxic ischemic encephalopathy (HIE). The development of reliable, non-invasive biomarkers would allow us to identify at an early stage, babies at risk of succumbing to developmental and neurological deficits and enable early intervention or prevention. Umbilical cord blood and plasma are a potential source of pertinent biological information following birth which could contain predictive biomarkers of neurological outcome, however little is known about the molecular profile of umbilical cord plasma.
MicroRNAs (miRNAs) are ubiquitously expressed, short non-coding RNAs which fine-tune gene expression by negatively regulating mRNA translation [5]. They are shuttled between cells via extracellular vesicles [6–8] and importantly are abundant in peripheral biofluids including plasma [9], urine [10], cerebrospinal fluid [11], tears, saliva and peritoneal fluid [12]. They were first profiled in human plasma, serum and microvescicles in 2008 [13–15] and since then, subsequent studies have found that their levels in peripheral biofluids often fluctuate in patients with various types of cancer, neurological disorders, sepsis, liver and cardiovascular disease (reviewed in [16–18]). As such miRNAs have received much interest as potential biomarkers and contain many characteristics which render them ideal biomarker candidates: they are more stable than mRNA as they are resistant to RNase cleavage [19]; expression profiles of miRNAs are often more informative and discriminatory than mRNA profiles; they are abundant and profiling miRNAs is rapid and economical [20]; and their levels often change more rapidly in response to an insult or pathophysiological processes allowing early detection of disease which is critical for progressive illnesses such as cancer, Alzheimer’s disease, epilepsy and early life insults [21–25].
We have previously shown that a number of miRNAs display altered expression in umbilical cord blood from infants following perinatal asphyxia [26]. These miRNAs may, in the future, aid clinicians in providing targeted neuroprotection. We have also shown that miRNA alterations may be used to examine downstream targets and elucidate pathogenesis [27]. Accordingly, identification of miRNAs associated with perinatal and neonatal injury is a priority.
Foetal Growth Restriction (FGR) is a disorder which manifests as a reduction or complete halt of genetically predetermined potential growth of a foetus [28]. The placenta plays a vital role in the correct development and growth of the foetus via provision of essential nutrients and protection from toxins which may affect development and growth and is believed to temporally and abundantly produce miRNAs which are involved in placental development and function [29–31]. The placenta can also produce exosomes in which miRNAs can be found [32]. Placentally-expressed miR-424 may play a crucial role in the development of the placenta and is believed therefore to be associated with FGR. Upregulation was noted in placentae with aberrant vascular development linked to FGR [33]. Downregulation of placental miR-16 and miR-21 has been linked to FGR also. MiR-16 has involvement in both apoptosis and regulation of the cell cycle and may display cell-specific functions and expression. PTEN, the target of miR-21, is normally expressed in the placenta and dysregulation may cause aberrant invasion of the placenta, and reduced migration and growth [30]. Trophoblastic miRNAs regulated by hypoxia are increased in maternal plasma and decreased in placental tissue from FGR cases [32].
Preeclampsia affects up to 8% of all live births worldwide and can result in a high risk of morbidity and mortality for both mother and offspring [29], with approx. 25% resulting in FGR [34]. Inadequate placental oxygenation/angiogenesis may result in consequential hypoxia-ischemia seen in the disorder. Zhu et al. [35] reported over 90 differentially expressed miRNAs between preeclamptic and healthy patients. Multiple miRNAs are dysregulated in severe cases of preeclampsia when compared to uncomplicated, healthy pregnancies. These included but are not limited to miR-210, miR-195, miR-181a, miR-411, and miR-377 [29, 34]. Master hypoxamir miR-210 expression levels are raised in both placental tissue and plasma samples from preeclamptic women [36] and can influence multiple pathways in preeclampsia, for example, angiogenesis, mitochondrial dysfunction and immunity [31]. Pineles et al. [37] demonstrated that overexpression of miR-210 and miR-182 may differentiate preeclampsia from healthy controls. miRNAs which are responsible for regulation of angiogenic factors such as VEGF are also dysregulated in preeclampsia [34].
Serum miR-323 levels differ from healthy controls in both ectopic pregnancy and spontaneous abortion [38, 39]. Conditions such as gestational diabetes may also be diagnosable with miRNA biomarkers, for example, miR-16, miR-17, miR-19a, miR-19b and miR-20a are dysregulated in the condition when compared to healthy subjects [40].
Many RNA species including miRNAs have been shown to be subjected to editing and modification processes including A-I editing, base modifications, as well as chemical modifications [41–45]. Analysis of deep sequencing data has revealed differences between genomic sequences and RNA sequences, including mRNAs, miRNAs and lncRNAs, the result of RNA editing mechanisms which is a critical function of gene regulation [46, 47]. MiRNAs are targeted by A-to-I editing enzymes (ADARs), where an A base is changed to an apparent G, as well as tailing and trimming modifications which involves the addition or removal of a nucleotide at the 3′ or 5′ end of the miRNA entity which is mediated by TUTases, however other forms of modification are also prominent including 2′O-methylation [47–50]. Modification of miRNAs has been shown to modify miRNA-mRNA targeting, stability and RISC-uptake which can alter gene network dynamics and cellular activity and function. Analysis of RNA editing in plasma is still poorly understood and limited by the lower yields of RNA obtained. However analysis of RNA editing, including miRNA editing in peripheral biofluids may confer additional biomarker potential and sensitivity which would allow greater confidence in biomarker identification as well as provide insight into the function of RNA editing in normal and disease processes.
MiRNA profiling has moved away from high throughput qRT-PCR-based platforms towards the use of RNA-sequencing (RNA-seq), however, few datasets on healthy umbilical cord blood plasma exist for reference purposes. In order to develop reliable biomarkers from umbilical cord plasma, it is important that we gain perspective on the naturally occurring miRNA profiles. Previous studies have used microarrays to profile miRNAs in cord blood from neonates with HIE [26], or used RNA-seq to profile miRNA expression from trios of samples from newborn babies and their parents [51], umbilical cord blood derived cells [52, 53], and cord blood buffy coat layers [54], However, a reference dataset of total miRNA profiles and miRNA editing analysis of healthy umbilical cord plasma has yet to be established.
Here we perform unbiased small RNA-seq on umbilical cord blood plasma from healthy new born infants. We compared the expression of miRNAs between sexes and between the maternity hospitals of origin. We also performed preliminary analyses on RNA editing differences which may exist between sexes in order to obtain a more comprehensive catalogue of miRNA profiles in umbilical cord blood plasma. Interrogation of the miRNA profiles from umbilical plasma revealed a largely stable miRNA profile between male and female, however differences in RNA editing were identified, indicating increased complexity in the miRNA makeup of umbilical cord blood plasma in females compared to males.
Materials and methods
Study population
Umbilical cord blood samples were collected from two maternity hospitals: Cork University Maternity Hospital (CUMH) and Karolinska University Hospital (KUH). Consent from parents or guardians of the infants included in the study was obtained according to the Declaration of Helsinki and ethical approval was granted from the Clinical Research Ethics Committee of the Cork Teaching Hospitals, Cork, Ireland and local ethical commitee approval in Karolinska University Hospital. This was a nested study of infants recruited to the BiHiVE 2 study (NCT02019147).
In total, 8 infants were included in this study; 4 males and 4 females (Table 1). All infants were of European descent, singleton, full term, uncomplicated vaginal births. Samples were collected from 4 infants in CUMH (2 males and 2 females) and from 4 infants in KUH (2 males and 2 females). We did not detect any significant difference between male and female infants based on maternal age, parity, gestation, birthweight, head circumference or length. The Apgar score for all infants was above 9 at 1 and 5 minutes.
Table 1. Summary of infant demographics and clinical findings, showing the mean and the range for each feature.
| Variable | Female (n = 4) | Male (n = 4) | p-value |
|---|---|---|---|
| CUMH (n) | 2 | 2 | |
| KUH (n) | 2 | 2 | |
| Maternal age (yrs) | 31.75 (25-37) | 34.25 (26-41) | 0.5733 |
| Parity (n) | 1.75 (1-3) | 2 (1-3) | 0.7049 |
| Gestation (weeks) | 41 (39-42) | 40 (39-40) | 0.2070 |
| Birthweight (g) | 3525 (2985-4240) | 3693.75 (3375-3980) | 0.5994 |
| Head circumference (cm) | 35.375 (34-36.2) | 35.05 (32-37) | 0.7940 |
| Length (cm) | 50.75 (48-53) | 52 (51-53) | 0.4122 |
| Apgar score (1 min) | 9 (9) | 9 (9-10) | 0.3559 |
| Apgar score (5 min) | 10 (10) | 10 (10) | – |
A two-tailed unpaired t-test was performed between males and females for each variable and the resulting p-value is shown. Gestation is rounded to the nearest week. The median Apgar score is shown rather than the mean.
Biofluid collection
Umbilical cord blood samples were collected immediately after delivery of all infants in this study and processed within 3 h following strict laboratory SOPs by a dedicated research team who were available 24 h a day. Samples were stored at −80°C in a monitored storage facility until analysis. 6 ml of umbilical cord blood was collected into vacutainer tubes from the infants and processed within 3 hours of delivery. The plasma was prepared by centrifuging the tubes at 2400 x g, for 10 minutes, at 4°C. The supernatant was collected into an RNAase free tube and extra care was taken not to disturb the buffy coat which contains the white blood cells. Plasma was then collected into 250 μl RNAase free eppendorfs and stored at −80°C. The level of haemolysis in the plasma samples was assessed by spectrophotometric analysis using a Nanodrop 2000 spectrophotometer. The absorbance at 414 nm was checked and a cut-off level of 0.25 was used to distinguish haemolysis free samples [55].
Small RNA library preparation and RNA-seq
200 μl of non-hemolyzed cord blood plasma was used to isolate RNA, using the miRCURY RNA isolation kit (Exiqon) according to the manufacturer’s protocol. RNA was eluted in 25 μl. A standard overnight RNA ethanol precipitation step was then performed and RNA was re-suspended in 10 μl RNase free water to concentrate. RNA integrity and quantity was determined using the small RNA detection kit (DNF-470) on a Fragment Analyzer (Advanced Analytical Technologies). RNA (5 μl) from each sample was then used to construct small RNA libraries using the Illumina TruSeq Small RNA library kit. Due to the small amount of input RNA the protocol was modified slightly (all kit reagents were halved) to prevent extensive adapter dimer formation. Libraries were size selected using a Pippin Prep with 3% agarose dye free cassettes and size selection was validated using a 2100 High sensitivity DNA Bioanalyzer chip (Agilent). The concentration of each library was determined using the HS-dsDNA kit for Qubit, libraries were pooled and pooled libraries were sequenced at the Trinseq Facility at the Institute for Molecular Medicine at St. James Hospital Dublin on an Illumina miSeq.
Sequencing data processing and differential miRNA analyses
The FastQC [56] program was employed to assess the quality of the reads. The fastq files were then upload to Chimira [44] where count-based miRNA expression data were generated. Sequences were adapter trimmed and mapped against miRBase v21 hairpin sequences [57] allowing up to two mismatches per sequence. Further analyses was performed using R/Bioconductor [58, 59]. The edgeR [60] package was used to identify significantly differentially expressed miRNAs following the protocol described by Law et al. [61]. The trimmed mean of M-values (TMM) normalisation method [62] was used for normalisation of the miRNA expression count data. Differential expression analysis was performed using voom [63] and limma [64]. P-values were adjusted for multiple testing by controlling the false discovery rate (FDR) according to the method of Benjamini and Hochberg [65]. A miRNA was considered to be differentially expressed if the adjusted p-value was ≤ 0.05. Expression data have been submitted to the gene expression Omnibus (GSE119002).
Modification analysis
Modification analysis of the umbilical cord blood samples was performed using Chimira [44]. Chimira allows for the detection of any non-templated sequences within the input small RNA-Seq samples that are not encoded in the genomic sequence of origin. The output of this pipeline is a comprehensive set of all identified 3′, 5′ and internal modifications (SNPs and ADAR-edits). Each modification is characterised by a non-templated sequence pattern and an index, which determines its position relative to the original sequence. In order to study the differential levels of adenylation, uridylation, guanylation and cytocylation between the female and male samples we have collapsed Chimira’s modification counts into either mono-nucleotide or poly-nucleotide patterns of the same nucleotide (e.g. A, UU, CCC, etc.).
Poly-nucleotide patterns refer to sequences of two or more identical nucleotides and are all grouped together into a single modification type. For example, any ‘CC’, ‘CCC’, and/or ‘CCCC’ modifications are considered collectively as poly-C modifications. A mono-nucleotide modification on the other hand, e.g. ‘C’, stands on its own as a distinct modification pattern. All other isoforms are not included in this analysis and their counts are merged with the counts of the corresponding templated sequences. Finally, we have defined as differentially expressed/modified miRNAs with a fold change in expression or modification level that is > 2 (or < -2) and an associated p-value < 0.05. Normalization of miRNA modification counts from male and female samples and identification of differentially modified miRNAs was performed using the DESeq2 software package [66].
Results and discussion
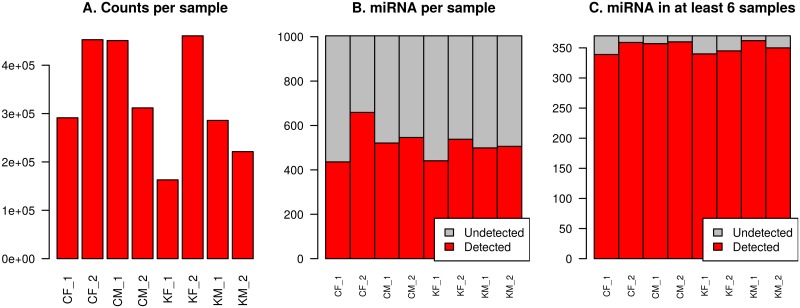
Count-based miRNA expression data was generated by mapping to human miRBase V21, resulting in an average of 329,721 counts per sample (range 162,954 to 460,783, Fig 1A). A total of 1,004 unique miRNAs were identified across all samples, ranging from 426 miRNAs in sample CF_1 to 659 in sample CF_2 with an average 486 miRNAs per sample (Fig 1B). 370 unique miRNAs were detected in at least six of the eight samples (Fig 1C) and 269 miRNAs were detected in all eight samples (S1 File).
Fig 1. Counts and miRNAs identified per sample.
A: Total number of counts per sample mapped to human miRBase V21. B: Number of unique miRNAs with at least one count in any sample. C: Number of unique miRNAs found in at least 6 of the 8 samples. Samples are coded as follows: C—CUMH; K—KUH; F—Female; M—Male.
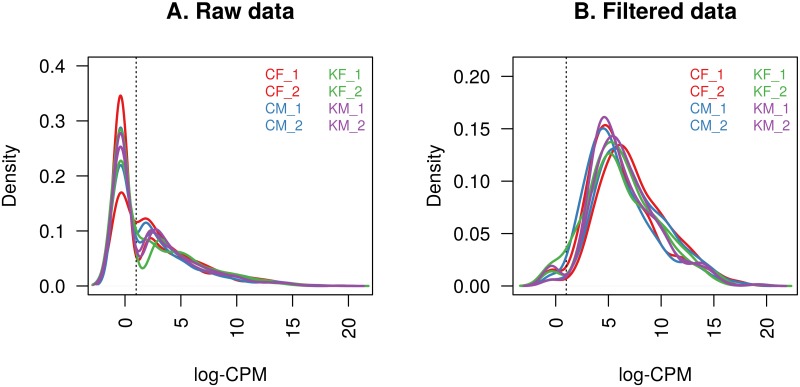
The raw counts were converted to CPM and log-CPM values and miRNAs were removed unless their CPM value was greater than 10 with expression in at least four of the eight samples. Fig 2 shows the density of the log-CPM values for raw pre-filtered data and post-filtered data for each sample. This includes the threshold for the log-CPM of one (equivalent to a CPM value of 10) used in the filtering step. This reduced the number of miRNAs from 1,004 to 300. This is substantially higher than results reported for high throughput qRT-PCR profiling platforms studies of adult plasma [9], and is one of the potential advantages of using an RNA-seq based approach to obtain genome-wide coverage. Lizarraga et al [54] used an EdgeSeq miRNA Whole Transcriptome Assay to profile the miRNAs in buffy coat of cord blood samples from 89 newborns, of which 564 miRNAs were retained for further analysis after filtering. Looney et al [26] used micorarray profiling of umbilical cord blood of 24 infants retaining 259 miRNAs for differential expression analysis after filtering [26]. Meanwhile, 395 miRNAs were detected in three pooled samples from Down syndrome and normal fetal cord blood mononuclear cells (CBMCs) using RNA-seq expression profiling [52].
Fig 2. The density of log-CPM values for each sample.
A: Raw pre-filtered data B: Post-filtered data The dotted vertical lines mark the log-CPM of one threshold (equivalent to a CPM value of 10) used in the filtering step.
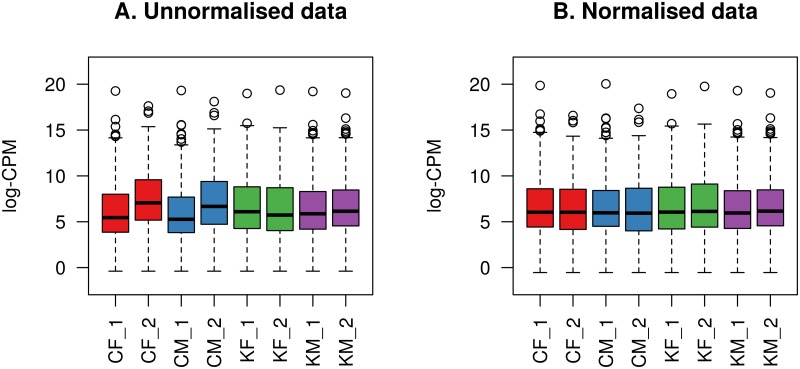
The filtered data were then normalised using the TMM method [62]. Boxplots of log-CPM values showing expression distributions for each sample before and after normallisation are shown in Fig 3.
Fig 3. Boxplots of log-CPM values showing expression distributions.
A: Filtered unnormalised data for each sample. B: Filtered and TMM normalised data for each sample. The line dividing the box represents the median of the data and top and bottom of the box shows the upper and lower quartiles respectively. The whiskers show the highest and lowest values, excluding outliers, which are show as circles.
Unsupervised clustering of samples
Principal Component Analysis (PCA), is a non-parametric method of reducing a complex data set to reveal hidden, simplified dynamics within it. This is accomplished by converting a set of observations of variables (which may be correlated) into a set of values of linearly uncorrelated principal components (PCs). These PCs may then reveal relationships between the variables. PC1 explains the largest proportion of variation in the data, with subsequent PCs having a smaller effect and being orthogonal to the ones before them. Ideally, samples should cluster by the condition of interest, and any outliers should be identified. If the samples cluster by anything other than the condition of interest in any dimensions then that factor can be included in the linear modelling.
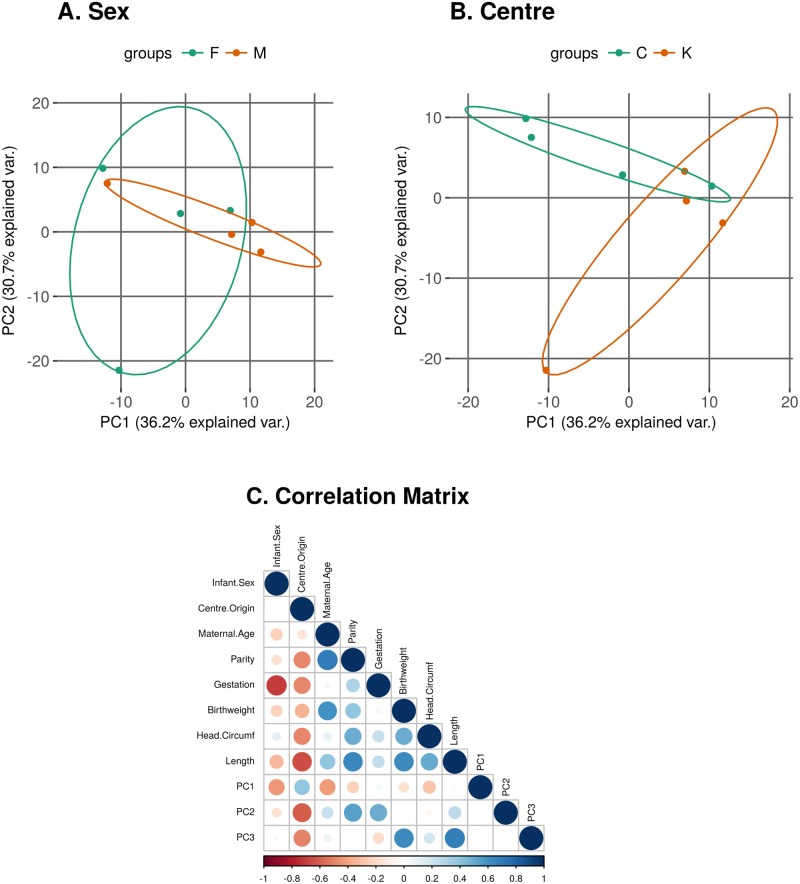
Fig 4 shows the principal components analysis plots of PC1 against PC2 for the normalised log-CPM values in our data. Each dot represents a sample and we have coloured and labelled the samples by the sex of the infant (Male or Female) (Fig 4A) or the centre of origin of the samples (CUMH or KUH) (Fig 4B). We can see from these plots that there is no obvious clustering by either sex or centre of origin. However, when we calculate the Pearson correlation coefficient (r) between the individual PCs and the available clinical/demographic variables (Table 1) we can observe that there is some correlation between the sex of the infant and the centre of origin of the sample and PC1 (r = -0.44 and 0.4 respectively) and between the centre of origin of the sample and PC2 and PC3 (r = -0.6 and -0.5 respectively) (Fig 4C). Therefore we conclude that there may be a slight “batch” effect due to the centre of origin of the sample and have included this factor in the linear model when looking for differentially expressed miRNAs between males and females. The PCs > 3 each account for less than 10% of the variation in the data and are not shown.
Fig 4. Principal components analysis plots of log-CPM values over PC1 and PC2 showing a 68% confidence ellipse where each point represents a sample.
Samples are coloured and labelled by A: Sex of the infant (M/F) and B: Centre of origin of the samples (C—CUMH; K—KUH). C: Correlation Matrix. Positive correlations (Pearson correlation coefficient (r)) are displayed in blue and negative correlations in red colour. Colour intensity and the size of the circle are proportional to the correlation coefficients. The PCs > 3 each account for less than 10% of the variation in the data and are not shown.
Differential expression analysis
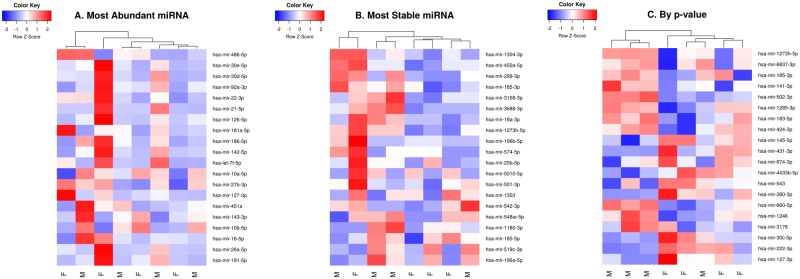
Differential expression analysis was performed using voom [63] and limma [64] as implemented in the R package, edgeR [60]. Subsequently, empirical Bayesian moderation was applied by borrowing information across all miRNAs to obtain more precise estimates of miRNA variability. Significance was defined using an adjusted p-value [65] cutoff that is set at 5% by default. No miRNAs were found to be significantly differentially expressed between male and female infants (Table 2). A heatmap of log-CPM values for the top 75 miRNAs ranked by p-value is shown in Fig 5.
Table 2. Differential expression analysis.
Males versus females.
| logFC | p-value | adj. p-value | |
|---|---|---|---|
| hsa-miR-145-5p | 1.536 | 0.025 | 0.894 |
| hsa-miR-141-3p | -1.999 | 0.031 | 0.894 |
| hsa-miR-660-5p | -1.699 | 0.035 | 0.894 |
| hsa-miR-380-3p | 2.805 | 0.037 | 0.894 |
| hsa-miR-3176 | -1.719 | 0.045 | 0.894 |
| hsa-miR-874-3p | 1.580 | 0.046 | 0.894 |
| hsa-miR-127-3p | 1.557 | 0.048 | 0.894 |
Table showing the log fold change (logFC), p-value and adjusted p-value (adj. p-value) of miRNAs with p-value < 0.05 (sorted by p-value) following linear modelling in limma with empirical Bayes moderation.
Fig 5. Heatmap of log-CPM values for top 20 miRNAs ranked by: A: Most abundant, B: Most stable and C: By p-value following differential expression analysis.
Expression across each miRNA has been scaled so that mean expression is zero and standard deviation is one. Samples with relatively high expression of a given miRNA are marked in red and samples with relatively low expression are marked in blue. Lighter shades and white represent genes with intermediate expression levels. Samples and genes have been reordered by the method of hierarchical clustering. A dendrogram is shown for the sample clustering.
Cellular origin of miRNAs in umbilical cord plasma samples and biomarker potential
The 10 most abundant miRNA (i.e. highest average miRNA expression across all samples) are: miR-486-5p, miR-10b-5p, miR-26a-5p, miR-191-5p, miR-16-5p, miR-22-3p, miR-181a-5p, miR-92a-3p, miR-451a and miR-30e-5p. Many of these are similar to those found in [51]. The most abundant miRNA, miR-486-5p, accounts for nearly 50% of all raw miRNA counts in our samples. miR-486-5p has previously been noted to be highly expressed in RNAseq studies [13, 51] and is detected in most profiling studies of adult plasma that we have seen (S2 File). This miRNA is abundant within red blood cells suggesting that this may be due to selective secretion to plasma or increased stability in plasma [51], however, it does not appear to be detected with such abundance in high-throughput qRT-PCR profiling [9].
Utilising Ensembl [67], the locations of the highest expression of these 300 miRNAs were determined. Of the most abundant miRNAs, interestingly, 49 were most highly expressed in “brain fragments”, 46 in the choroid plexus, 27 in the hindbrain, 21 in the forebrain/forebrain fragment and 11 in the spinal cord. While others were most abundant in the cerebellum (8), the cerebral cortex (8), the medulla oblongata (7), the basal ganglia (6), the temporal lobe (5) or in the midbrain (3). Other miRNAs of note were most abundant elsewhere such as skeletal muscle, ovaries, testis, adrenal gland and stomach. This is interesting as a number of placental miRNAs have been linked to brain development: miR-16-5p, miR-21-5p, miR-93-5p, miR-182-5p, miR-146a-5p and miR-135b-5p [68]. All of these, except miR-135b-5p, were found to be abundantly expressed in our samples (S3 File).
Haider et al. [18] have created a miRNA expression matrix spanning 18 cell types, reflecting a broad range of most major cell types (epithelial, endothelial, mesenchymal, hematopoietic, and muscle). We examined the possible cellular origin of the 100 most highly expressed miRNAs in the umbilical cord plasma samples by cross checking them against the 100 most highly expressed miRNAs in each of 18 unique cell types (S3 File). Thirty of the 100 most highly expressed miRNAs were not found in any of the 18 cell types and 19 miRNAs were ubiquitously expressed: miR-26a-5p, miR-16-5p, miR-22-3p, miR-21-5p, let-7f-5p, miR-25-3p, miR-103a-3p, miR-93-5p, miR-26b-5p, let-7a-5p, miR-19b-3p, miR-107, miR-29a-3p, miR-15a-5p, let-7g-5p, miR-27a-3p, miR-23a-3p, let-7d-5p and miR-29c-3p. Thirteen of these ubiquitously expressed miRNAs were also found in our profiling study of adult plasma (let-7d-5p, let-7g-5p, miR-103a-3p, miR-15b-5p, miR-16-5p, miR-19b-3p, miR-21-5p, miR-25-3p, miR-26a-5p, miR-26b-5p, miR-27a-3p, miR-29a-3p and miR-93-5p) [9]. Sixty nine miRNAs were found in at least one and 23 miRNAs were found in all of the seven of the hematopoietic cell types (centroblast, memory B cell, monocyte, naive B cell, NK cell, plasma B cell and red blood cell) (S3 File).
The three most studied pregnancy-associated miRNA-clusters [69] are the chromosome 14 miRNA cluster (C14MC), the chromosome 19 microRNA cluster (C19MC) and miR-371-3 cluster, which is also localized on chromosome 19. The C14MC (also called the miR-379/miR-656 or miR-379/miR-410 cluster) is the largest comprising 52 miRNA genes and is placental mammal lineage specific [69]. C19MC (also known as miR-498(46)) contains 46 miRNA genes. It is primate-specific and is expressed in placenta, embryonic stem cell (ESC), and certain tumors [69–71]. Williams et al. found high expression of C19MC in placental tissue and in samples from umbilical cord and mothers but low expression in nonpregnant women and fathers [51]. C19MC is also highly expressed in infantile hemangioma [71]. The third cluster, miR-371-3 cluster, consists mainly of three miRNAs, miR-371a-3p, miR-372 and miR-373-3p, located on chromosome 19 within a region adjacent to the C19MC cluster. Similar to C14MC and C19MC, this cluster is conserved in mammals and is predominantly expressed in the placenta [69]. 10% of the 300 miRNA in our filtered dataset map to these three clusters.
Indeed, many miRNAs identified in our study have been linked with processes associated with pregnancy and pregnancy related complications suggesting that umbilical cord plasma miRNA profiles may reflect pregnancy status and potential complications. For example, miRNA associated with preeclampsia (let-7a-3p, miR-24-3p, miR-26a-5p, miR-29a-3p, miR-103a-3p, miR-125a-5p, miR-125b-5p, miR-130b-3p, miR-181a-5p, miR-342-3p miR-542-3p and miR-574-5p) [72–74] and ectopic pregnancy (miR-323a-3p) [39].
Comparison of umbilical cord plasma miRNA profiles to adult miRNA plasma profiles
In a previous study of miRNA expression profiles in adult plasma we found that although there is a similar number of miRNAs being detected across studies, there is a large degree of variation between the lists of miRNAs being detected by the different platforms (e.g. high throughput qRT-PCR profiling or RNA-seq, Exiqon or TaqMan, etc) [9]. However, we observed that there was a set of 40 miRNAs that were common to at least six of the seven studies that were compared [9, 12, 13, 15, 75, 76]. In this case, of the 300 miRNAs that remained in our study after filtering, 192 of these have been previously detected in at least one other profiling study of adult plasma (S2 File).
Of the 108 miRNAs which were not detected in adult plasma 11 of these miRNA are also in the top 100 most abundant miRNA in our samples: miR-381-3p, miR-378a-3p, miR-92b-3p, miR-654-3p, miR-106b-3p, miR-6131, miR-340-5p, miR-151b, miR-1307-5p, miR-421 and miR-3182. This could indicate that umbilical cord plasma has a unique miRNA profile which may contain biomarkers more selective for pre/post-natal development or disease. This may also reflect the temporal changes in miRNA expression throughout ageing and future studies may compare miRNA profiles from adolescents and elderly people to determine this further.
The NCBI GeneRIF database [77] was used to determine whether any of the 108 miRNAs that were not found in adult plasma played specific roles in pregnancy or pregnancy complications. We identified miRNA that are involved throughout pregnancy from the preparation of the endometrium for pregnancy (miR-181a-3p) [78], to embryo attachment and early development (miR-145) [79, 80], embryonic stem cell differentiation and renewal (miR-181a-2-3p and the let-7 family of miRNAs) [81, 82], umbilical cord derived mesenchymal stem cells proliferation (miR-26b-3p) [83] and placental growth (miR-377-3p) [84]. Additionally, miRNAs including miR-141, miR-145, miR-378a-3p and miR-424 were identified that are regulators of trophoblast invasion, proliferation, survival and differentiation [79, 84–87].
Alterations in many of the miRNA that are present in our samples that were not detected in adult plasma have been linked to complications in both mothers and foetuses. These miRNA include: miR-18a, miR-136, miR-221, miR-141 and miR-145, which have been implicated in preeclampsia [88–91]. Additionally, miR-141 has been implicated in unexplained recurrent spontaneous abortions [92], miR-424 and miR-141 have been linked with fetal growth restriction [33, 93]) and miR-374a-3p is downregulated in HIE [26].
Using miRTarBase [94], we identified 75 validated targets (supported by strong experimental evidence) of the most abundant miRNA in our samples that were not found in adult plasma (summarised in Table 3)). We found evidence to support the role of 50 of these genes in pregnancy and pregnancy related complications.
Table 3. Validated gene targets of most abundant miRNA.
| hsa-miR-378a-3p | hsa-miR-340-5p | hsa-miR-92b-3p | hsa-miR-381-3p |
| CDK6 [95, 96] | AKT1 [97, 98] | CDKN1C [99–101] | ANO1 |
| CYP19A1 [102] | CCND1 | DAB2IP [103, 104] | CD1C [105] |
| GALNT7 | CCND2y [96] | DKK3 [106, 107] | GJA1 [108, 109] |
| GLI3 [110] | CCNG2 [111, 112] | ITGA6 [113, 114] | HDAC4 |
| GOLT1A | CDK6 [95] | ITGAV [115] | ID1 |
| GRB2 [116, 117] | HNRNPA2B1 [118] | NLK | NFKBIA |
| IGF1R [119] | IL4 [120] | PRMT5 [121] | P2RX5 |
| KSR1 | KRAS [122] | PTEN [123, 124] | TBC1D9 |
| MAPK1 [125, 126] | MDM2 [127, 128] | RAB23 [113, 129] | TWIST1 [130, 131] |
| MSC | MECP2 [132] | RECK [133, 134] | WEE1 |
| MYC [135] | MET | SLC15A1 | |
| NPNT | MITF [130] | SMAD3 [136] | |
| PGR [137] | PTBP1 | SMAD7 [88] | |
| RUNX1 [138] | PUM1 [139, 140] | ||
| SUFU | PUM2 | ||
| TGFB2 [141] | RHOA [142, 143] | ||
| TOB2 [144] | ROCK1 [115] | ||
| TUSC2 [145] | SKP2 [146] | ||
| VEGFA [147] | SOX2 | ||
| VIM | STAT3 [148–150] | ||
| WNT10A | |||
| hsa-miR-421 | hsa-miR-106b-3p | hsa-miR-654-3p | |
| ATM | BMP2 [123] | CDKN1A [151–153] | |
| CASP3 [154] | PTEN [123, 124] | ||
| CBX7 | |||
| CDH1 [155] | |||
| FOXO4 [156–158] | |||
| RBMXL1 | |||
| SIRT3 [159] | |||
| SMAD4 [160] |
Table showing the 75 validated targets, found using miRTarBase [94], for the most abundant miRNA in our samples that were not found in adult plasma. Where available, the citation which supports a link between the gene and pregnancy is show.
Many of the genes that we identified as targets of these miRNA are believed to play a role in the preparation and development of the uterus in early pregnancy (TWIST1 [130], CDK6 [95], RUNX1 [138], CDK6 [95], MITF [130], HNRNPA2B1 [118] and BMP2 [123]), decidualisation (CDKN1A [153] and STAT3 [150]), implantation of the embryo (RECK [134], KRAS [122], CDH1 [155] and SMAD4 [160]) and the activation of the migration, invasion, proliferation and differentiation of the trophoblast (MAPK1 [125], MYC [135], TGFB [141], DAB2IP [103], RECK [133], SMAD7 [88] and STAT3 [149]). They continue to play roles during pregnancy and are important for the healthy progression of pregnancy and fetal growth (CASP3 [154], GLI3 [110], VEGFA [147], CCND2 [96], CDKN1C [99], IL-4 [120], IGF1R [119] and Akt1 [98]). DKK3 [107], ITGAV [115], TWIST1 [131] and ROCK1 [115] upregulation has also been reported in the myometrium in healthy pregnancies at full-term. While CCNG2 [96] and FOXO4 [156] are downregulated in the placenta at full term. A number of these genes have been implicated in complications of preganacy including: miscarriage (VEGFA [147], DAB21P [103], CDKN1A [151, 151, 152], PTEN [124] and MDM2 [128]); preeclampsia (CD1C [105], CYP19A1 [102], VEGFA [147], CDKN1C [100, 101], DAB2IP [104], CCNG2 [111] and GRB2 [116]); pre-term labour (RhoA [142, 143]); gestational diabetes (CCNG2 [111, 112], ITGA6 [113], RAB23 [113] and FOXO4 [158]); gestational trophoblastic disease (MDM2 [127]); male-specific neonatal encephalopathy (MECP2 [132]), neural tube defects (ITGA6 [113] and RAB23 [113]); Carpenter Syndrome (RAB23 [129]); intrahepatic cholestasis of pregnancy (PUM1 [139]); ectopic pregnancies (DKK3 [106]); and foetal conotruncal anomalies (MAPK1 [126].
Analysis of miRNA editing
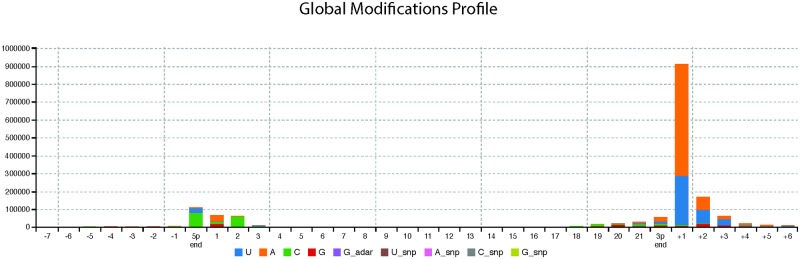
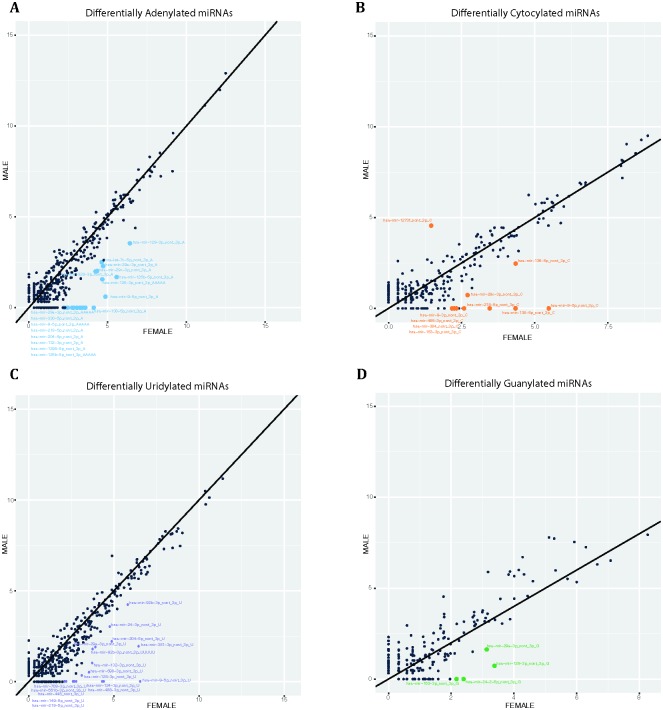
RNA editing enzymes including ADAR proteins have been shown to function aberrantly in various types of cancers and neurological disorders [161–164]. Additionally, it has recently been demonstrated that it is possible to distinguish between different cancer types based on the presence or absence of alternatively modified miRNAs (isomiRs) [165]. As such it is intuitive that the search for biomarkers should include criteria which would allow the identification of alternatively edited RNAs, as they may correlate with, and therefore aid in diagnosis of disease development, progression and prognosis [165]. MiRNAs have been shown to be subjected to edits and modifications and we therefore expanded our interrogation of umbilical cord plasma miRNA profiles to investigate the prevalence of editing and whether differences exist between sexes. Using Chimira [44], we performed editing analysis on our sequencing data, initially analysed global modification profile of all samples and differential expression of adenylated, uridylated, guanylated and cytocylated miRNAs (Fig 6). Statistically significant differential expression was taken as a fold change in expression of > 2 (or < -2) and an associated p-value < 0.05. We found there were consistently more differentially expressed edited miRNAs in female cord blood compared to males (Fig 7). However, expression of the differentially edited miRNAs is very low and thus further evidence is required in order to infer any functional implications from the differential modification profiles in this particular study (S4 File). Of the four types of editing analysed adenylation and uridylation were the most abundant differential modification identified, which is in line with previous editing analysis [45, 166–169]. Of note, miR-128-3p, miR-29a-3p, miR-9-5p, miR-218-5p, 204-5p and miR-132-3p were consistently both uridylated and adenylated in female cord blood plasma (S1 Fig).
Fig 6. Global modification profile of all samples showing high levels or adenylation and uridylation at the 3′ end of the miRNA.
The x-axis corresponds to the index positions across a miRNA molecule. They y-axis corresponds to the raw counts of the identified modification patterns. The start of a miRNA on the x-axis is at index ‘0’ (5′ end) while its end is at index ‘22’ (3′ end).
Fig 7. Differential expression of modified miRNAs between female and male samples (log2 of count data).
Statistically significant differentially expressed miRNAs are highlighted in non-black. A: Adenyated, B: Cytocylated, C: Uridylated and D: Guanylated.
Of further interest was the origin of miRNAs which were both differentially adenylated and uridylated in females. With the exception of miR-29a-3p (which is enriched in brain) 5 of the 6 miRNAs which underwent differential adenylation and uridylation are brain specific [170]. Editing of RNA has been reported to be highly prominent in the central nervous system [171]. It is interesting to speculate on the origin of these miRNAs, as these are healthy infants who experienced routine vaginal births, leakage via a disrupted blood brain barrier is unlikely. It is possible however that these brain specific miRNAs were encapsulated in signalling micro-vesicles and transported out of the brain in order to elicit a peripheral cell response [172]. Site-of-editing analysis revealed that editing occurred most frequently at the 3′ end of the miRNA molecule. Specifically adenylation and uridylation occurred most frequently at the +1 site suggesting 3′ tailing of the miRNAs. MiRNAs have been shown to be selectively uridylated by 3′ terminal uridylyl transferases (TUTases) TUT7 and TUT4. This editing can modify miRNA-gene regulatory networks by affecting the stability of the miRNA [50, 173–175].
Conclusion
miRNAs play a role in multiple key processes throughout pregnancy; including preparation of endometrial tissue for implantation, management of immune-associated genes, development of the placenta and angiogenesis [28]. Dysregulation of the expression of these miRNAs may therefore be associated with complications in pregnancy [29], making them good candidate biomarkers for not only HIE [26, 176], but for many pregnancy-related disorders. Furthermore, due to the relative stability of miRNAs under normal conditions [177], they appear to be potentially useful diagnostic biomarkers of multiple disordered states [178] in pregnancy and beyond. More research is required however, to decipher their target pathways and mechanisms of action.
While overall miRNA expression did not differ between male and female cord blood plasma, we did detect differentially edited miRNAs in female plasma compared to male. Editing of miRNAs is now known to be altered in disease [161–164] and can affect miRNA-mRNA targeting, indeed Choudhury et al, identified that A-I editing of miR-376 was reduced in glioma and had an effect on the repertoire of target mRNAs [179]. This allowed an increase in cell invasiveness. As such it is intuitive that future miRNA biomarker studies profile changes in miRNA editing as it may correlate with disease development, progression and outcome. Of note, and consistent with other studies of this type, adenylation and uridylation were the two most prominent forms of editing. Analysis of the sites of adenylation and uridylation along the miRNA molecule revealed that editing was most prominent at the 3′ end of the miRNAs at the +1 position, indicating 3′ tailing, a common modification of miRNAs. Analysis of the expression patterns of these miRNAs revealed that all except miR-29a-3p are expressed almost exclusively in brain [170]. Although only a few miRNAs were differentially edited in females and expression levels were low, it is an interesting finding as the effects of sex on RNA editing is poorly understood and warrants further investigation.
This study is the first to profile miRNA editing in cord blood plasma from healthy infants. Although we did not detect a difference between male and female miRNA expression, possibly due to the small sample size, and expression of the differentially edited miRNAs is very low, this study can be used as comparative data for future biomarker profiles from complicated births or those with developmental disorders including those initiated by HIE.
Supporting information
A: miR-128-3p, B: miR-29a-3p, C: miR-9-5p, D: miR-218-5p, E: 204-5p and F: miR-132-3p. Analysis of the expression patterns of these miRNAs revealed that all except miR-29a-3p (although it is enriched) are expressed almost exclusively in brain (highlighted with green boxes) [170].
(PNG)
miRNA are ranked by average abundance across all samples.
(CSV)
miRNA names were mapped to the mature human sequences from miRBase version 21 [57]. Blondal [75], Chen [13], Mitchell [15], Mooney [9], Wang–Exiqon [76], Wang–Taqman [76] and Weber [12].
(CSV)
a—acinar cell; b—adipocyte; c—ductal cell; d—endothelial; e—epithelial cell; f—fibroblast; g—hepatocyte; h—lymphatic EC; i—myocyte; j—neutrophil; k—smooth muscle cell; l—centroblast; m—memory B cell; n—monocyte; o—naïve B cell; p—NK cell; q—plasma B cell; and r—red blood cell. Cell types a—k are hematopoietic; Cell types l—r are hematopoietic. An asterix is plased in the column if the miRNA is found in the top 100 of miRNAs expressed in that cell type. Expression profiles for all cells taken from [18]. MiRNAs are included if they are identified in at least one cell type and are listed in order of average abundance across all samples (mean). SD—standard deviation.
(CSV)
(CSV)
Data Availability
All MicroRNA profiling data files are available from the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) (accession number(s) GSE119002).
Funding Statement
This publication has emanated from research supported in part by research grants from Science Foundation Ireland (SFI) under Grant Numbers SFI/12/RC/2272 (to D.M.M. and G.B.B.), SFI/16/RC/3948, SFI/13/IA/1891, SFI/12/COEN/18, SFI/14/ADV/RC2721 (to D.C.H. and C.M.) and co-funded under the European Regional Development Fund and by FutureNeuro and INFANT industry partners, the Royal College of Surgeons in Ireland seed fund (“PLASMIR-Dx”), the Irish Research Council Epilepsy Ireland/Medical Research Charities Group (to D.C.H.), CURE taking flight, a H2020 Marie Skłowdowksa-Curie Actions Individual Fellowship (Project no. 707530) (to G.P.B.) and EMBL core funding (to D.M.V). The BiHiVE 2 study was funded by the HRB Clinician Scientist Award CSA 2012/40 (to D.M.M.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Battin MR, Dezoete JA, Gunn TR, Gluckman PD, Gunn AJ. Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics. 2001;107(3):480–484. 10.1542/peds.107.3.480 [DOI] [PubMed] [Google Scholar]
- 2. McKinlay CJ, Cutfield WS, Battin MR, Dalziel SR, Crowther CA, Harding JE, et al. Cardiovascular risk factors in children after repeat doses of antenatal glucocorticoids: an RCT. Pediatrics. 2015;135(2):e405–e415. 10.1542/peds.2014-2408 [DOI] [PubMed] [Google Scholar]
- 3. Conway J, Walsh B, Boylan G, Murray D. Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome—A systematic review. Early human development. 2018;120:80–87. 10.1016/j.earlhumdev.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 4. O’Driscoll D, Felice VD, Kenny LC, Boylan GB, O’Keeffe GW. Mild prenatal hypoxia-ischemia leads to social deficits and central and peripheral inflammation in exposed offspring. Brain, Behavior, and Immunity. 2018;69:418–427. 10.1016/j.bbi.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 6. Simeoli R, Montague K, Jones HR, Castaldi L, Chambers D, Kelleher JH, et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nature Communications. 2017;8(1):1778 10.1038/s41467-017-01841-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luarte A, Cisternas P, Caviedes A, Batiz LF, Lafourcade C, Wyneken U, et al. Astrocytes at the Hub of the Stress Response: Potential Modulation of Neurogenesis by miRNAs in Astrocyte-Derived Exosomes. Stem Cells International. 2017;1719050 10.1155/2017/1719050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bayraktar R, Van Roosbroeck K, Calin GA. Cell to cell communication: microRNAs as hormones. Molecular Oncology. 2017;11(12):1673–86. 10.1002/1878-0261.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mooney C, Raoof R, El-Naggar H, Sanz-Rodriguez A, Jimenez-Mateos EM, Henshall DC. High Throughput qPCR Expression Profiling of Circulating MicroRNAs Reveals Minimal Sex-and Sample Timing-Related Variation in Plasma of Healthy Volunteers. PLOS ONE. 2015;10(12):e0145316 10.1371/journal.pone.0145316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Argyropoulos C, Wang K, McClarty S, Huang D, Bernardo J, Ellis D, et al. Urinary microRNA profiling in the nephropathy of type 1 diabetes. PLOS ONE. 2013;8(1):e54662 10.1371/journal.pone.0054662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raoof R, Jimenez-Mateos EM, Bauer S, Tackenberg B, Rosenow F, Lang J, et al. Cerebrospinal fluid microRNAs are potential biomarkers of temporal lobe epilepsy and status epilepticus. Scientific Reports. 2017;7(1):3328 10.1038/s41598-017-02969-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clinical Chemistry. 2010;56(11):1733–1741. 10.1373/clinchem.2010.147405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Research. 2008;18(10):997–1006. 10.1038/cr.2008.282 [DOI] [PubMed] [Google Scholar]
- 14. Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLOS ONE. 2008;3(11):e3694 10.1371/journal.pone.0003694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences. 2008;105(30):10513–10518. 10.1073/pnas.0804549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reid G, Kirschner MB, van Zandwijk N. Circulating microRNAs: Association with disease and potential use as biomarkers. Critical Reviews in Oncology/Hematology. 2011;80(2):193–208. 10.1016/j.critrevonc.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 17. Moldovan L, Batte KE, Trgovcich J, Wisler J, Marsh CB, Piper M. Methodological challenges in utilizing miRNAs as circulating biomarkers. Journal of Cellular and Molecular Medicine. 2014;18(3):371–390. 10.1111/jcmm.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haider BA, Baras AS, McCall MN, Hertel JA, Cornish TC, Halushka MK. A critical evaluation of microRNA biomarkers in non-neoplastic disease. PLOS ONE. 2014;9(2):e89565 10.1371/journal.pone.0089565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aryani A, Denecke B. In vitro application of ribonucleases: comparison of the effects on mRNA and miRNA stability. BMC Research Notes. 2015;8(1):164 10.1186/s13104-015-1114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods. 2010;50(4):298–301. 10.1016/j.ymeth.2010.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frères P, Wenric S, Boukerroucha M, Fasquelle C, Thiry J, Bovy N, et al. Circulating microRNA-based screening tool for breast cancer. Oncotarget. 2016;7(5):5416 doi: 10.18632/oncotarget.6786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yuan T, Huang X, Woodcock M, Du M, Dittmar R, Wang Y, et al. Plasma extracellular RNA profiles in healthy and cancer patients. Scientific Reports. 2016;6:19413 10.1038/srep19413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riancho J, Vázquez-Higuera JL, Pozueta A, Lage C, Kazimierczak M, Bravo M, et al. MicroRNA profile in patients with Alzheimer’s disease: analysis of miR-9-5p and miR-598 in raw and exosome enriched cerebrospinal fluid samples. Journal of Alzheimer’s Disease. 2017;57(2):483–491. 10.3233/JAD-161179 [DOI] [PubMed] [Google Scholar]
- 24. Raoof R, Bauer S, El Naggar H, Connolly NM, Brennan GP, Brindley E, et al. Dual-center, dual-platform microRNA profiling identifies potential plasma biomarkers of adult temporal lobe epilepsy. EBioMedicine. 2018. 10.1016/j.ebiom.2018.10.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ponnusamy V, Kapellou O, Yip E, Evanson J, Wong LF, Michael-Titus A, et al. A study of microRNAs from dried blood spots in newborns after perinatal asphyxia: a simple and feasible biosampling method. Pediatric Research. 2016;79(5):799 10.1038/pr.2015.276 [DOI] [PubMed] [Google Scholar]
- 26. Looney AM, Walsh BH, Moloney G, Grenham S, Fagan A, O’keeffe GW, et al. Downregulation of umbilical cord blood levels of miR-374a in neonatal hypoxic ischemic encephalopathy. The Journal of Pediatrics. 2015;167(2):269–273. 10.1016/j.jpeds.2015.04.060 [DOI] [PubMed] [Google Scholar]
- 27. Looney A, Ahearne C, Hallberg B, Boylan G, Murray D. Downstream mRNA target analysis in neonatal hypoxic-ischaemic encephalopathy identifies novel marker of severe injury: A proof of concept paper. Molecular Neurobiology. 2017;54(10):8420–8428. 10.1007/s12035-016-0330-4 [DOI] [PubMed] [Google Scholar]
- 28. Chiofalo B, Laganà AS, Vaiarelli A, La Rosa VL, Rossetti D, Palmara V, et al. Do miRNAs play a role in fetal growth restriction? A fresh look to a busy corner. BioMed research international. 2017;2017 10.1155/2017/6073167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lycoudi A, Mavreli D, Mavrou A, Papantoniou N, Kolialexi A. miRNAs in pregnancy-related complications. Expert review of molecular diagnostics. 2015;15(8):999–1010. 10.1586/14737159.2015.1053468 [DOI] [PubMed] [Google Scholar]
- 30. Maccani MA, Padbury JF, Marsit CJ. miR-16 and miR-21 expression in the placenta is associated with fetal growth. PLOS ONE. 2011;6(6):e21210 10.1371/journal.pone.0021210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bounds KR, Chiasson VL, Pan LJ, Gupta S, Chatterjee P. MicroRNAs: new players in the pathobiology of preeclampsia. Frontiers in cardiovascular medicine. 2017;4:60 10.3389/fcvm.2017.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks W, Sadovsky Y. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta. 2010;31(9):781–784. 10.1016/j.placenta.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang L, Shen Z, Xu Q, Huang X, Chen Q, Li D. Increased levels of microRNA-424 are associated with the pathogenesis of fetal growth restriction. Placenta. 2013;34(7):624–627. 10.1016/j.placenta.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 34. Lagana AS, Vitale SG, Sapia F, Valenti G, Corrado F, Padula F, et al. miRNA expression for early diagnosis of preeclampsia onset: hope or hype? The Journal of Maternal-Fetal & Neonatal Medicine. 2018;31(6):817–821. 10.1080/14767058.2017.1296426 [DOI] [PubMed] [Google Scholar]
- 35. Zhu Xm, Han T, Sargent IL, Yin Gw, Yao Yq. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. American journal of obstetrics and gynecology. 2009;200(6):661–e1. 10.1016/j.ajog.2008.12.045 [DOI] [PubMed] [Google Scholar]
- 36. Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li L, et al. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. Journal of cellular and molecular medicine. 2012;16(2):249–259. 10.1111/j.1582-4934.2011.01291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. American journal of obstetrics and gynecology. 2007;196(3):261–e1. 10.1016/j.ajog.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 38. Xu T, Li L, Huang C, Li X, Peng Y, Li J. MicroRNA-323-3p with clinical potential in rheumatoid arthritis, Alzheimer’s disease and ectopic pregnancy. Expert opinion on therapeutic targets. 2014;18(2):153–158. 10.1517/14728222.2014.855201 [DOI] [PubMed] [Google Scholar]
- 39. Zhao Z, Zhao Q, Warrick J, Lockwood CM, Woodworth A, Moley KH, et al. Circulating microRNA miR-323-3p as a biomarker of ectopic pregnancy. Clinical chemistry. 2012; p. 179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu Y, Tian F, Li H, Zhou Y, Lu J, Ge Q. Profiling maternal plasma microRNA expression in early pregnancy to predict gestational diabetes mellitus. International Journal of Gynecology & Obstetrics. 2015;130(1):49–53. 10.1016/j.ijgo.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 41. Blow MJ, Grocock RJ, van Dongen S, Enright AJ, Dicks E, Futreal PA, et al. RNA editing of human microRNAs. Genome Biology. 2006;7(4):R27 10.1186/gb-2006-7-4-r27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Velazquez-Torres G, Shoshan E, Ivan C, Huang L, Fuentes-Mattei E, Paret H, et al. A-to-I miR-378a-3p editing can prevent melanoma progression via regulation of PARVA expression. Nature Communications. 2018;9(1):461 10.1038/s41467-018-02851-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paul D, Sinha AN, Ray A, Lal M, Nayak S, Sharma A, et al. A-to-I editing in human miRNAs is enriched in seed sequence, influenced by sequence contexts and significantly hypoedited in glioblastoma multiforme. Scientific Reports. 2017;7(1):2466 10.1038/s41598-017-02397-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vitsios DM, Enright AJ. Chimira: analysis of small RNA sequencing data and microRNA modifications. Bioinformatics. 2015;31(20):3365–3367. 10.1093/bioinformatics/btv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vitsios DM, Davis MP, van Dongen S, Enright AJ. Large-scale analysis of microRNA expression, epi-transcriptomic features and biogenesis. Nucleic Acids Research. 2017;45(3):1079–1090. 10.1093/nar/gkw1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heale BS, Keegan LP, O’Connell MA. The effect of RNA editing and ADARs on miRNA biogenesis and function In: Regulation of microRNAs. Springer; 2010. p. 76–84. [PubMed] [Google Scholar]
- 47. Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, et al. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406(6791):78 10.1038/35017558 [DOI] [PubMed] [Google Scholar]
- 48. Chawla G, Sokol NS. ADAR mediates differential expression of polycistronic microRNAs. Nucleic Acids Research. 2014;42(8):5245–5255. 10.1093/nar/gku145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tomaselli S, Galeano F, Alon S, Raho S, Galardi S, Polito VA, et al. Modulation of microRNA editing, expression and processing by ADAR2 deaminase in glioblastoma. Genome Biology. 2015;16(1):5 10.1186/s13059-014-0575-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim B, Ha M, Loeff L, Chang H, Simanshu DK, Li S, et al. TUT7 controls the fate of precursor microRNAs by using three different uridylation mechanisms. The EMBO Journal. 2015;34(13):1801–1815. doi: 10.15252/embj.201590931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Williams Z, Ben-Dov IZ, Elias R, Mihailovic A, Brown M, Rosenwaks Z, et al. Comprehensive profiling of circulating microRNA via small RNA sequencing of cDNA libraries reveals biomarker potential and limitations. Proceedings of the National Academy of Sciences. 2013;110(11):4255–4260. 10.1073/pnas.1214046110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xu Y, Li W, Liu X, Ma H, Tu Z, Dai Y. Analysis of microRNA expression profile by small RNA sequencing in Down syndrome fetuses. International Journal of Molecular Medicine. 2013;32(5):1115–1125. 10.3892/ijmm.2013.1499 [DOI] [PubMed] [Google Scholar]
- 53. Merkerova M, Vasikova A, Belickova M, Bruchova H. MicroRNA expression profiles in umbilical cord blood cell lineages. Stem Cells and Development. 2010;19(1):17–26. 10.1089/scd.2009.0071 [DOI] [PubMed] [Google Scholar]
- 54. Lizarraga D, Huen K, Combs M, Escudero-Fung M, Eskenazi B, Holland N. miRNAs differentially expressed by next-generation sequencing in cord blood buffy coat samples of boys and girls. Epigenomics. 2016;8(12):1619–1635. 10.2217/epi-2016-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kirschner MB, Kao SC, Edelman JJ, Armstrong NJ, Vallely MP, van Zandwijk N, et al. Haemolysis during sample preparation alters microRNA content of plasma. PLOS ONE. 2011;6(9):e24145 10.1371/journal.pone.0024145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrews S. FASTQC. A quality control tool for high throughput sequence data; 2010.
- 57. Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Research. 2014;42(D1):D68 10.1093/nar/gkt1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.R Core Team. R: A Language and Environment for Statistical Computing; 2016. Available from: http://www.R-project.org/.
- 59. Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nature Methods. 2015;12(2):115–121. 10.1038/nmeth.3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Law CW, Alhamdoosh M, Su S, Smyth GK, Ritchie ME. RNA-seq analysis is easy as 1-2-3 with limma, Glimma and edgeR. F1000Research. 2016;5 doi: 10.12688/f1000research.9005.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biology. 2010;11(3):R25 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Law CW, Chen Y, Shi W, Smyth GK. Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biology. 2014;15(2):R29 10.1186/gb-2014-15-2-r29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43(7):e47 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 66. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15(12):550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hubbard T, Barker D, Birney E, Cameron G, Chen Y, Clark L, et al. The Ensembl genome database project. Nucleic Acids Research. 2002;30(1):38–41. 10.1093/nar/30.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Maccani MA, Padbury JF, Lester BM, Knopik VS, Marsit CJ. Placental miRNA expression profiles are associated with measures of infant neurobehavioral outcomes. Pediatric research. 2013;74(3):272 10.1038/pr.2013.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Morales-Prieto DM, Ospina-Prieto S, Chaiwangyen W, Schoenleben M, Markert UR. Pregnancy-associated miRNA-clusters. Journal of Reproductive Immunology. 2013;97(1):51–61. 10.1016/j.jri.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 70. Noguer-Dance M, Abu-Amero S, Al-Khtib M, Lefevre A, Coullin P, Moore GE, et al. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Human Molecular Genetics. 2010;19(18):3566–3582. 10.1093/hmg/ddq272 [DOI] [PubMed] [Google Scholar]
- 71. Mong EF, Akat KM, Canfield J, Lockhart J, VanWye J, Matar A, et al. Modulation of LIN28B/Let-7 Signaling by Propranolol Contributes to Infantile Hemangioma Involution. Arteriosclerosis, Thrombosis, and Vascular biology. 2018;38(6):1321–1332. 10.1161/ATVBAHA.118.310908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang Q, Lu J, Wang S, Li H, Ge Q, Lu Z. Application of next-generation sequencing technology to profile the circulating microRNAs in the serum of preeclampsia versus normal pregnant women. Clinica Chimica Acta. 2011;412(23-24):2167–2173. 10.1016/j.cca.2011.07.029 [DOI] [PubMed] [Google Scholar]
- 73. Gunel T, Zeybek Y, Akçakaya P, Kalelioglu I, Benian A, Ermis H, et al. Serum microRNA expression in pregnancies with preeclampsia. Genet Mol Res. 2011;10(4):4034–4040. 10.4238/2011.November.8.5 [DOI] [PubMed] [Google Scholar]
- 74. Wu L, Zhou H, Lin H, Qi J, Zhu C, Gao Z, et al. Circulating microRNAs are elevated in plasma from severe pre-eclamptic pregnancies. Reproduction. 2011; p. REP–11. [DOI] [PubMed] [Google Scholar]
- 75. Blondal T, Jensby Nielsen S, Baker A, Andreasen D, Mouritzen P, Wrang Teilum M, et al. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59(1):S1–S6. 10.1016/j.ymeth.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 76. Wang K, Yuan Y, Cho JH, McClarty S, Baxter D, Galas DJ. Comparing the MicroRNA spectrum between serum and plasma. PLOS ONE. 2012;7(7):e41561 10.1371/journal.pone.0041561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jimeno-Yepes AJ, Sticco JC, Mork JG, Aronson AR. GeneRIF indexing: sentence selection based on machine learning. BMC bioinformatics. 2013;14(1):171 10.1186/1471-2105-14-171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang Q, Zhang H, Jiang Y, Xue B, Diao Z, Ding L, et al. MicroRNA-181a is involved in the regulation of human endometrial stromal cell decidualization by inhibiting Krüppel-like factor 12. Reproductive Biology and Endocrinology. 2015;13(1):23 10.1186/s12958-015-0019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kang YJ, Lees M, Matthews LC, Kimber SJ, Forbes K, Aplin JD. MiR-145 suppresses embryo-epithelial juxtacrine communication at implantation by modulating maternal IGF1R. J Cell Sci. 2015; p. jcs–164004. 10.1242/jcs.164004 [DOI] [PubMed] [Google Scholar]
- 80. Lozoya T, Domínguez F, Romero-Ruiz A, Steffani L, Martínez S, Monterde M, et al. The Lin28/Let-7 system in early human embryonic tissue and ectopic pregnancy. PLOS ONE. 2014;9(1):e87698 10.1371/journal.pone.0087698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xu Z, Jiang J, Xu C, Wang Y, Sun L, Guo X, et al. MicroRNA-181 regulates CARM1 and histone aginine methylation to promote differentiation of human embryonic stem cells. PLOS ONE. 2013;8(1):e53146 10.1371/journal.pone.0053146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tran ND, Kissner M, Subramanyam D, Parchem RJ, Laird DJ, Blelloch RH. A miR-372/let-7 Axis Regulates Human Germ Versus Somatic Cell Fates. Stem Cells. 2016;34(7):1985–1991. 10.1002/stem.2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang Q, Xu C, Zhao Y, Xu Z, Zhang Y, Jiang J, et al. miR-26b-3p regulates human umbilical cord-derived mesenchymal stem cell proliferation by targeting estrogen receptor. Stem cells and development. 2016;25(5):415–426. 10.1089/scd.2015.0267 [DOI] [PubMed] [Google Scholar]
- 84. Farrokhnia F, Aplin JD, Westwood M, Forbes K. MicroRNA regulation of mitogenic signaling networks in the human placenta. Journal of Biological Chemistry. 2014; p. jbc–M114. 10.1074/jbc.M114.587295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ospina-Prieto S, Chaiwangyen W, Herrmann J, Groten T, Schleussner E, Markert UR, et al. MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Translational Research. 2016;172:61–72. 10.1016/j.trsl.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 86. Luo L, Ye G, Nadeem L, Fu G, Yang BB, Honarparvar E, et al. MicroRNA-378a-5p promotes trophoblast cell survival, migration and invasion by targeting Nodal. J Cell Sci. 2012;125(13):3124–3132. 10.1242/jcs.096412 [DOI] [PubMed] [Google Scholar]
- 87. Mouillet JF, Donker RB, Mishima T, Cronqvist T, Chu T, Sadovsky Y. The unique expression and function of miR-424 in human placental trophoblasts. Biology of reproduction. 2013;89(2):25–1. 10.1095/biolreprod.113.110049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li Yx, et al. Variations of microRNAs in human placentas and plasma from preeclamptic pregnancy. Hypertension. 2014;63(6):1276–1284. 10.1161/HYPERTENSIONAHA.113.02647 [DOI] [PubMed] [Google Scholar]
- 89. Ji L, Zhang L, Li Y, Guo L, Cao N, Bai Z, et al. MiR-136 contributes to pre-eclampsia through its effects on apoptosis and angiogenesis of mesenchymal stem cells. Placenta. 2017;50:102–109. 10.1016/j.placenta.2017.01.102 [DOI] [PubMed] [Google Scholar]
- 90. Li H, Ge Q, Guo L, Lu Z. Maternal plasma miRNAs expression in preeclamptic pregnancies. BioMed research international. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Han L, Zhao Y, Luo Q, Liu X, Lu S, Zou L. The significance of miR-145 in the prediction of preeclampsia. Bratislavske lekarske listy. 2017;118(9):523–528. 10.4149/BLL_2017_101 [DOI] [PubMed] [Google Scholar]
- 92. Li D, Li J. Association of miR-34a-3p/5p, miR-141-3p/5p, and miR-24 in decidual natural killer cells with unexplained recurrent spontaneous abortion. Medical science monitor: international medical journal of experimental and clinical research. 2016;22:922 doi: 10.12659/MSM.895459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tang Q, Wu W, Xu X, Huang L, Gao Q, Chen H, et al. miR-141 contributes to fetal growth restriction by regulating PLAG1 expression. PLOS ONE. 2013;8(3):e58737 10.1371/journal.pone.0058737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Research. 2017;46(D1):D296–D302. 10.1093/nar/gkx1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mechanisms of development. 2002;111(1-2):99–113. 10.1016/S0925-4773(01)00614-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Assou S, Haouzi D, De Vos J, Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. MHR: Basic science of reproductive medicine. 2010;16(8):531–538. 10.1093/molehr/gaq032 [DOI] [PubMed] [Google Scholar]
- 97. Boxer RB, Stairs DB, Dugan KD, Notarfrancesco KL, Portocarrero CP, Keister BA, et al. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell metabolism. 2006;4(6):475–490. 10.1016/j.cmet.2006.10.011 [DOI] [PubMed] [Google Scholar]
- 98. Kent LN, Ohboshi S, Soares MJ. Akt1 and insulin-like growth factor 2 (Igf2) regulate placentation and fetal/postnatal development. The International journal of developmental biology. 2012;56(4):255 10.1387/ijdb.113407lk [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dória S, Sousa M, Fernandes S, Ramalho C, Brandão O, Matias A, et al. Gene expression pattern of IGF2, PHLDA2, PEG10 and CDKN1C imprinted genes in spontaneous miscarriages or fetal deaths. Epigenetics. 2010;5(5):444–450. 10.4161/epi.5.5.12118 [DOI] [PubMed] [Google Scholar]
- 100. Romanelli V, Belinchon A, Campos-Barros A, Heath K, Garcia-Minaur S, Martinez-Glez V, et al. CDKN1C mutations in HELLP/preeclamptic mothers of Beckwith–Wiedemann syndrome (BWS) patients. Placenta. 2009;30(6):551–554. 10.1016/j.placenta.2009.03.013 [DOI] [PubMed] [Google Scholar]
- 101. Lim A, Ferguson-Smith A. Genomic imprinting effects in a compromised in utero environment: implications for a healthy pregnancy In: Seminars in cell & developmental biology. vol. 21 Elsevier; 2010. p. 201–208. [DOI] [PubMed] [Google Scholar]
- 102. Shimodaira M, Nakayama T, Sato I, Sato N, Izawa N, Mizutani Y, et al. Estrogen synthesis genes CYP19A1, HSD3B1, and HSD3B2 in hypertensive disorders of pregnancy. Endocrine. 2012;42(3):700–707. 10.1007/s12020-012-9699-7 [DOI] [PubMed] [Google Scholar]
- 103. Li S, Zhai J, Liu J, Hong Y, Zhao W, Zhao A, et al. BMAL1 facilitates trophoblast migration and invasion via SP1-DNMT1/DAB2IP pathway in recurrent spontaneous abortion. Oncotarget. 2017;8(52):89451 doi: 10.18632/oncotarget.20702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shan N, Xiao X, Chen Y, Luo X, Yin N, Deng Q, et al. Expression of DAB2IP in human trophoblast and its role in trophoblast invasion. The Journal of Maternal-Fetal & Neonatal Medicine. 2016;29(3):393–399. 10.3109/14767058.2014.1001974 [DOI] [PubMed] [Google Scholar]
- 105. DARMOCHWAL-KOLARZ D, Rolinski J, Tabarkiewicz J, Leszczynska-Gorzelak B, Buczkowski J, Wojas K, et al. Myeloid and lymphoid dendritic cells in normal pregnancy and pre-eclampsia. Clinical & Experimental Immunology. 2003;132(2):339–344. 10.1046/j.1365-2249.2003.02136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Savaris RF, Hamilton AE, Lessey BA, Giudice LC. Endometrial gene expression in early pregnancy: lessons from human ectopic pregnancy. Reproductive Sciences. 2008;15(8):797–816. 10.1177/1933719108317585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Rehman KS, Yin S, Mayhew BA, Word RA, Rainey WE. Human myometrial adaptation to pregnancy: cDNA microarray gene expression profiling of myometrium from non-pregnant and pregnant women. MHR: Basic science of reproductive medicine. 2003;9(11):681–700. [DOI] [PubMed] [Google Scholar]
- 108. Li SH, Lin MH, Hwu YM, Lu CH, Yeh LY, Chen YJ, et al. Correlation of cumulus gene expression of GJA1, PRSS35, PTX3, and SERPINE2 with oocyte maturation, fertilization, and embryo development. Reproductive Biology and Endocrinology. 2015;13(1):93 10.1186/s12958-015-0091-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Tong D, Lu X, Wang HX, Plante I, Lui E, Laird DW, et al. A dominant loss-of-function GJA1 (Cx43) mutant impairs parturition in the mouse. Biology of reproduction. 2009;80(6):1099–1106. 10.1095/biolreprod.108.071969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bethin KE, Nagai Y, Sladek R, Asada M, Sadovsky Y, Hudson TJ, et al. Microarray analysis of uterine gene expression in mouse and human pregnancy. Molecular endocrinology. 2003;17(8):1454–1469. 10.1210/me.2003-0007 [DOI] [PubMed] [Google Scholar]
- 111. Uusküla L, Männik J, Rull K, Minajeva A, Kõks S, Vaas P, et al. Mid-gestational gene expression profile in placenta and link to pregnancy complications. PLOS ONE. 2012;7(11):e49248 10.1371/journal.pone.0049248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lamadrid-Romero M, Solís K, Cruz-Reséndiz M, Pérez J, Díaz N, Flores-Herrera H, et al. Central nervous system development-related microRNAs levels increase in the serum of gestational diabetic women during the first trimester of pregnancy. Neuroscience research. 2018;130:8–22. 10.1016/j.neures.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 113. Salbaum JM, Kappen C. Neural tube defect genes and maternal diabetes during pregnancy. Birth Defects Research Part A: Clinical and Molecular Teratology. 2010;88(8):601–611. 10.1002/bdra.20680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Salilew-Wondim D, Hölker M, Rings F, Ghanem N, Ulas-Cinar M, Peippo J, et al. Bovine pretransfer endometrium and embryo transcriptome fingerprints as predictors of pregnancy success after embryo transfer. Physiological genomics. 2010;42(2):201–218. 10.1152/physiolgenomics.00047.2010 [DOI] [PubMed] [Google Scholar]
- 115. Breuiller-Fouche M, Germain G. Gene and protein expression in the myometrium in pregnancy and labor. Reproduction. 2006;131(5):837–850. 10.1530/rep.1.00725 [DOI] [PubMed] [Google Scholar]
- 116. Anteby EY, Ayesh S, Shochina M, Hamani Y, Schneider T, Al-Shareef W, et al. Growth factor receptor-protein bound 2 (GRB2) upregulation in the placenta in preeclampsia implies a possible role for ras signalling. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2005;118(2):174–181. 10.1016/j.ejogrb.2004.04.029 [DOI] [PubMed] [Google Scholar]
- 117. Enquobahrie DA, Qiu C, Muhie SY, Williams MA. Maternal peripheral blood gene expression in early pregnancy and preeclampsia. International journal of molecular epidemiology and genetics. 2011;2(1):78 [PMC free article] [PubMed] [Google Scholar]
- 118. Forde N, Bazer FW, Spencer TE, Lonergan P. ‘Conceptualizing’ the endometrium: identification of conceptus-derived proteins during early pregnancy in cattle. Biology of reproduction. 2015;92(6):156–1. 10.1095/biolreprod.115.129296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pringle K, Roberts C. New light on early post-implantation pregnancy in the mouse: roles for insulin-like growth factor-II (IGF-II)? Placenta. 2007;28(4):286–297. 10.1016/j.placenta.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 120. Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, et al. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. 2011;53(2):170–177. 10.1016/j.cyto.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wang Y, Li Q, Liu C, Han F, Chen M, Zhang L, et al. Protein arginine methyltransferase 5 (Prmt5) is required for germ cell survival during mouse embryonic development. Biology of reproduction. 2015;92(4). 10.1095/biolreprod.114.127308 [DOI] [PubMed] [Google Scholar]
- 122. Long X, Zhang M, Chen X, He J, Ding Y, Zhang C, et al. Expression of KRAS in the endometrium of early pregnant mice and its effect during embryo implantation. Reproductive biomedicine online. 2015;31(1):51–61. 10.1016/j.rbmo.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 123. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nature Medicine. 2012;18(12):1754 10.1038/nm.3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Tokyol C, Aktepe F, Husniye Dilek F, Yilmazer M. Comparison of Placental PTEN and β1 integrin expression in early spontaneous abortion, early and late normal pregnancy. Upsala journal of medical sciences. 2008;113(2):235–242. 10.3109/2000-1967-231 [DOI] [PubMed] [Google Scholar]
- 125. Du MR, Zhou WH, Dong L, Zhu XY, He YY, Yang JY, et al. Cyclosporin A promotes growth and invasiveness in vitro of human first-trimester trophoblast cells via MAPK3/MAPK1-mediated AP1 and Ca2+/calcineurin/NFAT signaling pathways. Biology of reproduction. 2008;78(6):1102–1110. 10.1095/biolreprod.107.063503 [DOI] [PubMed] [Google Scholar]
- 126. Contro E, Stefani L, Berto S, Lapucci C, Arcelli D, Prandstraller D, et al. Circulating mRNA in maternal plasma at the second trimester of pregnancy: a possible screening tool for cardiac conotruncal and left ventricular outflow tract abnormalities. Molecular diagnosis & therapy. 2017;21(6):653–661. 10.1007/s40291-017-0295-7 [DOI] [PubMed] [Google Scholar]
- 127. Fulop V, Mok SC, Genest DR, Gati I, Doszpod J, Berkowitz RS. p53, p21, Rb and mdm2 oncoproteins. Expression in normal placenta, partial and complete mole, and choriocarcinoma. The Journal of reproductive medicine. 1998;43(2):119–127. [PubMed] [Google Scholar]
- 128. Fraga LR, Boquett JA, Dutra CG, Vianna FS, Heck C, Gonçalves RO, et al. Interaction between TP63 and MDM2 genes and the risk of recurrent pregnancy loss. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2014;182:7–10. 10.1016/j.ejogrb.2014.07.044 [DOI] [PubMed] [Google Scholar]
- 129. Haye D, Collet C, Sembely-Taveau C, Haddad G, Denis C, Soulé N, et al. Prenatal findings in carpenter syndrome and a novel mutation in RAB23. American Journal of Medical Genetics Part A. 2014;164(11):2926–2930. 10.1002/ajmg.a.36726 [DOI] [PubMed] [Google Scholar]
- 130. Li Yw, Hou Xm, Ni H. Expression of Six bHLH Superfamily Members in Mouse Uterus During Early Pregnancy. Journal of Northeast Agricultural University (English Edition). 2013;20(4):39–45. 10.1016/S1006-8104(14)60045-3 [Google Scholar]
- 131. O’Brien M, Morrison JJ, Smith TJ. Upregulation of PSCDBP, TLR2, TWIST1, FLJ35382, EDNRB, and RGS12 gene expression in human myometrium at labor. Reproductive sciences. 2008;15(4):382–393. 10.1177/1933719108316179 [DOI] [PubMed] [Google Scholar]
- 132. Lynch S, Whatley S, Ramesh V, Sinha S, Ravine D. Sporadic case of fatal encephalopathy with neonatal onset associated with a T158M missense mutation in MECP2. Archives of Disease in Childhood-Fetal and Neonatal Edition. 2003;88(3):F250–F252. 10.1136/fn.88.3.F250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Guo J, Zou L. Correlation of RECK with matrix metalloproteinase-2 in regulation of trophoblast invasion of early pregnancy. Journal of Huazhong University of Science and Technology. 2006;26(6):738–740. 10.1007/s11596-006-0631-3 [DOI] [PubMed] [Google Scholar]
- 134. Hu SJ, Ren G, Liu JL, Zhao ZA, Yu YS, Su RW, et al. MicroRNA expression and regulation in mouse uterus during embryo implantation. Journal of Biological Chemistry. 2008;283(34):23473–23484. 10.1074/jbc.M800406200 [DOI] [PubMed] [Google Scholar]
- 135. Maruo T, Mochizuki M. Immunohistochemical localization of epidermal growth factor receptor and myc oncogene product in human placenta: implication for trophoblast proliferation and differentiation. American Journal of Obstetrics & Gynecology. 1987;156(3):721–727. 10.1016/0002-9378(87)90086-X [DOI] [PubMed] [Google Scholar]
- 136. Zhao KQ, Lin HY, Zhu C, Yang X, Wang H. Maternal Smad3 deficiency compromises decidualization in mice. Journal of cellular biochemistry. 2012;113(10):3266–3275. 10.1002/jcb.24204 [DOI] [PubMed] [Google Scholar]
- 137. Levy C, Robel P, Gautray J, De Brux J, Verma U, Descomps B, et al. Estradiol and progesterone receptors in human endometrium: normal and abnormal menstrual cycles and early pregnancy. American Journal of Obstetrics & Gynecology. 1980;136(5):646–651. 10.1016/0002-9378(80)91018-2 [DOI] [PubMed] [Google Scholar]
- 138.Athilakshmi K, Shanmugasundaram N, Li Q, DeMayo FJ, Lydon JP, Bagchi MK, et al. Runx1 Functions Downstream of BMP2 to Regulate Uterine Stromal Differentiation and Blood Vessel Formation at the Maternal-Fetal Interface.; 2011.
- 139. Li Y, Lu H, Ji Y, Wu S, Yang Y. Identification of genes for normalization of real-time RT-PCR data in placental tissues from intrahepatic cholestasis of pregnancy. Placenta. 2016;48:133–135. 10.1016/j.placenta.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 140. Gray CA, Abbey CA, Beremand PD, Choi Y, Farmer JL, Adelson DL, et al. Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biology of reproduction. 2006;74(2):383–394. 10.1095/biolreprod.105.046656 [DOI] [PubMed] [Google Scholar]
- 141. Prossler J, Chen Q, Chamley L, James J. The relationship between TGFβ, low oxygen and the outgrowth of extravillous trophoblasts from anchoring villi during the first trimester of pregnancy. Cytokine. 2014;68(1):9–15. 10.1016/j.cyto.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 142. Cario-Toumaniantz C, Reillaudoux G, Sauzeau V, Heutte F, Finet M, Chardin P, et al. Modulation of RhoA—Rho kinase-mediated Ca2+ sensitization of rabbit myometrium during pregnancy—role of Rnd3. The Journal of physiology. 2003;552(2):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lartey J, Smith M, Pawade J, Strachan B, Mellor H, Bernal AL. Up-regulation of myometrial RHO effector proteins (PKN1 and DIAPH1) and CPI-17 (PPP1R14A) phosphorylation in human pregnancy is associated with increased GTP-RHOA in spontaneous preterm labor. Biology of reproduction. 2007;76(6):971–982. 10.1095/biolreprod.106.058982 [DOI] [PubMed] [Google Scholar]
- 144. Musavi SA, Yamashita S, Fujihara T, Masaka H, Islam MR, Kim S, et al. Analysis of differentially expressed genes and the promoters in bovine endometrium throughout estrus cycle and early pregnancy. Animal Science Journal. 2018. 10.1111/asj.13091 [DOI] [PubMed] [Google Scholar]
- 145. Takahashi H, Ohkuchi A, Usui R, Takizawa T, Matsubara S, Suzuki M. Importance of chromosome 19 miRNA cluster in pregnancy. Obstet Gynecol. 2014;2(2):1032. [Google Scholar]
- 146. Fotovati A, Abu-Ali S, Nakayama K, Nakayama KI. Impaired ovarian development and reduced fertility in female mice deficient in Skp2. Journal of anatomy. 2011;218(6):668–677. 10.1111/j.1469-7580.2011.01370.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Su MT, Lin SH, Chen YC, Kuo PL. Gene-gene interactions and gene polymorphisms of VEGFA and EG-VEGF gene systems in recurrent pregnancy loss. Journal of assisted reproduction and genetics. 2014;31(6):699–705. 10.1007/s10815-014-0223-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ladyman S, Grattan D. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology. 2004;145(8):3704–3711. 10.1210/en.2004-0338 [DOI] [PubMed] [Google Scholar]
- 149. Poehlmann TG, Fitzgerald JS, Meissner A, Wengenmayer T, Schleussner E, Friedrich K, et al. Trophoblast invasion: tuning through LIF, signalling via Stat3. Placenta. 2005;26:S37–S41. 10.1016/j.placenta.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 150. Teng CB, Diao HL, Ma H, Cong J, Yu H, Ma XH, et al. Signal transducer and activator of transcription 3 (Stat3) expression and activation in rat uterus during early pregnancy. Reproduction. 2004;128(2):197–205. 10.1530/rep.1.00053 [DOI] [PubMed] [Google Scholar]
- 151. Lv X, Cai Z, Li S. Increased apoptosis rate of human decidual cells and cytotrophoblasts in patients with recurrent spontaneous abortion as a result of abnormal expression of CDKN1A and Bax. Experimental and therapeutic medicine. 2016;12(5):2865–2868. 10.3892/etm.2016.3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Shang W, Shu M, Liu M, Wang A, Lv L, Zhao Y, et al. Elevated expressions of p53, CDKNA1, and Bax in placental villi from patients with recurrent spontaneous abortion. Eur Rev Med Pharmacol Sci. 2013;17(24):3376–3380. [PubMed] [Google Scholar]
- 153. Li F, Devi YS, Bao L, Mao J, Gibori G. Involvement of cyclin D3, CDKN1A (p21), and BIRC5 (Survivin) in interleukin 11 stimulation of decidualization in mice. Biology of reproduction. 2008;78(1):127–133. 10.1095/biolreprod.107.063313 [DOI] [PubMed] [Google Scholar]
- 154. Shynlova O, Dorogin A, Lye SJ. Stretch-induced uterine myocyte differentiation during rat pregnancy: involvement of caspase activation. Biology of reproduction. 2010;82(6):1248–1255. 10.1095/biolreprod.109.081158 [DOI] [PubMed] [Google Scholar]
- 155. Kiewisz J, Kaczmarek MM, Andronowska A, Blitek A, Ziecik AJ. Gene expression of WNTs, β-catenin and E-cadherin during the periimplantation period of pregnancy in pigs-involvement of steroid hormones. Theriogenology. 2011;76(4):687–699. 10.1016/j.theriogenology.2011.03.022 [DOI] [PubMed] [Google Scholar]
- 156. Lim R, Riley C, Barker G, Rice G, Lappas M. Human labour is associated with decreased cytoplasmic FoxO4. Placenta. 2012;33(1):52–59. 10.1016/j.placenta.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 157. Lappas M, Lim R, Riley C, Menon R, Permezel M. Expression and localisation of FoxO3 and FoxO4 in human placenta and fetal membranes. Placenta. 2010;31(12):1043–1050. 10.1016/j.placenta.2010.09.009 [DOI] [PubMed] [Google Scholar]
- 158. Sati L, Soygur B, Celik-Ozenci C. Expression and localization of FoxO4 in normal term and gestational diabetic placentas. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2016;206:e104 10.1016/j.ejogrb.2016.07.274 [Google Scholar]
- 159. Boyle KE, Newsom SA, Janssen RC, Lappas M, Friedman JE. Skeletal muscle MnSOD, mitochondrial complex II, and SIRT3 enzyme activities are decreased in maternal obesity during human pregnancy and gestational diabetes mellitus. The Journal of Clinical Endocrinology & Metabolism. 2013;98(10):E1601–E1609. 10.1210/jc.2013-1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Liu G, Lin H, Zhang X, Li Q, Wang H, Qian D, et al. Expression of Smad2 and Smad4 in mouse uterus during the oestrous cycle and early pregnancy. Placenta. 2004;25(6):530–537. 10.1016/j.placenta.2003.11.006 [DOI] [PubMed] [Google Scholar]
- 161. Cesarini V, Silvestris DA, Tassinari V, Tomaselli S, Alon S, Eisenberg E, et al. ADAR2/miR-589-3p axis controls glioblastoma cell migration/invasion. Nucleic Acids Research. 2018;46(4):2045–2059. 10.1093/nar/gkx1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pinto Y, Buchumenski I, Levanon EY, Eisenberg E. Human cancer tissues exhibit reduced A-to-I editing of miRNAs coupled with elevated editing of their targets. Nucleic Acids Research. 2017;46(1):71–82. 10.1093/nar/gkx1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Zipeto MA, Sadarangani A, Santos NPD, Balaian L, Chun HJ, Pineda G, et al. ADAR1 activation drives leukemia stem cell self-renewal by impairing Let-7 biogenesis. Cell Stem Cell. 2016;19(2):177–191. 10.1016/j.stem.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Nigita G, Acunzo M, Romano G, Veneziano D, Laganà A, Vitiello M, et al. microRNA editing in seed region aligns with cellular changes in hypoxic conditions. Nucleic Acids Research. 2016;44(13):6298–6308. 10.1093/nar/gkw532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Telonis AG, Magee R, Loher P, Chervoneva I, Londin E, Rigoutsos I. Knowledge about the presence or absence of miRNA isoforms (isomiRs) can successfully discriminate amongst 32 TCGA cancer types. Nucleic Acids Research. 2017;45(6):2973–2985. 10.1093/nar/gkx082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Burroughs AM, Ando Y, de Hoon MJ, Tomaru Y, Nishibu T, Ukekawa R, et al. A comprehensive survey of 3’ animal miRNA modification events and a possible role for 3′ adenylation in modulating miRNA targeting effectiveness. Genome Research. 2010;20(10):1398–1410. 10.1101/gr.106054.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Newman MA, Mani V, Hammond SM. Deep sequencing of microRNA precursors reveals extensive 3’ end modification. RNA. 2011;17(10):1795–1803. 10.1261/rna.2713611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Boele J, Persson H, Shin JW, Ishizu Y, Newie IS, Søkilde R, et al. PAPD5-mediated 3’ adenylation and subsequent degradation of miR-21 is disrupted in proliferative disease. Proceedings of the National Academy of Sciences. 2014;111(31):11467–11472. 10.1073/pnas.1317751111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Thornton JE, Du P, Jing L, Sjekloca L, Lin S, Grossi E, et al. Selective microRNA uridylation by Zcchc6 (TUT7) and Zcchc11 (TUT4). Nucleic Acids Research. 2014;42(18):11777–11791. 10.1093/nar/gku805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ludwig N, Leidinger P, Becker K, Backes C, Fehlmann T, Pallasch C, et al. Distribution of miRNA expression across human tissues. Nucleic Acids Research. 2016;44(8):3865–3877. 10.1093/nar/gkw116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Gallo A, Vukic D, Michalík D, O’Connell MA, Keegan LP. ADAR RNA editing in human disease; more to it than meets the I. Human Genetics. 2017;136(9):1265–1278. 10.1007/s00439-017-1837-0 [DOI] [PubMed] [Google Scholar]
- 172. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9(6):654–659. 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 173. Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Molecular Cell. 2008;32(2):276–284. 10.1016/j.molcel.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 174. Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, et al. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138(4):696–708. 10.1016/j.cell.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 175. Heo I, Ha M, Lim J, Yoon MJ, Park JE, Kwon SC, et al. Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II let-7 microRNAs. Cell. 2012;151(3):521–532. 10.1016/j.cell.2012.09.022 [DOI] [PubMed] [Google Scholar]
- 176. Looney A, O’Sullivan M, Ahearne C, Finder M, Felderhoff-Mueser U, Boylan G, et al. Altered Expression of Umbilical Cord Blood Levels of miR-181b and Its Downstream Target mUCH-L1 in Infants with Moderate and Severe Neonatal Hypoxic-Ischaemic Encephalopathy. Molecular Neurobiology. 2018; p. 1–7. [DOI] [PubMed] [Google Scholar]
- 177. Bail S, Swerdel M, Liu H, Jiao X, Goff LA, Hart RP, et al. Differential regulation of microRNA stability. Rna. 2010;16(5):1032–1039. 10.1261/rna.1851510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wang J, Chen J, Sen S. MicroRNA as biomarkers and diagnostics. Journal of cellular physiology. 2016;231(1):25–30. 10.1002/jcp.25056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Choudhury Y, Tay FC, Lam DH, Sandanaraj E, Tang C, Ang BT, et al. Attenuated adenosine-to-inosine editing of microRNA-376a* promotes invasiveness of glioblastoma cells. The Journal of Clinical Investigation. 2012;122(11):4059–4076. 10.1172/JCI62925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: miR-128-3p, B: miR-29a-3p, C: miR-9-5p, D: miR-218-5p, E: 204-5p and F: miR-132-3p. Analysis of the expression patterns of these miRNAs revealed that all except miR-29a-3p (although it is enriched) are expressed almost exclusively in brain (highlighted with green boxes) [170].
(PNG)
miRNA are ranked by average abundance across all samples.
(CSV)
miRNA names were mapped to the mature human sequences from miRBase version 21 [57]. Blondal [75], Chen [13], Mitchell [15], Mooney [9], Wang–Exiqon [76], Wang–Taqman [76] and Weber [12].
(CSV)
a—acinar cell; b—adipocyte; c—ductal cell; d—endothelial; e—epithelial cell; f—fibroblast; g—hepatocyte; h—lymphatic EC; i—myocyte; j—neutrophil; k—smooth muscle cell; l—centroblast; m—memory B cell; n—monocyte; o—naïve B cell; p—NK cell; q—plasma B cell; and r—red blood cell. Cell types a—k are hematopoietic; Cell types l—r are hematopoietic. An asterix is plased in the column if the miRNA is found in the top 100 of miRNAs expressed in that cell type. Expression profiles for all cells taken from [18]. MiRNAs are included if they are identified in at least one cell type and are listed in order of average abundance across all samples (mean). SD—standard deviation.
(CSV)
(CSV)
Data Availability Statement
All MicroRNA profiling data files are available from the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) (accession number(s) GSE119002).