Abstract
We tested whether oxidized linoleic acid metabolites (OXLAM) are associated with pediatric metabolic syndrome (MetS) and a proatherogenic lipoprotein profile in 122 obese adolescents. Furthermore, we examined whether genetic and metagenomic factors can modulate plasma OXLAM concentrations by genotyping the fatty acid desaturase 1/2 (FADS) gene and by characterizing the gut microbiota. Subjects with MetS (n = 50) showed higher concentrations of 9- and 13-oxo-octadecadienoic acid (9- and 13-oxo-ODE) than subjects without MetS (n = 72). Both metabolites were associated with an adverse lipoprotein profile that was characterized by elevated very small-dense low-density lipoprotein (p < 0.005) and large very low-density lipoprotein particles (p = 0.01). Plasma 9- and 13-oxo-ODE were higher in subjects carrying the haplotype AA of the FADS gene cluster (p = 0.030 and p = 0.048, respectively). Furthermore, the reduced gut bacterial load was associated with higher 9-oxo-ODE concentrations (p = 0.035). This is the first study showing that high plasma OXLAM concentrations are associated with MetS and suggesting that the leading factors for high plasma concentrations of OXLAM might be the genetic background and the composition of the gut microbiota. In conclusion, high concentrations of 9- and 13-oxo-ODE, which may be the result of a genetic predisposition and a reduced gut bacterial load, are associated with MetS and with a proatherogenic lipoprotein profile in obese adolescents.
Keywords: genetic predisposition, gut microbiota, linoleic acid, metabolic syndrome, oxidized low-density lipoproteins, oxidized metabolites of linoleic acid, pediatric obesity
Introduction
Several studies have shown that pediatric metabolic syndrome (MetS) confers an increased risk for developing cardiovascular diseases in adulthood (2). The rising prevalence of MetS in the pediatric population, estimated to be about 30% in the United States, is closely linked to an unabated rise of childhood obesity and to deleterious changes in dietary habits occurring in Western countries over the past decades (2, 8). Although over-nutrition is fundamental to the development of obesity, the excess or deficiency of particular dietary components may contribute to the development of its complications, such as the MetS. In fact, recent studies showed that an increased ratio between dietary omega 6 (n-6) and omega 3 (n-3) polyunsaturated fatty acids (PUFAs) can lead to the development of an adverse cardiometabolic profile. In particular, linoleic acid (LA), an essential PUFA, and its oxidized metabolites (OXLAM) have been associated with intrahepatic fat accumulation, liver injury, impaired insulin secretion, and type 2 diabetes in the context of pediatric obesity (9). Despite this robust evidence, the role of LA and OXLAM, namely 9- and 13-hydroxy-octadecadienoic acid (9- and 13-HODE) and 9- and 13-oxo-octadecadienoic acid (9- and 13-oxo-ODE), in the development of MetS in youth has not been explored.
Innovation.
For the first time, this study shows that plasma concentrations of oxidized linoleic acid metabolites (OXLAM) are the result of genetic and metagenomic factors and that OXLAM might be responsible for the cardiometabolic derangement that some obese children and adolescents experience. These data are clinically relevant, as they pose the bases for nutritional therapies of the metabolic complications of pediatric obesity targeting both the human genotype and the gut microbiota.
To fill this knowledge void, in this study we assessed whether OXLAM levels are associated with MetS and with a proatherogenic lipoprotein profile in obese adolescents. Furthermore, to gain insights into potential causes that are relevant to changes in plasma OXLAM, we genotyped genetic variants in the fatty acid desaturase (FADS) gene cluster, a rate-limiting enzyme in the biosynthesis of n-6 PUFA byproducts (e.g., arachidonic acid), and explored the putative role of gut microbiota in modulating plasma OXLAM concentrations. We hypothesize that OXLAM would be increased in obese adolescents with MetS and that genetic variants in the FADS1/2 gene cluster as well as alterations of gut microbiota would modulate the changes in OXLAM levels.
To achieve these aims, we measured OXLAM concentrations by liquid chromatography-mass spectrometry (LC-MS) and lipoprotein profile by nuclear magnetic resonance (NMR) spectroscopy, and we genotyped the two major haplotypes (A and D) in the FADS1/2 genes cluster in a cohort of 122 obese children and adolescents (Fig. 1). Moreover, the gut microbiota was characterized by sequencing the region V4 of the 16S RNA gene in a subgroup of 60 subjects.
FIG. 1.
Study flowchart. Study participants were stratified according to the presence or absence of MetS. FADS, fatty acid desaturase; MetS, metabolic syndrome; rRNA, ribosomal RNA.
Plasma OXLAM Are Increased in Obese Adolescents with MetS
The main anthropometric and metabolic characteristics of the study population stratified according to the presence or absence of MetS are shown in Table 1. Among the 122 obese adolescents recruited, 52 subjects met the criteria of MetS and 70 did not. As expected, the group with MetS showed lower high-density lipoprotein (HDL) cholesterol and higher body mass index (BMI), z-score BMI, waist circumference, systolic and diastolic blood pressure, and triglyceride levels than subjects without MetS (p < 0.05 for all) (Table 1).
Table 1.
Clinical Features of the Study Population Stratified by the Presence or Absence of Metabolic Syndrome
| With MetS | Without MetS | p | |
|---|---|---|---|
| Anthropometric features | |||
| Age (years) | 13.05 ± 3.07 | 12.80 ± 2.89 | 0.94 |
| Gender (male/female)% | 47/53 | 57/43 | 0.72 |
| Race (Caucasian/African American/Hispanic/Asian)% | 48/8/36/8 | 26/28/40/6 | 0.03 |
| BMI (kg/m2) | 34.90 ± 5.86 | 31.77 ± 6.03 | 0.008 |
| BMI z-score | 2.43 ± 0.29 | 2.18 ± 0.45 | 0.004 |
| Body fat (%) | 44.7 ± 9.0 | 42.6 ± 9.6 | 0.23 |
| Waist circumference (cm) | 106.76 ± 11.99 | 99.07 ± 14.59 | 0.003 |
| Systolic pressure (mmHg) | 122.30 ± 9.55 | 118.35 ± 10.33 | 0.03 |
| Diastolic pressure (mmHg) | 71.24 ± 7.40 | 67.47 ± 7.32 | 0.01 |
| Glucose metabolism | |||
| Fasting glucose (mg/dL) | 94.92 ± 11.18 | 92.82 ± 6.96 | 0.69 |
| Fasting insulin (μU/mL) | 47.54 ± 24.05 | 32.02 ± 19.69 | <0.0001 |
| 2 h glucose (mg/dL) | 130.80 ± 36.17 | 125.14 ± 26.82 | 0.69 |
| HbA1C (%) | 5.50 ± 0.34 | 5.54 ± 0.32 | 0.55 |
| WBISI | 1.19 ± 0.58 | 2.01 ± 1.58 | 0.0005 |
| IGI | 5.23 ± 2.95 | 4.79 ± 3.53 | 0.10 |
| DI | 6.09 ± 5.37 | 7.71 ± 6.45 | 0.05 |
| Lipid profile | |||
| Total cholesterol (mg/dL) | 158.19 ± 30.36 | 150.24 ± 32.09 | 0.16 |
| HDL cholesterol (mg/dL) | 36.23 ± 6.56 | 46.10 ± 8.42 | <0.0001 |
| LDL cholesterol (mg/dL) | 91.56 ± 26.35 | 85.76 ± 23.35 | 0.31 |
| Triglycerides (mg/dL) | 160.94 ± 91.12 | 89.75 ± 55.76 | <0.0001 |
| Liver function | |||
| Alanine transaminase (U/L) | 53.76 ± 50.13 | 25.10 ± 18.34 | <0.0001 |
| Aspartate transaminase (U/L) | 38.28 ± 32.33 | 23.88 ± 8.92 | 0.001 |
Statistical comparisons between the two groups of obese adolescents with MetS (n = 52) or without MetS (n = 70) were made by either Student's t-tests for continuous variables or chi-square tests for categorical variables. A p value <0.05 was considered statistically significant and is indicated in bold italic.
BMI, body mass index; DI, disposition index; HDL, high-density lipoprotein; IGI, insulinogenic index; LDL, low-density lipoproteins; MetS, metabolic syndrome; WBISI, whole body insulin sensitivity index.
Plasma concentrations of LA (p = 0.045) as well as the ratio between LA and arachidonic acid (p = 0.0049) were significantly higher in obese adolescents with MetS compared with those without MetS, independent of age, gender, ethnicity, and z-score BMI (Fig. 2), despite similar dietary intakes of n-6 PUFAs (13.5 ± 6.3 g/day and 11.2 ± 4.6 g/day, respectively; p = 0.27). Among the oxidized fatty acids derived from LA metabolism (hydroxy-eicosatetraenoic acid [HETE], HODE, and oxo-ODE), only the concentrations of 9-oxo-ODE and 13-oxo-ODE were higher in adolescents with MetS (p = 0.002 and p = 0.0006, respectively) after adjustment for confounding factors (Fig. 2). Moreover, subjects with MetS showed a lower LA/oxo-ODE ratio than those without MetS (845 ± 493 and 1135 ± 669, respectively; p = 0.03).
FIG. 2.
Plasma OXLAM concentrations are higher in obese adolescents with MetS. Subjects with MetS (black bars, n = 52) compared with subjects without MetS (white bars, n = 70) showed higher Linoleic Acid concentrations (A; p = 0.045), Linoleic Acid to Arachidonic Acid ratio (B; p = 0.0049), 9-oxo-ODE concentrations (C; p = 0.004), and 13-oxo-ODE concentrations (D; p = 0.0006). Statistical comparisons between groups were made by using a general linear model after adjusting for age, gender, ethnicity, and z-score BMI. Data are expressed as mean ± SEM. BMI, body mass index; OXLAM, oxidized linoleic acid metabolites; Oxo-ODE, oxo-octadecadienoic acid; SEM, standard error of the mean.
These data clearly suggest a link between the amount of circulating OXLAM and the features of MetS in obese youth.
Plasma Concentrations of 9- and 13-oxo-ODE are Associated with a Proatherogenic Lipoprotein Profile
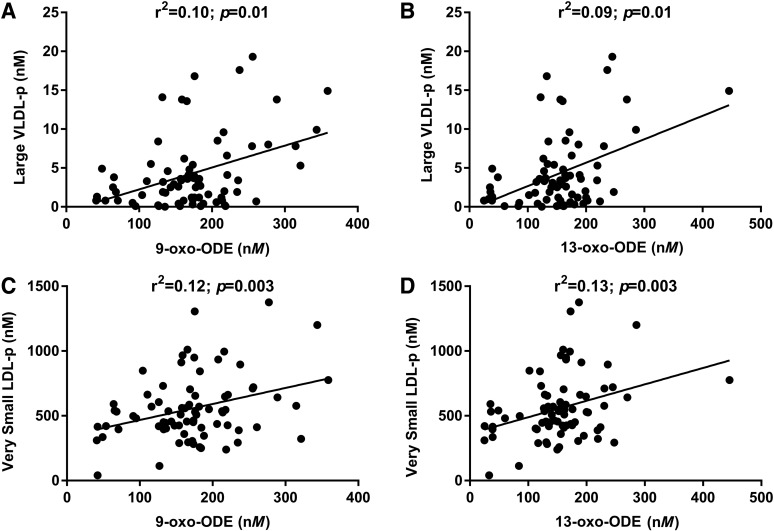
Since 9-oxo-ODE and 13-oxo-ODE were associated with MetS, we then tested whether they might be also associated with a proatherogenic lipoprotein profile. We observed that plasma levels of 9-oxo-ODE and 13-oxo-ODE were associated with large very low-density lipoprotein (VLDL) particles (both p = 0.01) and very small LDL particles (p = 0.003 and p = 0.003, respectively) (Fig. 3). Significant associations were also found between 9-oxo-ODE and 13-oxo-ODE and plasma concentrations of Apolipoprotein B-100 (p = 0.002 and 0.003, respectively), intermediate-density lipoprotein (IDL) particles (p = 0.05 and p = 0.02, respectively), total LDL particles (both p = 0.001), medium-small LDL particles (p = 0.003 and p = 0.001, respectively), and small LDL particles (p = 0.003 and p = 0.002, respectively) (Table 2). Moreover, both OXLAM were associated with circulating levels of triglycerides (9-oxo-ODE: r2 = 0.016, p = 0.01; 13-oxo-ODE: r2 = 0.016, p = 0.015) and total cholesterol (9-oxo-ODE: r2 = 0.08, p = 0.005; 13-oxo-ODE: r2 = 0.08, p = 0.01) after adjustment for confounding factors.
FIG. 3.
Plasma OXLAM concentrations are associated with lipoprotein levels. 9- and 13-oxo-ODE were associated with large VLDL particles (A, B; r2 = 0.10, p = 0.01 and r2 = 0.09, p = 0.01, respectively) and with very small LDL particles (C, D; r2 = 0.12, p = 0.003 and r2 = 0.13, p = 0.003, respectively). Correlations between variables were tested by using Spearman's rank correlations (n = 122). LDL, low-density lipoproteins; VLDL, very LDL.
Table 2.
Association Between Lipoprotein Particles and Concentrations of 9- and 13-Oxo-Octadecadienoic Acid
| 9-oxo-ODE | 13-oxo-ODE | |||||
|---|---|---|---|---|---|---|
| r | r2 | p | r | r2 | p | |
| Apolipoprotein B-100 | 0.36 | 0.13 | 0.003 | 0.39 | 0.15 | 0.002 |
| VLDL & chylomicron particles | 0.40 | 0.16 | 0.02 | 0.36 | 0.13 | 0.07 |
| Large | 0.31 | 0.10 | 0.01 | 0.30 | 0.09 | 0.01 |
| Medium | 0.37 | 0.13 | 0.09 | 0.33 | 0.11 | 0.19 |
| Small | 0.19 | 0.04 | 0.69 | 0.15 | 0.02 | 0.93 |
| IDL particles | 0.25 | 0.06 | 0.05 | 0.32 | 0.10 | 0.02 |
| LDL particles | 0.34 | 0.11 | 0.001 | 0.41 | 0.17 | 0.001 |
| Large | 0.03 | 0.001 | 0.51 | 0.04 | 0.002 | 0.38 |
| Medium-small | 0.23 | 0.06 | 0.003 | 0.31 | 0.09 | 0.001 |
| Small | 0.25 | 0.09 | 0.003 | 0.32 | 0.10 | 0.002 |
| Very small | 0.25 | 0.10 | 0.003 | 0.32 | 0.11 | 0.003 |
| HDL particles | 0.22 | 0.05 | 0.04 | 0.25 | 0.06 | 0.06 |
| Large | −0.05 | 0.003 | 0.53 | −0.05 | 0.003 | 0.42 |
| Medium | 0.19 | 0.03 | 0.13 | 0.20 | 0.04 | 0.10 |
| Small | 0.08 | 0.01 | 0.48 | 0.06 | 0.004 | 0.65 |
Correlations between variables were tested by using Spearman's rank correlations (n = 122). A p value <0.05 was considered statistically significant and is indicated in bold italic.
IDL, intermediate-density lipoproteins; VLDL, very low-density lipoproteins.
Based on this evidence, it is reasonable to speculate that, in the long term, obese adolescents with elevated OXLAM concentrations might experience a much higher risk of cardiovascular diseases than those with lower plasma OXLAM. In fact, high concentrations of circulating lipoproteins that are rich in oxidized fatty acids affect the cardiac structure and function and might participate in the development of atherosclerosis and hypertension through different mechanisms (7).
Haplotype in the FADS1/2 Gene Cluster is Associated with OXLAM Plasma Concentrations
To assess whether genetic variants in the FADS genes might affect OXLAM levels, we genotyped 17 single nucleotide polymorphisms (SNPs) in the FADS region and built the two most common haplotypes in the FADS1/2 genes cluster (D and A). Out of 122 subjects, 80 carried the two major haplotypes A or D in the FADS genes cluster (age 12.29 ± 3.10 years; gender 47 men/33 women; BMI 30.96 ± 6.78 kg/m2; z-score BMI 2.07 ± 0.74). Consistent with previous studies (1), the distribution of the two haplotypes was different among ethnic groups (p = 0.004). Among the Caucasians, 23 subjects were homozygous for the D haplotype (DD), 13 were heterozygous (DA), and 2 were homozygous for the A haplotype (AA); among the African Americans, 1 was AA and 1 was DD; in the group of Hispanics, 7 were DD, 13 were DA, and 14 were AA; and of the Asian Americans, 3 were DD, 1 was DA, and 2 were AA. Subjects carrying the haplotype AA showed lower concentrations of arachidonic acid (p = 0.040) and higher plasma concentrations of 9-oxo-ODE (p = 0.030) and 13-oxo-ODE (p = 0.044) (Fig. 4). Moreover, subjects carrying the haplotype AA showed a higher LA to arachidonic acid ratio (p < 0.0001) (Fig. 4). LA is an essential fatty acid, introduced in the human body exclusively through the diet. The conversion of LA to its derivatives, primarily the arachidonic acid, is done through a series of processes, including the desaturation after the fifth and sixth carbon atom introduced by the rate-limiting enzymes D-5 and D-6 FADS (1). In this study, we show that subjects with the AA haplotype in the region containing the genes FADS1 and FADS2 encoding the D-5 and D-6 FADS show a higher LA to arachidonic acid ratio and higher 9- and 13-oxo-ODE plasma levels. This finding is consistent with recent data obtained by genome-wide genotyping and targeted resequencing of the region containing the FADS gene in humans showing that the A haplotype in the FADS gene is much less efficient than the D haplotype in converting the precursor LA to arachidonic acid (1).
FIG. 4.
Plasma polyunsaturated fatty acid concentrations are modulated by the FADS haplotype. Subjects homozygous for the A haplotype (black bars, n = 19) compared with the heterozygous (gray bars, n = 27) and the D homozygous (white bars, n = 34) showed lower concentrations of Arachidonic Acid (A; p = 0.04), a lower ratio between the Linoleic and the Arachidonic Acid (B; p < 0.0001), and higher plasma concentrations of 9-oxo-ODE (C; p = 0.03) and 13-oxo-ODE (D; p = 0.04). Statistical comparisons between groups were made by using a general linear model after adjusting for age, gender, ethnicity, and z-score BMI. Data are expressed as mean ± SEM.
These observations corroborate the hypothesis that individuals carrying the A haplotype in the FADS gene cluster have a lower ability to convert LA in long-chain PUFAs as compared with those homozygous for the D haplotype, thereby increasing plasma LA availability for alternative metabolic pathways such as oxidation.
Plasma OXLAM Concentrations are Associated with Gut Bacterial Load
To explore the potential link between the gut microbiota and plasma OXLAM, stool samples were collected in a subgroup of 60 subjects (45 without and 15 with MetS; age 13.3 ± 3.1 years; gender 32 men/28 women; ethnicity 20 Caucasian/16 African American/23 Hispanic/1 Asian; BMI 32.7 ± 6.1 kg/m2; z-score BMI 2.2 ± 0.5). The average bacterial load was 5.5 × 105 ± 3.0 × 105 copies of 16S/ng of DNA in all subjects. Obese adolescents with MetS showed a significantly lower bacterial load (4.8 × 105 ± 3.1 × 105 copies/ng) than subjects without MetS (5.8 × 105 ± 3.0 × 105 copies/ng, p = 0.02). Notably, lower bacterial loads were associated with higher 9-oxo-ODE concentrations (r2 = 0.093; p = 0.035), and a similar trend was observed for 13-oxo-ODE (r2 = 0.048; p = 0.09) and LA (r2 = 0.067; p = 0.065). Among the genera examined, 9-oxo-ODE and 13-oxo-ODE concentrations showed a nominal association with Actinomyces, Bifidobacterium, Lactobacillus, Ruminococcus, Sarcina, Turicibacter, Varibaculum, Haemophilus, Lachnobacterium, and Veillonella (Table 3). However, none of them remained statistically significant after applying a Bonferroni correction for multiple comparisons (p < 9.8 × 10−4), probably owing the small sample size. Plasma 9- and 13-oxo-ODE were associated with lower bacterial load and with a lower prevalence in the gut of genera composed by bacteria that avidly convert LA to conjugated LA (CLA), such as Bifidobacterium and Lactobacillus (5).
Table 3.
Association Between the Genera Composing the Gut Microbiota and Plasma Concentrations of 9- and 13-Oxo-Octadecadienoic Acid
| 9-oxo-ODE | 13-oxo-ODE | |||||
|---|---|---|---|---|---|---|
| Genus (%) | r | r2 | p | r | r2 | p |
| Ruminococcus | −0.329 | 0.108 | 0.003 | −0.206 | 0.042 | 0.030 |
| Haemophilus | 0.081 | 0.006 | 0.013 | 0.139 | 0.019 | 0.011 |
| Varibaculum | −0.299 | 0.089 | 0.015 | −0.209 | 0.043 | 0.086 |
| Veillonella | 0.095 | 0.009 | 0.018 | 0.140 | 0.019 | 0.016 |
| Sarcina | −0.285 | 0.081 | 0.019 | −0.222 | 0.049 | 0.026 |
| Lactobacillus | −0.247 | 0.061 | 0.020 | −0.267 | 0.071 | 0.008 |
| Turicibacter | −0.292 | 0.085 | 0.023 | −0.235 | 0.055 | 0.038 |
| Actinomyces | −0.230 | 0.053 | 0.026 | −0.306 | 0.093 | 0.002 |
| Bifidobacterium | −0.310 | 0.096 | 0.031 | −0.226 | 0.051 | 0.053 |
| Lachnobacterium | 0.252 | 0.063 | 0.046 | 0.325 | 0.106 | 0.031 |
| Catenibacterium | 0.090 | 0.008 | 0.073 | 0.078 | 0.006 | 0.141 |
| Acidaminococcus | −0.043 | 0.001 | 0.133 | 0.026 | 0.001 | 0.085 |
| Perphyromonas | −0.178 | 0.031 | 0.135 | −0.061 | 0.004 | 0.666 |
| Dorea | −0.068 | 0.004 | 0.139 | −0.094 | 0.009 | 0.221 |
| Bacteroides | 0.081 | 0.006 | 0.169 | 0.188 | 0.035 | 0.060 |
| Clostridium | −0.190 | 0.036 | 0.182 | −0.157 | 0.024 | 0.142 |
| Parabacterioides | 0.100 | 0.010 | 0.234 | 0.188 | 0.035 | 0.132 |
| Odoribacter | 0.105 | 0.011 | 0.266 | 0.179 | 0.032 | 0.099 |
| Lactococcus | −0.174 | 0.030 | 0.280 | −0.143 | 0.020 | 0.309 |
| Peptoniphilus | −0.157 | 0.024 | 0.294 | −0.053 | 0.002 | 0.716 |
| Faecalibacterium | 0.040 | 0.001 | 0.301 | 0.051 | 0.002 | 0.322 |
| Butyricimonas | −0.129 | 0.016 | 0.356 | −0.112 | 0.012 | 0.511 |
| Coprococcus | −0.077 | 0.005 | 0.365 | −0.078 | 0.006 | 0.459 |
| Enterobacter | −0.189 | 0.035 | 0.381 | −0.102 | 0.010 | 0.411 |
| Sutterella | −0.044 | 0.020 | 0.385 | 0.199 | 0.039 | 0.100 |
| Holdemania | −0.205 | 0.042 | 0.425 | −0.090 | 0.008 | 0.707 |
| Coprobacillus | 0.087 | 0.007 | 0.448 | 0.069 | 0.004 | 0.958 |
| Roseburia | 0.188 | 0.035 | 0.465 | 0.218 | 0.047 | 0.278 |
| Streptococcus | −0.137 | 0.018 | 0.478 | −0.206 | 0.042 | 0.214 |
| Peptococcus | −0.048 | 0.002 | 0.493 | −0.011 | 0.0001 | 0.555 |
| Methanobrevibacter | −0.080 | 0.006 | 0.504 | −0.117 | 0.013 | 0.375 |
| Synergistes | 0.045 | 0.002 | 0.506 | 0.055 | 0.003 | 0.778 |
| Prevotella 2 | −0.074 | 0.005 | 0.517 | −0.012 | 0.0002 | 0.335 |
| Anaerostipes | 0.086 | 0.007 | 0.522 | 0.093 | 0.008 | 0.355 |
| Collinsella | −0.123 | 0.015 | 0.527 | −0.103 | 0.010 | 0.434 |
| Akkermansia | −0.126 | 0.015 | 0.561 | −0.042 | 0.001 | 0.405 |
| Phascolarctobacterium | −0.171 | 0.029 | 0.586 | −0.058 | 0.003 | 0.542 |
| Paraprevotella | −0.068 | 0.004 | 0.618 | −0.009 | 0.0001 | 0.804 |
| Eubacterium | −0.021 | 0.0004 | 0.621 | 0.053 | 0.002 | 0.711 |
| Megasphaera | −0.018 | 0.0003 | 0.625 | −0.066 | 0.004 | 0.580 |
| Eggerthella | 0.047 | 0.002 | 0.635 | 0.065 | 0.004 | 0.475 |
| Bilophila | 0.001 | <0.0001 | 0.646 | 0.021 | 0.0005 | 0.447 |
| Megamonas | 0.095 | 0.009 | 0.690 | 0.092 | 0.008 | 0.634 |
| Blautia | 0.082 | 0.006 | 0.789 | 0.026 | 0.006 | 0.982 |
| Oscillospira | −0.012 | 0.0001 | 0.891 | 0.043 | 0.001 | 0.896 |
| Prevotella | −0.010 | 0.0001 | 0.892 | 0.034 | 0.001 | 0.800 |
| Dialister | −0.108 | 0.011 | 0.931 | 0.033 | 0.001 | 0.418 |
| Lachnospira | −0.016 | 0.002 | 0.932 | 0.018 | 0.0003 | 0.942 |
| Escherichia | 0.016 | 0.0002 | 0.934 | −0.002 | <0.0001 | 0.869 |
| Desulfovibrio | −0.062 | 0.003 | 0.952 | −0.095 | 0.009 | 0.937 |
Correlations between variables were tested by using Spearman's rank correlations (n = 60). A Bonferroni-corrected p value of <9.8·10−4 was considered statistically significant and is indicated by bold italic.
The conversion of LA to CLA represents a possible detoxification mechanism protecting healthy gut bacteria from the inhibiting effect of LA (6), which, in turn, may favor the growth of endotoxin-producing bacteria that may trigger a systemic low-grade inflammation. Based on these observations, it could be speculated that a reduced conversion of LA into CLA by gut microbiota increases intestinal absorption and plasma concentrations of LA, although an effect of the dietary LA in shaping the gut microbiota cannot be excluded (3).
Strengths and Limitations
Strengths of our study are as follows: (i) the young age of the study population, which is therefore free from some confounders present in adults, such as smoking, alcohol consumption, and aging; (ii) the accurate clinical evaluation of the study population; and (iii) the combined assessment of genetic and metagenomic factors contributing to LA metabolism. The most important limitation of our study is the lack of longitudinal data in a large population of youth. Indeed, the longitudinal follow-up of a large cohort of youngsters would allow: (i) to document the long-term implications of high levels of plasma OXLAM; (ii) to define the plasma concentrations beyond which the cardiovascular risk is increased (clinical thresholds); and (iii) to individuate those patients who would benefit the most from nutritional interventions that are aimed at lowering OXLAM concentrations.
Concluding Remarks and Future Directions
In this study, we observed that obese adolescents with MetS have higher plasma concentrations of OXLAM, namely 9-oxo-ODE and 13-oxo-ODE, than those without MetS. In addition, we showed that plasma 9- and 13-oxo-ODE were associated with a proatherogenic lipoprotein profile. Also, plasma levels of OXLAM were associated with the AA haplotype in the FADS1/2 region and with a lower intestinal bacterial load. Altogether, these findings suggest that OXLAM may lead to the development of MetS early on and that elevated 9- and 13-oxo-ODE concentrations may be sustained by an increased bioavailability of LA in obese adolescents with MetS. Higher plasma bioavailability of LA may be due to the AA haplotype in FADS gene and to a lower bacterial load.
In the presence of diets that are rich in LA, such as the American diet, the cooccurrence of a less efficient conversion of LA to arachidonic acid and CLA may represent the perfect storm leading to the metabolic derangement in obese adolescents. In fact, in the context of a dysmetabolic environment that is characterized by a subtle inflammation, typical of the obesity and MetS, the increased bio-availability of LA would result in an increased formation of OXLAM, particularly 9- and 13-oxo-ODE, which, in turn, would enrich circulating lipoproteins and accumulate in atherogenic plaques and tissues, such as the liver (9), therefore worsening the overall metabolic state of the individual.
These data suggest that therapeutic opportunities targeting OXLAM might be useful in the treatment of obese youth with MetS. There is a compelling need to conduct studies that are focused on youth, both to understand disease etiology and, related to the personalized/precision medicine theme, to better tailor prevention and treatment approaches to this age group.
Notes
Study cohort
We enrolled 122 obese adolescents who were recruited from the Yale Pediatric Obesity Clinic (4). The clinical features of the study population stratified according to the presence of MetS are shown in Table 1. The MetS was defined by the presence of three or more of the following criteria: waist circumference ≥90th percentile, fasting glucose ≥110 mg/dL, blood pressure ≥90th percentile, triglyceride ≥110 mg/dL, and HDL ≤40 mg/dL. All subjects underwent a standard oral glucose tolerance test by using 1.75 g glucose/kg body weight (up to 75 g glucose) (4), the measurement of OXLAM by LC-MS, the assessment of lipoprotein profile by magnetic resonance spectroscopy, and the genotyping of the two major haplotypes (A and D) in the FADS gene cluster, and they completed a 3-day food record. A subgroup of 60 subjects underwent the characterization of gut microbiota by genotyping the 16S RNA gene. All metabolic studies were performed at the Yale Center for Clinical Investigation at 8:00 AM after a 10- to 12-h overnight fast.
The study was conducted according to the principles expressed in the Declaration of Helsinki and approved by the Yale University Human Investigation Committee. Written parental informed consent and written child assent were obtained from all participants.
Biochemical analyses
Plasma glucose was determined by using a glucose analyzer by the glucose oxidase method (Beckman Instruments, Brea, CA). Plasma insulin was measured by the Linco radioimmunoassay, lipid levels were determined with an auto-analyzer (model 747-200), and liver enzymes were measured by using standard automated kinetic enzymatic assays (4).
Lipoprotein analyses
Fasting plasma samples were obtained to determine lipoprotein particle concentrations and sizes. The analyses were conducted with a 400-MHz proton NMR analyzer at Liposcience (Raleigh, NC). The concentration of each lipoprotein subclass was measured by the amplitude of its characteristic lipid-methyl group NMR signal and reported in particle concentration units (nM for VLDL, IDL, and LDL and μM for HDL particles). Plasma lipoproteins were separated into 10 subclass categories: large VLDL (including chylomicrons) (>60 nm), medium VLDL (35–60 nm), small VLDL (27–35 nm), IDL (23–27 nm), large LDL (21.2–23 nm), medium-small LDL (19.8–21.2 nm), very small LDL (18–19.8 nm), large HDL (8.8–13 nm), medium HDL (8.2–8.8 nm), and small HDL (7.3–8.2 nm).
Lipid extraction from human plasma
Lipid extractions and protein hydrolyses were performed by using disposable threaded borosilicate glass test tubes with PTFE-lined caps. Before use, all glassware tubes, caps, and pipette tips were washed with nitric acid to remove trace transition metals, extensively rinsed with Chelex-treated water containing 1 μM diethylenetriaminepentaacetic acid (DTPA; pH 7.0 in H2O), and finally rinsed with pure Chelex-treated water. Plastic tips were further rinsed in methanol and air-dried before use. Test tubes were also baked at 500°C overnight to remove residual potential organics. All plasma samples for analyses contained antioxidant cocktail (DTPA [2 mM final] and butylated hydroxytoluene [500 μM final]), with head space overlaid with argon. Samples were thawed in an ice/water bath immediately before sample handling for LS/tandem MS analysis. Fatty acids and oxidized fatty acids in plasma were extracted as previously described. Briefly, plasma (50 μL), internal standard (synthetic 15(S)-HETE-d8), and potassium hydroxide were added to the glass test tubes, overlaid with argon, and sealed. Lipids were hydrolyzed at 60°C under an argon atmosphere for 2 h; then, the released fatty acids were extracted into the hexane layer twice by liquid/liquid extraction. After each extraction, argon was used to purge the head space of the tube before sealing and vortexing/centrifuging. The combined hexane layers were dried under nitrogen gas and then resuspended in 200 μL of 85% methanol/water (v/v).
LS online electrospray ionization tandem MS
Plasma levels of multiple products of fatty acid oxidation (free and esterified) were quantified by using LS online electrospray ionization tandem MS (LC/ESI/MS/MS). Briefly, lipid extract was injected onto a high-performance LC (Waters 2690 Separations Module, Franklin, MA), and the oxidized fatty acids and their precursors were separated through a C18 column (Phenomenex ODS, 2 × 150 mm, 5 μm; Rancho Palos Verdes, CA) by using a gradient starting from 85% methanol containing 0.2% acetic acid over 10 min and then to 100% methanol containing 0.2% acetic acid over 2 min, followed by 100% methanol containing 0.2% acetic acid for 15 min. The oxidized fatty acids and their precursors were quantified on a triple quadrupole mass spectrometer (Quattro Ultima; Micromass, Manchester, UK) by using ESI in the negative ion mode and multiple reaction monitoring using characteristic parent/daughter ion transitions for the specific molecular species monitored. The lipid peroxidation products analyzed included structurally specific species of HODEs (9 and 13), oxo-ODEs (9 and 13), and their precursor LA. Next, 15-HETE-d8 (Cayman Chemicals, Ann Arbor, MI) was used as an internal standard for the calibration of oxidized fatty acids in plasma, as previously described. One of the main products of LA metabolism, the Arachidonic Acid was also measured.
FADS gene analysis
Seventeen SNPs (rs1535, rs174545, rs174546, rs174547, rs174548, rs174549, rs174550, rs174555, rs174556, rs174560, rs174561, rs174562, rs174568, rs174574, rs174576, rs174577, and rs174581) were genotyped by Sequenom Massarray to characterize the FADS gene cluster haplotypes. The A and D FADS haplotype were assigned based on genotypes of all SNPs by using PHASE. Individuals carrying rare or ambiguous (confidence <95%) haplotypes were excluded from the subsequent analyses.
Microbial DNA extraction, polymerase chain reaction amplification, and sequencing of taxonomic marker
DNA was extracted from a 0.25 g fecal sample by using the MoBio PowerMag Soil 96-well kit (MoBio Laboratories, Inc.). DNA extracts were quantified by using the Quant-iT PicoGreen Kit (Invitrogen; ThermoFisher Scientific). Partial bacterial 16S ribosomal RNA (rRNA) genes (V4) were amplified by using 30 ng extracted DNA as a template. The V4 region of the 16S rRNA gene was amplified by using 515F and 806R with Illumina adapters and golay indices on the 3′ end. Samples were amplified in triplicate by using Phusion High-Fidelity polymerase chain reaction (PCR) master mix (New England BioLabs), with the addition of 10 μg Bovine serum albumin (New England BioLabs). PCR products were pooled for quantification and visualization by using the QIAxcel DNA Fast Analysis (Qiagen). The pooled PCR products were cleaned by using the Gene Read Size Selection kit (Qiagen) and then sequenced on the MiSeq by using v2 2 × 250 base pair kit. Total 16S rRNA sequences for each sample were quantified by using universal bacteria primers 27F (3′-AGAGTTTGATCMTGGCTCAG-5′) and 519R (3′-GWATTACCGCGGCKGCTG-5′). Reactions contained 1x SsoAdvanced™ Universal Inhibitor-Tolerant SYBR® Green Supermix (BioRad), 0.4–0.5 μM of each primer, and 1 μL sample. Data were processed by using CFX Manager™ (BioRad). Reported values are an average of ≥4 replicates for each sample.
Dietary composition
Subjects were instructed on completing a 3-day food record, including 2 week days and 1 weekend day. No dietary instruction was given to the participants or their families before the completion of the food records. A registered dietitian checked the food records with the subjects for accuracy, including specific quantity, product brand name, and cooking method details. The dietary fat composition was analyzed by using the Nutrition Data System for Research software program (NDS-R version 2011), which was developed by the Nutrition Coordinating Center at the University of Minnesota (www.ncc.umn.edu).
Statistical analyses
The primary goal of this study was to test the null hypothesis that mean concentrations of plasma OXLAM are equal between obese adolescents with and without MetS. Based on our previous study (9), a sample size of 50 subjects for each group was calculated to provide at least 80% power to detect a difference in plasma OXLAM levels of 50 nM, deemed clinically significant, by using a two-tailed test and a significance level of 5%. Before data analysis, all variables were tested for normal distribution and appropriately log-transformed, when required, to approximate univariate normality before association analyses, except for the populations composing the gut microbiota, for which an inverse normal transformation was used. Statistical comparisons between clinical and metabolic characteristics of the two groups of adolescents with and without MetS and with different haplotypes were made by using a general linear model after adjusting for age, gender, ethnicity, and z-score BMI when needed. A chi-square test was used to compare prevalences between groups. Correlations between variables were tested by using either Spearman's rank correlations or Pearson's correlations, as appropriate. Statistical tests were conducted by using a two-sided α level of 0.05. A Bonferroni correction for multiple comparisons was applied when appropriate. Data are expressed as mean ± standard deviation, unless otherwise specified. Statistical analyses were performed by using SAS® 9.4 (SAS Institute, Inc., Cary, NC).
Acknowledgments
The authors are grateful to the patients and their families as well as to the Yale Center for Genome Analysis and the Yale Center for Clinical Investigation and Hospital Research Unit personnel. The authors thank Zhipeng Liu from Purdue University in Prof. Wanqing Liu's laboratory for providing assistance with fatty acid desaturase haplotype data. The authors also thank Mary Savoye from the Department of Pediatrics at the Yale School of Medicine for providing assistance with the collection and analysis of diet composition data. N.S. is funded by the American Heart Association (Grant Nos. 13SDG14640038 and 16IRG27390002) and by the Allen foundation. S.C. is funded by the National Institutes of Health (NIH) (Grant Nos. R01-HD-40787 and R01-HD-28016) and the American Diabetes Association (Distinguished Clinical Scientist Awards from the American Diabetes Association, DK-49230). This work was also made possible by DK-045735 to the Yale Diabetes Endocrinology Research Center and by Clinical and Translational Science Awards Grant UL1-RR-024139 from the National Center for Advancing Translational Sciences, a component of the NIH, and the NIH Roadmap for Medical Research. The contents of this scientific contribution are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Abbreviations Used
- BMI
body mass index
- CLA
conjugated linoleic acids
- DTPA
diethylenetriaminepentaacetic acid
- FADS
fatty acid desaturase
- HDL
high-density lipoprotein
- HETE
hydroxy-eicosatetraenoic acid
- HODE
hydroxy-octadecadienoic acid
- IDL
intermediate-density lipoproteins
- LA
linoleic acid
- LC-MS
liquid chromatography-mass spectrometry
- LDL
low-density lipoproteins
- MetS
metabolic syndrome
- NIH
National Institutes of Health
- NMR
nuclear magnetic resonance
- OXLAM
oxidized linoleic acid metabolites
- PCR
polymerase chain reaction
- PUFAs
polyunsaturated fatty acids
- rRNA
ribosomal RNA
- SEM
standard error of the mean
- SNPs
single nucleotide polymorphisms
- VLDL
very low-density lipoproteins
Authors' Contributions
D.T., A.D.S., S.C., and N.S. analyzed and reviewed the data and wrote and edited the article. R.I.H. and G.R.U. helped with the data analysis. N.G., W.L., and T.L. genotyped the single nucleotide polymorphisms composing the fatty acid desaturase haplotype. J.G. performed the microbiome analyses. A.E.F. and C.D.J. measured the OXLAM. D.T., A.D.S., S.C., and N.S. are responsible for the integrity of the data and the accuracy of the data analysis.
References
- 1. Ameur A, Enroth S, Johansson A, Zaboli G, Igl W, Johansson AC, Rivas MA, Daly MJ, Schmitz G, Hicks AA, Meitinger T, Feuk L, van Duijn C, Oostra B, Pramstaller PP, Rudan I, Wright AF, Wilson JF, Campbell H, and Gyllensten U. Genetic adaptation of fatty-acid metabolism: a human-specific haplotype increasing the biosynthesis of long-chain omega-3 and omega-6 fatty acids. Am J Hum Genet 90: 809–820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D'Adamo E, Santoro N, and Caprio S. Metabolic syndrome in pediatrics: old concepts revised, new concepts discussed. Pediatr Clin North Am 58: 1241–1255, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Ghosh S, Molcan E, DeCoffe D, Dai C, and Gibson DL. Diets rich in n-6 PUFA induce intestinal microbial dysbiosis in aged mice. Br J Nutr 110: 515–523, 2013 [DOI] [PubMed] [Google Scholar]
- 4. O'Malley G, Santoro N, Northrup V, D'Adamo E, Shaw M, Eldrich S, and Caprio S. High normal fasting glucose level in obese youth: a marker for insulin resistance and beta cell dysregulation. Diabetologia 53: 1199–1209, 2010 [DOI] [PubMed] [Google Scholar]
- 5. O'Shea EF, Cotter PD, Stanton C, Ross RP, and Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol 152: 189–205, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Polan CE, McNeill JJ, and Tove SB. Biohydrogenation of Unsaturated Fatty Acids by Rumen Bacteria. J Bacteriol 88: 1056–1064, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rietzschel ER, Langlois M, De Buyzere ML, Segers P, De Bacquer D, Bekaert S, Cooman L, Van Oostveldt P, Verdonck P, De Backer GG, and Gillebert TC. Oxidized low-density lipoprotein cholesterol is associated with decreases in cardiac function independent of vascular alterations. Hypertension 52: 535–541, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Santoro N, Amato A, Grandone A, Brienza C, Savarese P, Tartaglione N, Marzuillo P, Perrone L, and Miraglia Del Giudice E. Predicting metabolic syndrome in obese children and adolescents: look, measure and ask. Obes Facts 6: 48–56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santoro N, Caprio S, Giannini C, Kim G, Kursawe R, Pierpont B, Shaw MM, and Feldstein AE. Oxidized fatty acids: A potential pathogenic link between fatty liver and type 2 diabetes in obese adolescents? Antioxid Redox Signal 20: 383–389, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]