Abstract
A laboratory zebrafish colony developed red masses, predominantly under the jaw, in a significant portion of the population. The masses were diagnosed histopathologically as thyroid follicular hyperplasia, adenoma, or carcinoma in accordance with published morphologic criteria. After switching to a higher iodine brand of salt used to maintain a low level of salinity within the water system and a small diet change, the thyroid lesions regressed dramatically. Within 5 months the masses were no longer grossly visible. At the population level, external evaluations and histopathological assessments of whole-body sections document a regression in the prevalence of thyroid neoplasia and hyperplasia to normal thyroid conformation by 11 months after salt change. These findings suggest that a wide range of proliferative thyroid lesions, including neoplasms, in zebrafish may be hormone-dependent, even following lesion development. In addition, these results suggest that zebrafish have an adaptive ability to absorb iodine from water and food, which should be considered in discussions to standardize diets and when describing environmental parameters in publications.
Keywords: thyroid, iodine, zebrafish, hyperplasia, neoplasia
Introduction
The thyroid gland produces thyroid hormone (TH), which has important roles in metabolism, development, and growth. Alternations in TH can have important consequences, and the occurrence of thyroid pathology is of concern in populations across the globe.1 Data from the World Health Organization indicates that in most countries the incidence of thyroid cancer has been increasing since 1970.2 Studies utilizing zebrafish (Danio rerio) have provided significant advances in our understanding of carcinogenesis,3 toxicology,4 and early-stage development.5 Zebrafish have also been utilized in thyroid studies.6,7 Several traits make zebrafish well suited for these studies; the hypothalamic-pituitary-thyroid axis is well conserved in teleosts, the genetic backgrounds of many strains are well established, and a wide variety of transgenic models are readily available.
Given the utility of laboratory zebrafish, it is important to appreciate the factors that could alter normal thyroid anatomy and function and the impacts that subclinical thyroid disease could have on research endpoints. Similarly, it is valuable to understand whether steps can be taken to reverse or limit these changes with a goal of minimizing background variables to enhance reproducibility of data.
Unlike in mammals, the thyroid tissue of teleosts is not organized as an encapsulated discrete organ. In most fishes, including zebrafish, the thyroid develops as a series of scattered follicles within the ventral pharynx and it typically follows the course of the ventral aorta between the first gill arch and the bulbus arteriosus.7 Thyroid follicles are normally composed of flattened to cuboidal follicular epithelial cells that line a round-to-oval, colloid-filled lumen. The follicular epithelial cells produce TH, release it into the colloid for storage, and control secretion of the hormone from the colloid into the bloodstream. In zebrafish reared at 28.5°C, the first follicle is expressing TH at 72 h post-fertilization and becomes the most anterior follicle, with subsequent follicles developing caudally.8,9
Ectopic thyroid follicles in non-pharyngeal anatomic locations have been observed occasionally in a variety of fish species.10 For example, non-pharyngeal follicles occur occasionally in Japanese medaka (Oryzias latipes) and are common in goldfish (Carassius auratus),11 but are a relatively infrequent finding in zebrafish.
In many vertebrate taxa, thyroid follicular hyperplasia (goiter) can be a response to a variety of different stimuli, the common result of which is elevated production and release of thyroid-stimulating hormone (TSH) by the pituitary. Follicular hyperplasia is often associated with iodine deficiency, because iodine is required for TH (thyroxine) synthesis. The reduction in circulating thyroxine that occurs as a consequence of iodine deficiency results in the release of increased TSH from the pituitary through a classic negative feedback mechanism. In coho salmon, elevated TSH induces a hyperplasic response characterized by increased thickening of the thyroid follicular epithelium and follicle proliferation.12
Reported instigators of thyroid hyperplasia in fish, other than iodine, include poor water quality, pollutants, genetic anomalies, goitrogens, and temperature.10,13,14 When the hyperplastic stimulus is removed, follicular hyperplasia is potentially reversible.15 However, there is also evidence that hyperplasia can progress to neoplasia in at least some vertebrate species. For example, this apparent progression has been noted in laboratory rodents and Japanese medaka.16,17
Interestingly, iodine deficiency, a common etiology of thyroid follicular hyperplasia, has also been linked to the development of thyroid neoplasia in both humans and animals.1 However, distinguishing between extensive thyroid follicular hyperplasia and thyroid neoplasia (adenomas and carcinomas) can be especially difficult in fish because of the propensity of thyroid hyperplasia to occur in non-pharyngeal sites (i.e., ectopic thyroid), which can mimic multicentric tumor development or metastasis.17
Fournie et al. proposed specific histological criteria for diagnosing proliferative thyroid lesions in teleosts. Such criteria were based solely on morphologic characteristics of the lesions rather than lesion behavior, which is difficult to monitor longitudinally over time in fish because lethal sampling is typically used for microscopic confirmation of the diagnosis. The proposed criteria are extremely useful as a starting point, however, because histopathology is the most common diagnostic and research endpoint for tumor characterization in small bony fishes.
This report concerns the widespread occurrence of proliferative thyroid lesions in a laboratory zebrafish colony. Fish in iodine-deficient conditions developed thyroid follicular hyperplasia and neoplasia. Histopathological lesions in the fish during iodine deficiency and replenishment are described. By examining fish at regular intervals following the onset of increased iodine supplementation, we document a dramatic decrease in the prevalence of both hyperplastic and morphologically neoplastic thyroid lesions in this closed population over the course of a year. The regressive responses observed during recovery suggest that zebrafish may absorb sufficient iodine from water to compensate for a deficient diet.
Materials and Methods
Zebrafish care and maintenance
All protocols for the care and maintenance of zebrafish were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Zebrafish were maintained in three recirculating multi-tank water systems (AHAB; Pentair Aquatic Eco-Systems, Apopka, FL) in a single facility. These three systems housed ∼37,000 fish distributed among 2040 tanks of 1.5, 3, and 10 L capacity.
Throughout the facility, system water consisted of reverse osmosis water with added salt and NaHCO3. Crystal Sea® Bioassay Marinemix salt (Marine Enterprises International, Baltimore, MD) was added to achieve a conductivity of 450 μS. UV irradiation was built into the recirculating systems and delivered at a dose of 90 μWs/cm2. Water temperature was maintained at 26°C–27°C, and the target pH was 7.0. Other parameters were measured weekly with the following results: ammonia 0 ppm, nitrites 0 ppm, nitrates average 40 ppm, hardness 30–40 ppm. During each 24-h period, fish were exposed to 10 h of darkness and 14 h of light.
Early larval fish were fed Paramecium spp. and were then transitioned to decapsulated Artemia spp. (brine shrimp; Brine Shrimp Direct, Ogden, UT) twice daily supplemented with Ziegler 100 micron larval diet (Zeigler Bros., Inc., Gardners, PA) and spirulina algae (Pentair Aquatic Eco-Systems) once daily. After 45 days post-fertilization, fish were fed decapsulated brine shrimp twice daily during the week and once daily on weekends. Some researchers gave an additional feeding of O.range GROW pellet (INVE Aquaculture, Inc., Salt Lake City, UT) for 1 or 2 days before spawning. The pellet food was 1 year past the expiration date and only given sporadically. The majority of adult fish were fed only brine shrimp.
Fish were monitored for morbidity and mortality, and three post-filtration fish were randomly selected from each water system and submitted biannually for histopathology. Salt and diet history before 2015 is unclear as the facility was under a different management.
Histopathology
Fish were euthanized by overdose of MS-222 (tricaine methanesulfonate), fixed in Dietrich's fixative, and placed on a laboratory rocker for at least 24 h. Fixed specimens were decalcified for 48 h in Cal-Ex II (water, formic acid, formaldehyde, and methyl alcohol) and bisected parasagittally, just off midline to preserve the spinal cord. Specimens were processed routinely for paraffin embedding, sectioned at 4 μm thickness, and stained with hematoxylin and eosin according to standard techniques.18 Two sagittal sections of each fish were examined through light microscopy by at least two pathologists (K.N.M., J.C.W., S.T.S., and M.L.K.), and the final recorded diagnoses represent their consensus opinions.
Results
Widespread thyroid lesions in a zebrafish colony
A high prevalence of grossly visible red masses in adult zebrafish (Fig. 1) occurred in a zebrafish facility. The masses were typically located under and embedded within the lower jaw, often protruding beneath the opercula, but were occasionally seen in other locations, including at the nares and vent. These lesions developed in wild-type, mutant, and transgenic lines, and ∼10% (220) of the tanks in the facility were overtly affected. Among these tanks, ∼25% of the fish had externally visible masses. There was no apparent relationship between lesion occurrence and sex, researcher, or room location. Besides the appearance of red masses, no other indications of morbidity emerged. Qualitatively, the mortality rate, which was estimated to be ∼30 out of 37,000 fish in the facility per week, was not increased relative to baseline. Fish with red masses were not selectively culled.
FIG. 1.

An adult zebrafish with a large red mass between the mandibles.
In June 2016, 12 fish with visible masses were submitted to the Zebrafish International Resource Center (ZIRC, University of Oregon, Eugene, OR) for histopathology. Submitted fish were selected to represent the range of masses observed, from very large lesions to single, small reddish lumps located between the mandibles. In all examined fish the masses were found to represent altered thyroid tissue as determined by histopathological evaluation. Lesions in two fish were diagnosed as thyroid follicular hyperplasia, one was diagnosed as follicular hyperplasia in the pharynx and follicular adenoma at the vent, seven were diagnosed as follicular adenomas, and two were diagnosed as follicular cell carcinomas (Table 1).
Table 1.
Diagnostic Evaluation of Thyroid Tissue in the Initial 12 Zebrafish with Red Masses Submitted for Histopathology
| Genotype | Age | Sex | Tank | Mass location | Thyroid tissue diagnosis |
|---|---|---|---|---|---|
| gata2blga14/UAS:GFP+/− | 412 days | Female | A | Vent | Follicular cell hyperplasia in pharynx, follicular adenoma at vent |
| LF:CFP-NTR+;p53ko+ | 566 days | Male | B | Exophthalmia | Follicular cell carcinoma |
| hsp70:KDR-DN2 | 552 days | Female | C | Under jaw | Follicular cell hyperplasia pharynx, kidney, and vent |
| fibronectin +/−xAB;fli:GFP +/− | 342 days | Female | D | Snout, under jaw | Follicular cell carcinoma |
| Runx23 | 362 days | Female | E | Top of head, under jaw | Follicular cell adenoma |
| AB | 153 days | Male | F | Under jaw | Follicular cell adenoma |
| AB | 153 days | Male | F | Under jaw | Follicular cell adenoma |
| AB | 153 days | Gonad not in sections | F | Under jaw | Follicular cell adenoma |
| topD:GFP | 2 years | Female | G | Under jaw | Follicular cell hyperplasia |
| topD:GFP | 2 years | Female | G | Under jaw | Follicular cell hyperplasia in pharynx, adenoma beneath eye |
| topD:GFP | 2 years | Male | H | Under jaw | Follicular cell adenoma |
| topD:GFP | 2 years | Female | H | Under jaw | Follicular cell adenoma |
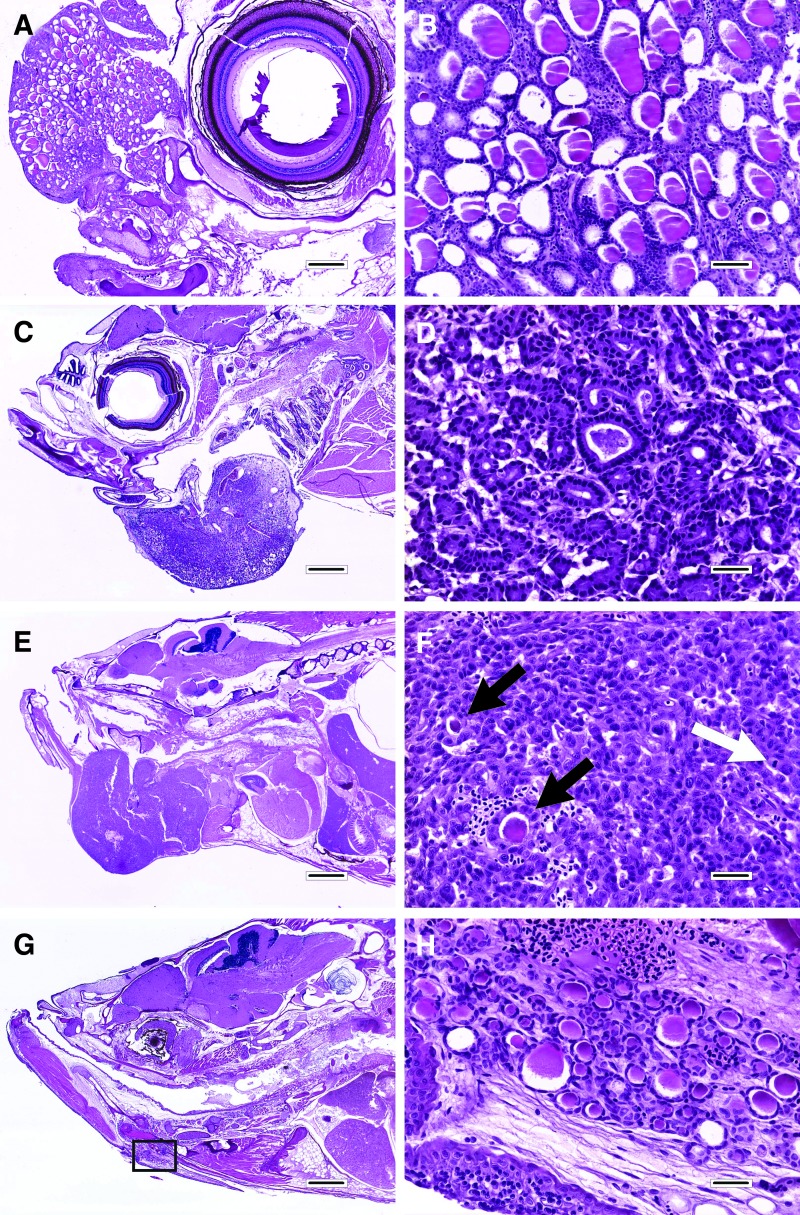
Follicular cell hyperplasia, adenoma, and carcinoma were diagnosed by previously defined criteria17 that are summarized briefly below. Follicular cell hyperplasia (Fig. 2A, B) occurred as diffuse or nodular lesions consisting of multiple round-to-oval follicles of various sizes that were each lined by a single layer of cuboidal epithelium without papillary infoldings. Hyperplastic follicles generally contained barely visible to abundant colloid. Follicular epithelial cells with mitotic figures were rare to non-existent. Ectopic follicular cell hyperplasia (i.e., hyperplasia in non-pharyngeal sites) occurred in a wide variety of sites near and distant to the pharynx and was qualitatively similar to hyperplasia in the pharyngeal region.
FIG. 2.
Thyroid lesions. (A) and (B) are ectopic follicular cell hyperplasia. (A) Hyperplastic lesion in the nasal region anterior to the eye. Follicular hyperplasia also occurred in the pharyngeal region and kidney of this fish to a lesser extent. H&E; scale bar, 250 μm. (B) Higher magnification of the lesion in A. H&E; scale bar, 50 μm. (C) and (D) are follicular cell adenoma. (C) The adenoma protrudes from the ventral jaw region, which was a common presentation for thyroid lesions in this report. H&E; scale bar, 500 μm. (D) Higher magnification of the lesion in (C). H&E; scale bar, 25 μm. (E) and (F) are follicular cell carcinoma. (E) This lesion also protrudes from the lower jaw, but compared to the adenoma in (C) and (D), this carcinoma is somewhat more irregular in outline and larger. H&E; scale bar, 800 μm. (F) Higher magnification of the lesion in (E). This tumor was characterized by sheets of cells with minimal follicle development (black arrow), cytologic atypia, and the presence of mitotic figures (white arrow). H&E; scale bar, 25 μm. (G) and (H) are recovering thyroid tissue. (G) Mass lesions are no longer present 5 months after iodine replenishment. The box indicates the location of (H). H&E; scale bar, 500 μm. (H) The thyroid tissue consists of numerous tiny follicles in the ventral jaw area. H&E; scale bar, 25 μm. H&E, hematoxylin and eosin.
In contrast, follicular adenomas (Fig. 2C, D), which were frequently located adjacent to or within areas of follicular hyperplasia, were nodular expansile lesions and featured follicles of various shapes (e.g., round, oval, or irregular) that were lined by columnar epithelial cells, often with papillary infoldings. Nuclei, which were invariably basilar, tended to be small relative to the amount of cytoplasm, and again mitotic figures were not generally evident.
The two follicular cell carcinomas (Fig. 2E, F) were much more heterogeneous and poorly-differentiated as compared to the adenomas. The tumor cells formed haphazardly-arranged sheets, with only very limited follicle development in most areas. The tumor cells themselves displayed heightened cytologic atypia, characterized by large but variably-sized and irregularly-shaped nuclei, clumped chromatin, and only moderate to small amounts of cytoplasm with indistinct borders. Tumor cells with mitotic figures were common (e.g., 1–3/40 × objective field).
In addition, the microsporidian parasite, Pseudoloma neurophilia, was diagnosed in one fish and the myxozoan, Myxidium streisingeri, was diagnosed in two fish.
Thyroid lesions regress when dissolved iodine is increased
In July 2016 the water system salt supply was changed from Crystal Sea Bioassay Marinemix (0.05 ppm iodine) to Instant Ocean® (Instant Ocean Spectrum Brands, Blacksburg, VA; 0.24 ppm iodide). Salt was added to achieve a conductivity of ∼450 μS. Adult zebrafish continued to be fed brine shrimp twice daily. The supplemental feed was changed from O.range GROW to Gemma micro (Skretting, Stavanger, Norway; 5 ppm iodine). This supplemental feed was still only occasionally given to a subset of the population for 1 or 2 days before spawning.
Fish were then evaluated at 1, 3, 5, and 11 months after the water system salt and diet were changed (Table 2). One month after the salt and diet were changed, red masses were still grossly visible, but at a population level they were reduced in size and no new masses were observed. At this time, 30 adult fish with red masses were selected arbitrarily from nine tanks, euthanized, and evaluated by histopathology. Twenty-three of these fish had thyroid follicular hyperplasia and three had follicular adenomas (Table 2). In 13 fish, hyperplastic follicles developed in non-pharyngeal tissues. Sites of ectopic follicular hyperplasia included kidney, mesentery, coelomic lining, spleen, head tissues, nares, and skeletal muscle. Additional findings included egg-associated inflammation (three fish), M. streisingeri infection (seven fish), ultimobranchial gland hypertrophy (one fish), P. neurophilia infection (one fish), and nephrocalcinosis (two fish).
Table 2.
Summary of Diagnostic Evaluation of Thyroid Tissue in Zebrafish Submitted for Histopathology During Iodine Deficiency (June 2016) and Recovery
| Date | No. of fish examined | No. of tanks represented | Background | Age (days) | Grossly visible masses? | Thyroid carcinoma | Thyroid adenoma | Thyroid hyperplasia | No abnormal thyroid findings | Notes and additional findings |
|---|---|---|---|---|---|---|---|---|---|---|
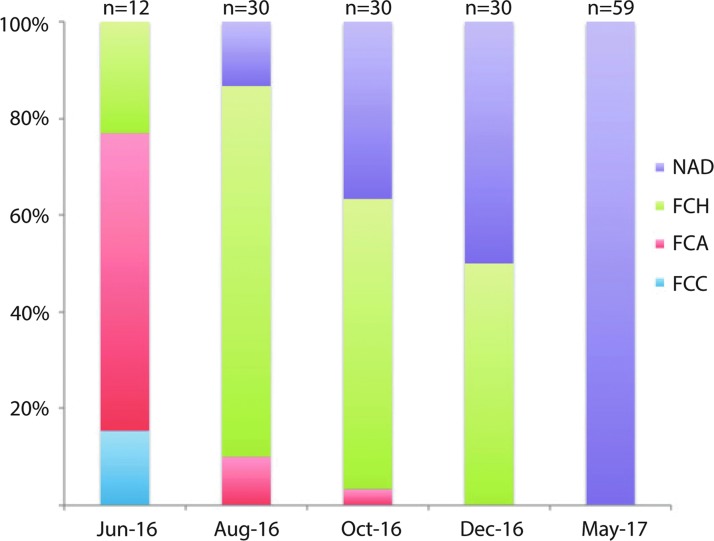
| June 2016 | 12 | 8 | WT, Mt, Tg | 153–522 | Yes | 2 | 8 | 3 | 0 | One fish hyperplasia in pharynx and adenoma at vent |
| August 2016 | 30 | 9 | WT, Mt, Tg | 251–909 | Yes | 0 | 3 | 23 | 4 | |
| October 2016 | 30 | 12 | WT, Mt, Tg | 227–970 | Yes | 0 | 1 | 18 | 11 | |
| December 2016 | 30 | 12 | WT, Mt, Tg | 311–879 | Not reported | 0 | 0 | 15 | 15 | |
| May 2017 | 19 | 3 | Original WT | 363–494 | Not reported | 0 | 0 | 0 | 19 | |
| May 2017 | 10 | 1 | Original p53 Mt | 550 | Not reported | 0 | 0 | 0 | 10 | Two peripheral nerve sheath tumors, two sarcomas, one carcinoma |
| May 2017 | 20 | 3 | New WT | 85–168 | Not reported | 0 | 0 | 0 | 20 | |
| May 2017 | 10 | 1 | New p53 Mt | 74 | Not reported | 0 | 0 | 0 | 10 |
Original WT and p53 Mt refers to fish that were in the facility before the change to a higher iodine salt. New WT and p53 Mt refers to fish that were fertilized after salt change.
Mt, mutant; Tg, transgenic; WT, wild-type.
Three months after salt and diet change, masses were less frequently observed macroscopically, and others had regressed to the point that they were no longer grossly visible. There was no evidence that new masses had developed during this period. Thirty fish were submitted for histopathological evaluation at this 3-month time point. Fish were selected arbitrarily from 12 tanks that all contained fish with visible red masses at the first sampling (June 2016). Six of the selected fish had visible masses and the other 24 fish had no visible lesions. Eighteen of these fish had follicular hyperplasia and 1 had a follicular adenoma. Ectopic thyroid tissue was noted in a variety of organs and locations, including Stannius corpuscle, kidney, mesentery, intestinal wall, adjacent to the heart, ventral to the brain, and at the vent. The thyroid tissue in the other 11 examined fish was unremarkable. Additional findings included nephrocalcinosis (two fish), egg-associated inflammation (one fish), periaerocystitis (one fish), and M. streisingeri infection (one fish).
Five months after salt and diet change, no visible masses were evident in the population. Thirty fish were randomly sampled for histopathology from 12 tanks that previously contained fish with red masses. Fifteen fish were diagnosed as having follicular hyperplasia and the rest had unremarkable thyroid follicles. In some cases, extensive hyperplastic tissue protruded from between the ventral mandibles in tissue sections. In several cases, follicular hyperplasia consisted of multiple very small colloid-filled follicles (Fig. 2G, H). Ectopic thyroid follicles occurred in the spleen, kidney, ovary, mesentery, heart, testis, caudal coelomic lining, and vent. Other changes included nephrocalcinosis (six fish), aerocystitis (three fish), M. streisingeri infection (six fish), egg-associated inflammation (three fish), testicular seminoma (one fish), and P. neurophilia infection (one fish).
Eleven months after iodine levels are corrected, zebrafish thyroid returns to normal
In May 2017 no visible thyroid masses were observed in the population. Fifty-nine fish were selected impartially from eight tanks and submitted for histopathology. The submission included 19 original wild-type fish (AB, TL, and TU strains) and 10 original p53-mutant fish that were in the facility before salt and diet change and were from tanks that previously had visible red masses. In addition, 20 newly added wild types (AB, TL, and TU) and 10 new p53-mutant fish that were fertilized after salt and diet change were also submitted. Microscopically, thyroid tissue was normally developed in all submitted fish at this time point. One small ectopic thyroid follicle occurred in the intestinal wall of an original AB-strain fish, and two small follicles developed in the pituitary gland of an original p53 mutant.
Non-thyroid lesions included nephrocalcinosis (one fish), hepatic megalocytosis (one fish), M. streisingeri (four fish), and aerocystitis (two fish). Several non-thyroid tumors were also diagnosed. A cholangioma developed in an original TL-strain fish. Neoplasms occurred in several of the 10 original p53-mutant fish. These included two peripheral nerve sheath tumors, two intra-abdominal sarcomas of undetermined lineage, and one poorly-differentiated intra-abdominal carcinoma. None of these tumors appeared to arise from or involve thyroid tissue.
Discussion
The cause of proliferative thyroid disease in this case study was presumptive environmental iodine deficiency. The amount of iodine required by zebrafish is unknown, although one reference suggests that fish need 1–4 ppm in a dry diet.19 However, fish also absorb iodine from water, the importance of which is often overlooked in studies of dietary requirements. In the present case, fish over 45 days post-fertilization were fed Artemia (brine shrimp) twice daily. Some researchers gave one additional feeding of expired O.range GROW pellets for a day or two before spawning, but this was not done routinely. Artemia produce their own version of TH, which was previously measured as 11 ± 0.58 ng/mg T3 and 107 ± 41 ng/mg T4.20 However, it is unknown if feeding Artemia results in appreciable increases in bioavailable iodine or active exogenous TH that can be utilized by the zebrafish.
The recirculating water system was treated with Crystal Sea® Bioassay Marinemix, which contains 0.05 ppm iodine, to achieve a conductivity of 450 μS. Levels of thyroxine in fish were not assayed; therefore, it is unknown whether the hyperplastic or neoplastic thyroid tissue was itself capable of producing TH. There were no changes in pigmentation or sex skewing in the population, effects that were previously associated with TH deficiency in zebrafish.6,21 However, during the first 45 days, when sex differentiation occurs in zebrafish, larval fish received increased amounts of iodine in the feed relative to adult stages due to the addition of Ziegler 100 μm larval diet (1.19 ppm iodine) and spirulina algae to the two brine shrimp meals.
Interestingly, among the proliferative thyroid lesions were masses with morphologic characteristics of adenomas and carcinomas. In a review of several studies and populations, Zimmermann and Galetti concluded that iodine deficiency is a strong tumor promoter, but a weak initiator and is a risk factor for follicular thyroid cancer in people. They speculated that increased replication of thyrocytes induced by iodine deficiency would increase their vulnerability to mutagens.
The progression from thyroid follicular hyperplasia to adenoma and carcinoma has been documented in laboratory rodents.16 Fournie et al. observed adenomas in normal and hyperplastic thyroid tissue in Japanese medaka, prompting the authors to conclude that hyperplasia might progress to neoplasia in fish as well. In the current case report, one of the most poorly-differentiated tumors, a follicular carcinoma, occurred in a p53-mutant zebrafish. While that line is predisposed to developing tumors, this is the first time the authors have observed thyroid follicular carcinoma in p53 mutants. It may be that a lack of p53 tumor suppression leads to neoplasms with higher degrees of malignancy in fish that have iodine deficiency-induced proliferation of thyroid tissue.
Following the change to a higher iodine salt mix and a minor diet change, the prevalence and severity of thyroid lesions decreased dramatically (Fig. 3). Whether the changes to the thyroid tissue occurred as a result of apoptosis of follicular cells or dedifferentiation cannot be determined from the results of this case study because sampling for histopathology was lethal, and thus the thyroid glands of individual fish were not assessed longitudinally over time. Furthermore, the final group of submitted fish included wild-type strains and p53 mutants that were in the facility before and after salt and diet change. As might be expected, the original p53 mutants developed neoplasms (Table 2), but none of those tumors originated from the thyroid gland. The new wild-type and p53-mutant fish were younger than their strain-matched controls sampled before salt and diet change. Histopathology was not performed at later stages to evaluate whether thyroid pathology would develop as the fish mature. However, gross appearance was monitored over the following year and red masses did not recur in any of the populations.
FIG. 3.
Distribution of thyroid lesions shifts to hyperplasia and normal thyroid after iodine deficiency is corrected. FCA, follicular cell adenoma; FCC, follicular cell carcinoma; FCH, follicular cell hyperplasia; NAD, no abnormalities detected.
Conclusions that can be drawn from this case report are somewhat limited because individual fish were not tracked over time, fish selected for histopathology were not sampled in an unbiased randomized manner, and there was no group of fish that continued to receive the previous diet and salt in water (i.e., to rule out the possibility of spontaneous lesion regression).
Information in this report is derived from what was essentially a series of diagnostic cases; if a prospective study had been performed, a higher level of control would have been incorporated. However, the disappearance of externally and microscopically evident thyroid lesions at the population level following an increase in available iodine was remarkable. Although it is possible that some of the fish with presumptive follicular carcinomas may have died before the first sampling after salt and diet change, there was no appreciable increase in facility mortality, and fish with red masses were not selectively culled. Therefore, the shrinking and disappearance of all externally-visible red lower-jaw masses and a lack of microscopically-evident proliferative lesions by 11 months later suggest that even the most malignant-appearing masses may have regressed.
Following a strict definition of neoplasia, that it involves permanent and irreversible genomic changes, one could claim that these lesions we describe with irrefutable histological characteristics of neoplasia were simply hyperplasia or dysplasia. However, there are other examples of regression of neoplasms in fish. Dermal sarcomas in walleye (Stizostedion vitreum) have been shown to be reversible, with their disappearance on individual fish during warm weather.22 Regression of epidermal papillomas in Atlantic salmon (Salmo salar) and white sucker (Catostomus commersoni) has also been reported.23–25
There is also precedence for a regression of thyroid neoplasia in mammalian literature. For example, in one rodent study, rat thyrocytes became increasingly aneuploid with follicular hyperplasia resulting from an iodine-deficient diet.26 Following iodine replenishment, lesions regressed and thyroid glands returned to near normal weight and histological appearance. However, most of the rat thyrocytes retained an acrocentric chromosome, which the authors proposed might indicate a permanent mutation. Hardisty and Boorman suggested that the hormone-dependent regression and reversal of thyroid neoplasms in laboratory rodents following removal of the hormonal (i.e., TSH) stimulus indicates a low potential for malignant behavior, but they contend that hormone dependency is not incompatible with neoplasia, as not all tumors exhibit truly autonomous growth and development.
In the current study, the histopathological characteristics of zebrafish adenomas and carcinomas included patterns of abnormal growth and degrees of cytologic atypia that were consistent with true neoplasia; hence the reversal and regression of these tumors in response to iodine replenishment and presumed reduction in TSH are noteworthy. In addition to pharyngeal thyroid tumors, many affected zebrafish had substantial development of hyperplastic thyroid follicles in ectopic locations. As previously postulated,17 it is suspected that these follicles arose from nascent stem cell populations at ectopic sites rather than by metastatic spread, as these hyperplastic ectopic foci lacked the morphologic characteristics of adenomas or carcinomas located at the primary sites.
In another rodent study, monoclonal thyroid adenomas and carcinomas, which were induced by a combination of mutagen and prolonged goitrogen exposure, regressed when goitrogen was removed compared to cohorts that were sacrificed before goitrogen removal.27 Regression was described as a decrease in the number of carcinomas relative to adenomas along with cellular morphological changes. In that study the tumors exhibited only two of the three characteristics required for spontaneous carcinogenesis; they lacked a growth-limiting mechanism and were invasive, but their growth was not TSH-independent. The authors concluded that when TSH levels are consistently elevated, as during chronic goitrogen exposure or iodine deficiency, a mutation that releases a cell from a growth-limiting mechanism will cause a cell to proliferate and be at risk for additional mutations. In that situation, a further mutation leading to TSH-independent growth would not provide any additional selective advantage. The authors went on to hypothesize that these tumors might even exhibit enhanced TSH dependence if a growth control gene was translocated to a TSH-inducible promoter.
Finally, data from this case report indirectly suggest that zebrafish may absorb a significant amount of iodine from water. Although changes were made to both water and diet, the addition of supplemental dry feed was not uniformly applied and was only given for a day or two before spawning to a small subset of the population. The change to a higher iodine salt (Instant Ocean brand), with maintenance of brine shrimp diet, was sufficient to reverse the thyroid lesions in the colony as a whole. Previously published studies of zebrafish administered strict brine shrimp diets have shown either no adverse thyroid effects28 or jaw dysmorphogenesis secondary to enlarged thyroid glands.29 However, neither of those studies listed the concentration or brand of salt added to the water. The findings in this case report suggest that unsupplemented dietary brine shrimp alone may not provide sufficient iodine for normal health and well-being, but zebrafish can compensate by absorbing iodine from water, if water is supplemented with adequate iodine in the correct form. We further speculate that thyroid tissue would also develop normally in low iodine water, if sufficient iodine was provided in the diet. Hence, it is possible that zebrafish adjust the relative amounts of iodine they absorb from diet and water according to physiological needs. Proliferative thyroid disease is then most likely to occur when a low iodine diet is combined with low iodine water.
Although the prevalence of thyroid lesions was exceptionally high in this case report, we have diagnosed qualitatively similar thyroid lesions in other laboratory zebrafish colonies. Since 2001, the ZIRC diagnostic service has diagnosed 25 cases of proliferative thyroid lesions (hyperplasia, adenoma, and carcinoma) from 11 zebrafish facilities out of ∼17,000 fish examined.
The zebrafish research community is currently considering which particular husbandry and health parameters should be included in the Methods sections of articles in an effort to minimize unintended variations that may affect research outcomes (https://orip.nih.gov/about-orip/workshop-reports). Other groups have proposed developing a standardized diet for use in zebrafish studies.30 Results from the current case support the need for further study of water and dietary factors and the influence that water chemistry may have on response to diet.
Acknowledgments
The authors would like to thank Olivia Weeks for providing details about genotypes and tank numbers. The Zebrafish International Resource Center is supported by the National Institutes of Health, Office of Research Infrastructure Programs (P40 OD011021).
Disclosure Statement
No competing financial interests exist.
References
- 1. Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res 2015;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer 2015;136:2187–2195 [DOI] [PubMed] [Google Scholar]
- 3. Yen J, White RM, Stemple DL. Zebrafish models of cancer: progress and future challenges. Curr Opin Genet Dev 2014;24:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat Rev Drug Discov 2015;14:721–731 [DOI] [PubMed] [Google Scholar]
- 5. Udvadia AJ, Linney E. Windows into development: historic, current, and future perspectives on transgenic zebrafish. Dev Biol 2003;256:1–17 [DOI] [PubMed] [Google Scholar]
- 6. McMenamin SK, Bain EJ, McCann AE, Patterson LB, Eom DS, Waller ZP, et al. Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science 2014;345:1358–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wendl T, Lun K, Mione M, Favor J, Brand M, Wilson SW, et al. pax2.1 is required for the development of thyroid follicles in zebrafish. Development 2002;129:3751–3760 [DOI] [PubMed] [Google Scholar]
- 8. Alt B, Reibe S, Feitosa NM, Elsalini OA, Wendl T, Rohr KB. Analysis of origin and growth of the thyroid gland in zebrafish. Dev Dynam 2006;235:1872–1883 [DOI] [PubMed] [Google Scholar]
- 9. Elsalini OA, von Gartzen J, Cramer M, Rohr KB. Zebrafish hhex, nk2.1a, and pax2.1 regulate thyroid growth and differentiation downstream of Nodal-dependent transcription factors. Dev Biol 2003;263:67–80 [DOI] [PubMed] [Google Scholar]
- 10. Hoover KL. Hyperplastic thyroid lesions in fish. Natl Cancer Inst Monogr 1984;65:275–289 [PubMed] [Google Scholar]
- 11. Chavin W. Thyroid distribution and function in the goldfish, Carassius auratus L. J Exp Zool 1956;133:259–279 [Google Scholar]
- 12. Nishioka RS, Grau EG, Lai KV, Bern HA. Effect of thyroid-stimulating hormone on the physiology and morphology of the thyroid-gland in Coho Salmon, Oncorhynchus-Kisutch. Fish Physiol Biochem 1987;3:63–71 [DOI] [PubMed] [Google Scholar]
- 13. Nigrelli RF, Ruggieri GD. Hyperplasia and neoplasia of thyroid in marine fishes. Mt Sinai J Med 1974;41:283–293 [PubMed] [Google Scholar]
- 14. Leatherland JF. Changes in thyroid hormone economy following consumption of environmentally contaminated Great Lakes fish. Toxicol Ind Health 1998;14:41–57 [DOI] [PubMed] [Google Scholar]
- 15. Leatherland JF, Sonstegard RA. Seasonal-changes in thyroid hyperplasia, serum thyroid-hormone and lipid concentrations, and pituitary-gland structure in Lake-Ontario Coho Salmon, Oncorhynchus-Kisutch Walbaum and a comparison with Coho Salmon from lakes Michigan and Erie. J Fish Biol 1980;16:539–562 [Google Scholar]
- 16. Hardisty JF, Boorman GA: Thyroid gland. In: Pathology of the Fischer Rat: Reference and Atlas. Boorman GA. (ed), pp. 519–536, Academic Press, Inc., San Diego, CA, 1990 [Google Scholar]
- 17. Fournie JW, Wolfe MJ, Wolf JC, Courtney LA, Johnson RD, Hawkins WE. Diagnostic criteria for proliferative thyroid lesions in bony fishes. Toxicol Pathol 2005;33:540–551 [DOI] [PubMed] [Google Scholar]
- 18. Humason GL: Animal Tissue Techniques. 4th ed. W. H. Freeman, San Francisco, 1979 [Google Scholar]
- 19. Watanabe T, Kiron V, Satoh S. Trace minerals in fish nutrition. Aquaculture 1997;151:185–207 [Google Scholar]
- 20. Hawkyard M, Saele O, Nordgreen A, Langdon C, Hamre K. Effect of iodine enrichment of Artemia sp on their nutritional value for larval zebrafish (Danio rerio). Aquaculture 2011;316:37–43 [Google Scholar]
- 21. Sharma P, Patino R. Regulation of gonadal sex ratios and pubertal development by the thyroid endocrine system in zebrafish (Danio rerio). Gen Comp Endocrinol 2013;184:111–119 [DOI] [PubMed] [Google Scholar]
- 22. Getchell RG, Wooster GA, Bowser PR. Temperature-associated regression of walleye dermal sarcoma tumors. J Aquat Anim Health 2000;12:189–195 [Google Scholar]
- 23. Smith IR, Zajdlik BA. Regression and development of epidermal papillomas affecting white suckers, Catostomus-commersoni (Lacepede), from Lake-Ontario, Canada. J Fish Dis 1987;10:487–494 [Google Scholar]
- 24. Carlisle JC, Roberts RJ. An epidermal papilloma of the Atlantic salmon I: epizootiology, pathology, and immunology. J Wildl Dis 1977;13:230–234 [DOI] [PubMed] [Google Scholar]
- 25. Coffee LL, Casey JW, Bowser PR. Pathology of tumors in fish associated with retroviruses: a review. Vet Pathol 2013;50:390–403 [DOI] [PubMed] [Google Scholar]
- 26. Alsaadi AA, Beierwaltes WH. Chromosomal changes in rat thyroid cells during iodine depletion and repletion. Cancer Res 1966;26:676–688 [PubMed] [Google Scholar]
- 27. Thomas GA, Williams D, Williams ED. Reversibility of the malignant phenotype in monoclonal tumors in the mouse. Brit J Cancer 1991;63:213–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonzales JM. Preliminary evaluation on the effects of feeds on the growth and early reproductive performance of zebrafish (Danio rerio). J Am Assoc Lab Anim 2012;51:412–417 [PMC free article] [PubMed] [Google Scholar]
- 29. Craig MP, Desai MB, Olukalns KE, Afton SE, Caruso JA, Hove JR: Unsupplemented artemia diet results in reduced growth and jaw dysmorphogenesis in zebrafish. In: Aquaculture. Muchlisin ZA. (ed). InTech, London, UK, 2012 [Google Scholar]
- 30. Watts SA, Powell M, D'Abramo LR. Fundamental approaches to the study of zebrafish nutrition. ILAR J 2012;53:144–160 [DOI] [PMC free article] [PubMed] [Google Scholar]