Abstract
Background
The cultivation of bananas and other plants is limited by environmental stresses caused by climate change. In order to recognize physiological, biochemical and molecular components indicated to confer tolerance to water stress in Musa spp. we present the first systematic review on the topic.
Methods
A systematic literature review was conducted using four databases for academic research (Google Academic, Springer, CAPES Journal Portal and PubMed Central). In order to avoid publication bias, a previously established protocol and inclusion and exclusion criteria were used.
Results
The drought tolerance response is genotype-dependent, therefore the most studied varieties are constituted by the “B” genome. Tolerant plants are capable of super-expressing genes related to reisistance and defense response, maintaining the osmotic equilibrium and elimination of free radicals. Furthermore, they have higher amounts of water content, chlorophyll levels, stomatic conductance and dry root matter, when compared to susceptible plants.
Conclusions
In recent years, few integrated studies on the effects of water stress on bananas have been carried out and none related to flood stress. Therefore, we highlight the need for new studies on the mechanisms of differentially expressed proteins in response to stress regulation, post-translational mechanisms and epigenetic inheritance in bananas.
Introduction
Bananas are among the oldest cultivated plants to be domesticated [1]. They are classified botanically as monocotyledons of the Musaceae family which include the genus Ensete of Asian and African origin, the genus Musella, genetically close to Asian origin, and the Musa of East Asia, which is divided into sections with 22 (Eumusa, Rhodochlamys) and 20 chromosomes (Australimusa, Callimusa) [2]. Most edible bananas belong to the Eumusa section and are hybrids from Musa acuminata (genome "A") or from crosses with Musa balbisiana (genome "B"). A smaller group, including "Fe'i" or "Fehi" bananas, is limited to the Pacific region and is derived from Australimusa species [3].
The cross between species and subspecies of M. acuminata and M. balbisiana contributed to plant sterility and the appearance of parthenocarpy in the triploid and tetraploid genotypes [4].
It is known that the varieties constituted with the genome "A" produce fruits of high yield and quality, with long fingers and durability in the green and mature phases [5]. Molecular studies indicate that the "A" genome harbors more genes that are important for banana production and quality and can be used as candidates in breeding programs [6, 5]. However, bananas constituted with the "B" genome were domesticated under more severe climatic conditions, such as wide temperature variation and soil water scarcity, supporting better environmental stresses [7]
Plants undergo successive abiotic stress events during its life cycle. When water is not available to roots or when the transpiration becomes intense it is said that the plant is under water stress. Water stress may be caused by water deficit or high salinity in the soil. In the case of high salinity in the soil, periods of inundation and low soil temperature, there is still water in the solution of the soil, however, the plants are not capable of absorbing it, leading to a phenomenon called “physiological drought”[8].
Water deficit is one of the main limiting factors for the cultivation of Musa spp. In the highlands of East Africa, when annual rainfall is less than 1,100 mm, there may be losses of 20–60% in yield compared to other, more humid areas of the region. The weight of the bunch is affected due to the effect of water deficit during the flowering period, which reduces the number of fingers produced [9].
The response of banana varieties during periods of drought can be genotype-dependent [1]. Studies indicate that the presence of the "B" genome contributes to a better drought tolerance [1, 10]. However, in the absence of a conclusive definition of drought, it is a challenge to identify correct parameters and stress intensities for assessing water deficit tolerance [11].
This paper contributes to this challenge, since through a systematic review of the studies carried out over the past 10 years on the effects of water deficit on bananas and with the genetic sequencing of the species already carried out [12], it is possible to recognize, gather, classify and identify relevant new knowledge produced by other researchers.
A systematic review of the literature is a formalized and repetitive process where the state of the art of a given subject is documented through systematized searches in specific databases. This type of review is very common in Medical Sciences, since it is able to gather in a single document detailed and high level conclusions about diseases that one wishes to study [13, 14].
This paper proposes the first systematic review on water deficit in bananas. To guarantee its efficiency, the search process was conducted around a general objective, which in this review, was to recognize physiological, biochemical and molecular components indicated to confer drought tolerance on Musa spp.
Materials and methods
The free software StArt (State of the Art through Systematic Review) v.3.3 Beta 03 was used to perform the systematic review. Developed by the Federal University of São Carlos (UFSCar), the tool provides computational support to researchers who seek answers to research questions using the systematic review technique. The software is available for download at the link: <http://lapes.dc.ufscar.br/tools/start_tool>.
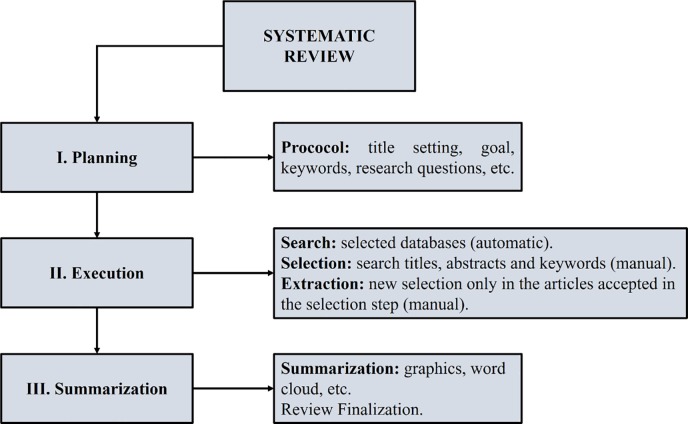
The review process was run on StArt in three steps: Planning, Execution and Summarization (Fig 1).
Fig 1. General systematic literature review flowchart.
Each step performed is described below:
I. Planning: A protocol was developed to be followed throughout the review process (http://doi.org/10.5281/zenodo.1465055). Title, objective, keywords, research questions, research sources, inclusion/exclusion criteria and the quality of the collected files were defined. The issues underlying this review are given in Table 1.
Table 1. List of review questions.
| Research Questions |
|---|
| Q1. In what countries has more knowledge been produced about water stress in bananas? |
| Q2. What are the main institutions and/or groups involved in the study of water deficit tolerance? |
| Q3. What are the main genotypes and varieties studied? |
| Q4. What types of trials are proposed for studies of water stress? |
| Q5. What are the types of stresses addressed in papers on water stress? |
| Q6. Has there been any mention of using the banana genome? |
| Q7. Which components confer drought tolerance on Musa spp.? |
| Q8. What are the stressors used in the drought studies? |
II. Execution: the searches were carried out in databases previously selected: Google Academic, Springer, CAPES Journal Portal and PubMed Central. The results were imported into BIBTEX, MEDILINE, RIS or Cochrane formats, compatible with StArt. The automatic search performed in the databases searches the central themes in the titles, abstracts and keywords. Relevant papers not found by searches were added later. To make the query expressive, the OR logical connector was used to group the synonymous keywords and AND to group the main parts. Thus, the search string used in all databases is represented in the following Box 1:
Box 1: Search string
("Musa" OR "banana") AND ("drought stress" OR "water deficit" OR "water stress")
In order to guarantee the international spectrum of the papers, only works written in English and available in academic channels were selected.
After automatic sorting, the manual selection and extraction phases were performed. In these phases, the inclusion and exclusion criteria are presented in the Table 2.
Table 2. Criteria used to include or exclude papers in the review process.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Papers that contain in the title, abstract or keywords, the terms banana (or Musa) and drought stress (or water deficit/stress), as detailed below in the search string. | Theses, dissertations, manuals and reports. |
| Review Papers. | |
| Papers published in journals with no impact factor (individually checked on the sites: https://sucupira.capes.gov.br and https://www.scimagojr.com)* | |
| Papers without clear contribution. | |
| Papers published before 2008. |
* Only one exception for the only article found on epigenetic studies in banana.
In the Selection, all papers imported to the software were classified as inclusion criteria: accepting, rejecting or excluding because they were duplicated. In Extraction, a new choice was made, considering only the papers accepted in the selection stage. In extraction, in addition to accepting, we can also reject and/or delete duplicate papers. PRISMA Checklist is presented in S2 Table.
III. Summarization: Graphs, Table and Word Cloud were generated to make up the systematic review.
Results
Search in databases
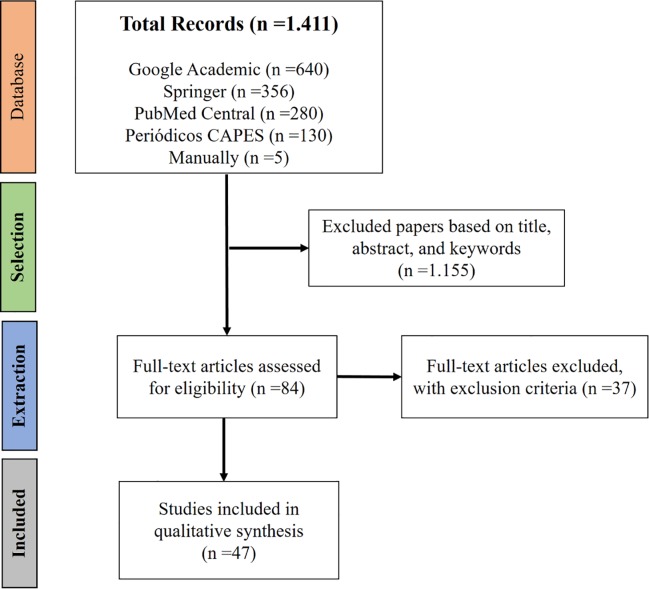
The StArt software selected 1,410 papers related to the search string. No papers were found on water stress due to flooding in bananas. Google Academic contributed with the largest number of papers for this review, 46% of the total (Fig 2A). The majority of the papers in this database not related to the theme, gray literature (theses, dissertations, reports, annals …) and/or duplication, due to small differences in titles, such as capital letters, italicized formatting and accentuation. The Springer, PubMed Central and CAPES journals represented, respectively, 25%, 20% and 9% of the papers found, and presented lower rejection criteria (Fig 2).
Fig 2. PRISMA flow diagram.
Papers collected considering the search string in the databases.
Other databases could have been used, including Musalit (http://www.musalit.org/), which is specific to bananas. However, when using the search string, the results were restricted or insufficient, so we opted for databases with broader search spectrum such as Google Academic.
After Selection phase, only 6% or 84 papers out of a total of 1, 411 were accepted. In the Extraction phase, of the 84 previously selected papers, 55% were accepted, 45% were rejected by the exclusion criteria. Thus, we followed the review with 47 papers. For consultation purposes, the manuscripts were stored in a free access digital library at the following link: http://doi.org/10.5281/zenodo.1454052.
A cloud of words was generated considering the frequency with which they appeared in papers of the Extraction phase (Fig 3). All the keywords used in database searches are represented and the other words are related to the research on water stress in banana plants.
Fig 3. Relevant search word cloud.
Research questions
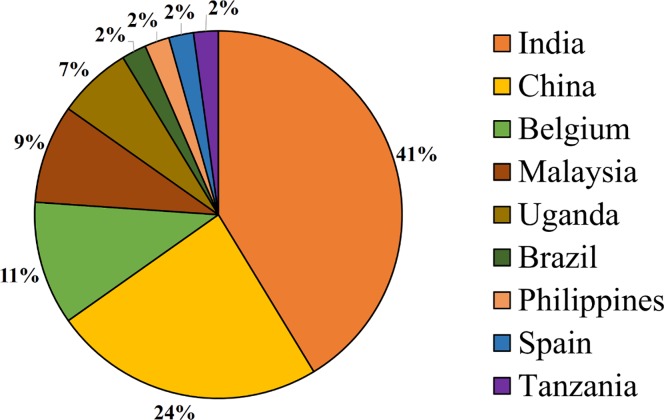
Most of the research works were developed in Asian countries, approximately 80% of the total of 47 (Fig 4). In Asia are also the main institutes and/or research institutions that are dedicated to the studies of Musa spp. under water deficit (S1 Table). In Europe, Africa and South America, 13%, 8% and 3% of the works were performed, respectively.
Fig 4. Main producing knowledge countries on water stress in banana plantations.
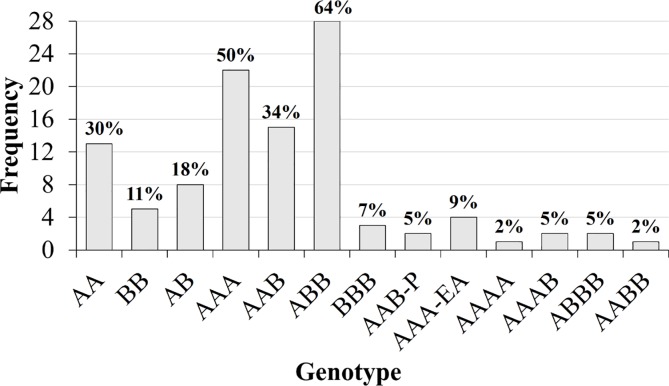
In the studies on water deficit, the main varieties studied are triploids AAA, AAB, ABB present in 50%, 34% and 64% of the papers, respectively (Fig 5). The diploids were studied in 59% of the works and the tetraploids in 14%. The total frequency of genotypes does not reflect the total number of papers in Fig 5, since some authors considered more than one genotype.
Fig 5. Frequency of genotypes of Musa spp. used in papers published in the last 10 years.
A list of cultivars and species used in the 47 papers is presented in Table 3. Among the most widely used varieties are triploids 'Saba', 'BaXi Jiao', 'Fen Jiao', 'Poovan', 'Karibale Monthan', 'Berangan', 'Cachaco' and 'Grande Naine', and the diploid 'Ney Poovan'.
Table 3. Musa spp. most used in studies on water deficit in the last 10 years.
| Genomic Group | Genotypes (varieties/types) |
|---|---|
| AA | M. acuminata, M. paradisiaca, Matti, Sanna Chenkathali, Anaikomban, Calcutta-4. |
| BB | Bee hee kela, Bhimaithia. |
| AB | Ney Poovan. |
| BBB | Gubao, Cardaba, Saba Puti. |
| AAA | Berangan, Berangan Intan, Tianbaojiao, BaXi Jiao, Grande Naine, Brazilian, Yangambi Km5, Mpologoma, Mbwazirume, Williams, Uganda. |
| AAA-EA | Kisansa, Mbwazirume. |
| AAB | Rasthali, Latundan, PK Malaccacina, Pokpok, Sukali Ndizi, Popoulou, Nendran, Poovan, Karpuravalli. |
| AAB-P | Obino l'Ewai |
| ABB | Cachaco, Fen Jiao, Prata anã, Saba, Karibale Monthan, K. Namwa, Maduranga, Matavia, Paa Dalaga, Pelipia, Tindok, Kayinja, Karpooravalli. |
| AAAB | BRS Tropical. |
| ABBB | Tiparot. |
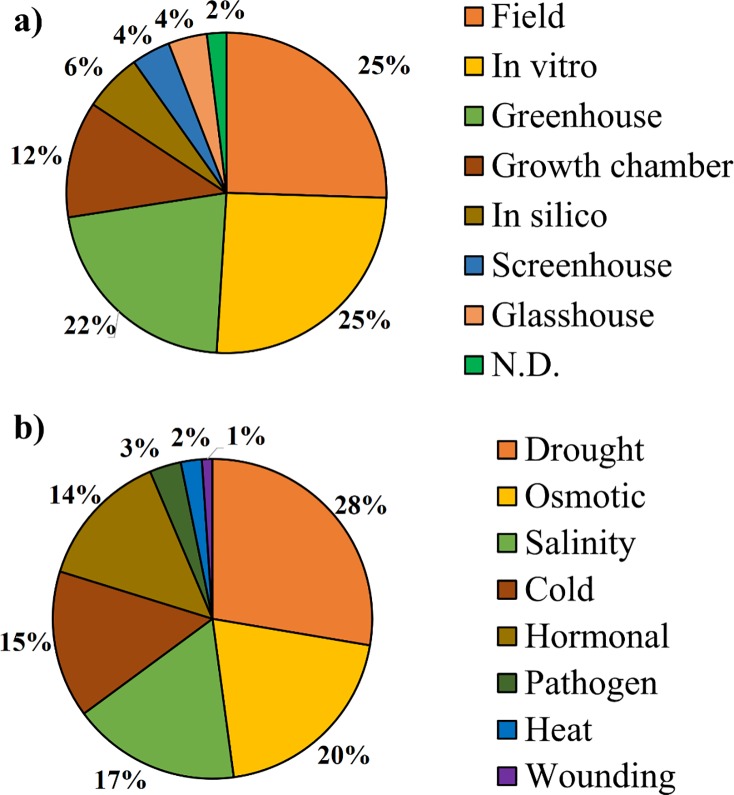
Most of the studies were performed in the field, in vitro, greenhouse and growth chamber, corresponding to 30%, 30%, 25% and 14%, respectively. The remaining studies accounted for 19% (Fig 6A). The main stresses observed were drought, osmotic, salinity, cold and hormonal stress (Fig 6B). The following discussions considered only osmotic stress and water deficit, although some papers addressed multiple stresses.
Fig 6.
a) Frequency of study environments for application of water stress in banana plants. b) Types of stress applied on Musa spp.
Stress applied on Musa spp. were grouped into physical, chemical and biotic (Table 4). The agents causing water stress in selected papers were water deficit, water deficit progressive, PEG6000, mannitol, mannitol+PEG, sucrose+sorbitol and methyl viologen (Table 4).
Table 4. Classification of stresses applied on Musa spp.
| Type of stress | Stressors |
|---|---|
| Physical | Water deficit, water deficit progressive, low temperature, high temperature, injury, NaCl. |
| Chemical | PEG6000, mannitol, mannitol+PEG, sucrose+sorbitol, methyl viologen, ethephon, ABA, AIA, MeJA, GI3, SA, CuSO4. |
| Biotic | Fusarium oxysporum f. sp. cubense, Xanthomonas campestris pv. musacearum (Xcm). |
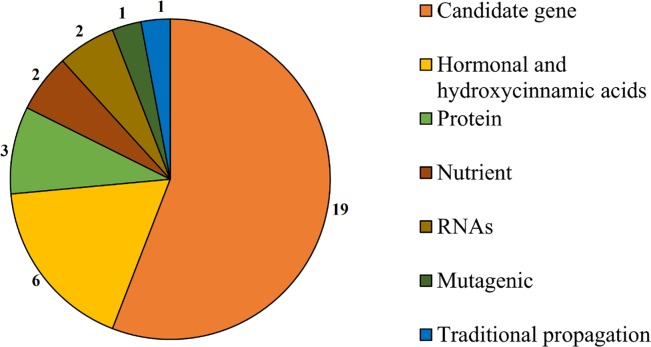
Some markers have been successfully used in drought tolerance research in banana plantations with different applications (Fig 7). There are 21 candidate genes, 6 hormones, 3 proteins, 2 nutrients, 2 RNAs and 1 mutagenic agent.
Fig 7. A summary of markers used in banana research.
Molecular markers and their effects under drought tolerance in Musa spp. are addressed in 21 papers (Table 5).
Table 5. Candidate gene used in research with Musa spp. and applications.
| Candidate Gene | Applications | Authors |
|---|---|---|
| AhSIPR10 | Improvement of photosynthetic efficiency and reduction of plasma membrane damage in the presence of NaCl and mannitol. | Rustagi et al., 2014 [15] |
| MaAGPase | Regulates the signaling pathway of biotic and abiotic stress and is involved in the development and maturation of fruits. | Miao et al., 2017 [6] |
| MaAQP | Promotes the early development of fruits, accelerating post-harvest banana maturation processes and plant resistance to saline and osmotic stress. | Hu et al., 2015 [16] |
| MaARFs | Involved in banana growth, fruit development, post-harvest maturation and responses to osmotic, saline and cold stress. | Hu et al., 2015 [17] |
| MabZIP | Involved in stages of organ development, fruit maturation and responses to abiotic stresses, including dry, cold and salt. | Hu et al., 2016 [5] |
| MaCCS | Induced in response to light, heat, drought stress, abscisic acid and indole-3-acetic acid. | Feng et al., 2016 [18] |
| MaHsfs | Involved in the growth of specific tissues or stages of development, such as fruit maturation and biotic and abiotic stress. | Wei et al., 2016 [19] |
| MaPIP1;1 | It imparts tolerance to saline and water stress, reducing membrane damage, improving ionic distribution (K+/Na+ ratio) and maintaining the osmotic balance. | Xu et al., 2014 [20] |
| MaSODs | Plays an important role in the elimination of reactive oxygen species caused by abiotic and hormonal stresses in banana. | Feng et al., 2015 [21] |
| MpASR | Demonstrates positive activity to F. oxysporum f. sp. cubense and cold stress, dehydration, ABA and high salt concentration. | Liu et al., 2010 [22] |
| MusaDHN-1 | Induced in leaves by drought, salinity, cold, oxidation, heavy metals, as well as by treatment with signaling molecules such as abscisic acid, ethylene and methyl jasmonate. | Shekhawat et al., 2011 [23] |
| MusaNAC042 | Modulates the response to abiotic stress in banana preserving high levels of total chlorophyll and maintaining lower MDA content (malondialdehyde). | Tak et al., 2017 [24] |
| MusaNAC68 | Regulates stress tolerance induced by NaCl and mannitol and root development. | Negi et al., 2015[25] |
| MusaPIP1;2 | It improves survival characteristics under abiotic stress by maintaining low levels of malondialdehyde and high concentrations of proline, relative water content and photosynthetic efficiency. | Sreedharan et al., 2013 [26] |
| MusaSAP1 | Involved in reducing malondialdehyde levels and regulating polyphenoloxidases (PPOs) that play important roles in multiple defense pathways. | Sreedharan et al., 2012 [27] |
| MusaWRKY71 | An important constituent in the transcriptional reprogramming involved in several responses to stress in bananas, such as improved photosynthetic efficiency and reduction in leaf membrane damage. | Shekhawat et al., 2013 [28] |
|
Non-redundant DEGs |
Involved mainly in protein modifications, lipid metabolism, alkaloid biosynthesis, carbohydrate degradation, glycan metabolism, amino acid biosynthesis, cofactor, sugar-nucleotide, hormone, terpenoids and other secondary metabolites. | Muthusamy et at., 2016 [29] |
| PYL-PP2C-SnRK2 | Regulates maturation and tolerance of banana fruits to cold, saline and osmotic stresses. | Hu et al., 2017 [8] |
| MaSWEETs | Increases sugar transport during initial fruit development and under abiotic and biotic stresses. | Miao et al., 2017 [30] |
| WRKY | Regulated in multiple stresses, involved in the growth, development and process of ripening fruits | Goel et al., 2016 [31] |
Some papers have addressed the existence of ARNs regulating the response to water deficit in banana (Table 6).
Table 6. ARNs involved in drought tolerance in Musa spp.
| RNAs | Applications | Authors |
|---|---|---|
| microRNAs | The miR169, miR156 and miR2118 were upregulated during stress due to water deficiency of the soil. MiR169 can control the expression of NFY and eventually the expression of AQN and DHN by reducing transcription, or by a mechanism of post-transcriptional degradation. | Muthusamy et al., 2014 [32] |
| LncRNAs | They are crucial regulators of gene transcription in plants in response to biotic and abiotic stress. | Muthusamy et al., 2015 [33] |
Only two papers of this review presented the proteomic study of Musa spp. The ATPase, Heat shock and Dehydrogenases, are among the main proteins observed in contrasting banana genotypes for tolerance to water deficit (Table 7).
Table 7. Important proteins in the response of banana plants to water stress.
| Protein | Applications | Authors |
|---|---|---|
| ATPase | Provides the main driving force for many cellular processes such as osmoregulation, signal transduction and reaction metabolism. | Mattos-Moreira et al., 2018 [34] |
| Heat shock | Promotes balance between antioxidants (AOX) and ROS during water stress. | |
| Dehydrogenases involved in NAD/NADH homeostasis | Limitation of ROS production by the NADPH matrix produced by NADP-isocitrate dehydrogenase and the non-proton-pumping transhydrogenase activities | Vanhove et al., 2012 [35] |
Three papers deal with the Hormonal and Hydroxycinnamic Acids study to confer drought tolerance on banana varieties (Table 8).
Table 8. Hormonal and Hydroxycinnamic Acids in bananas under water déficit and its applications.
| Hormonal and Hydroxycinnamic Acids |
Applications | Authors |
|---|---|---|
| Methyl jasmonate | Improves drought tolerance, since it moderates the effects of oxidative stress, leading to better plant performance, fresh weight and shoot proliferation rate. | Mahmood et al., 2012 [36] |
| Abscisic Acid | Trigger adaptation of the plant to drought, as well as reduction of stomatal conductance, photosynthetic rate, plant growth in height, circumference, number of leaves and area. | Mahouachi et a., 2014 [37] |
| Indole-3-acetic acid | It can alleviate leaf senescence, improve survival levels and maintain cell stretching. | |
| Cinnamic acids | They are photoprotectors, since they are involved in the folding mechanism of the leaves, which reduces the area of irradiation and loss of water. | |
| Ferulic acids | ||
| Salicylic acid | They may be limited to a rapid and transient increase at the beginning of the first stress period, as they did not respond to consecutive stresses. | |
| Jasmonic acid | ||
| Salicylic acid | Promotes increase in the rate of proliferation, fresh weight gain, maintenance of the relative water content and reduction of H2O2. | Bidabadi et al., 2012 [38] |
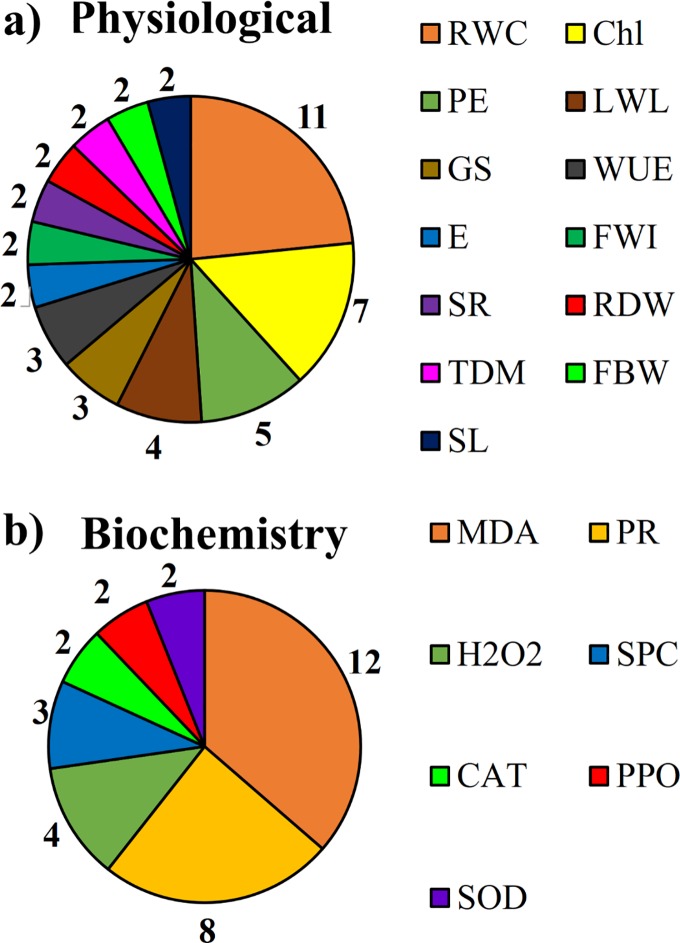
Relative water content, chlorophyll levels, photosynthetic efficiency and leaf water loss are among the main physiological analyzes used to measure of the effects of water stress on banana genotypes (Fig 8A). The content of Malondialdehyde, Proline, Hydrogen peroxide and Soluble protein are among the main biochemical analysis (Fig 8B)
Fig 8. Main biochemical and physiological parameters measured.
a) RWC—Relative water content, Chl—chlorophyll levels, PE—Photosynthetic efficiency, LWL—leaf water loss, GS—Stomatal conductance, WUE—Water Use Efficiency, E—Transpiration rate, FWI- fresh weight increase, SR- survival rates, RDW- Root dry weight, TDM- Total dry matter yield, FBW- fresh bunch weight, SL- Shoot Length. b) MDA—Malondialdehyde, PR- Proline, H2O2—Hydrogen peroxide, SPC—Soluble protein content, AN—Acid ninhydrin, CAT—Catalase, PPO—Polyphenol oxidase, SOD- Superoxide dismutase.
Discussion
This systematic review presentes a compilation of quality articles and main institutions involved in the study of tolerance to water stress in Musa spp. The great interest of Asian countries in the study of the water deficit in Musa sp. probably stems from the fact that these countries are at the center of origin and world banana trade. Molecular evidence confirmed that edible banana genotypes originated in the islands of Southeast Asia and developed through complex pathways of geodomestication [39]. India is by far the world's largest banana producer, and China is the fourth largest importer. Malaysia is also among the domestic market consumers in Asia. The Philippines was the world's second largest banana exporter by 2015, when they lost their position due to the long period of drought caused by El Niño [40]. Brazil is among the five largest producers in the world and the European Union and the United States are among the largest importers of this fruit, approximately 60% of the global distribution [40].
The triploid cultivars are the most studied. In natural germplasm, there are few ABB triploid varieties that have good palatability and high productivity, and usually the most consumed are the Cavendish (AAA) subgroup, such as Grande Naine and Williams [39, 41]. Therefore, an improvement strategy is to combine edible AB cultivars with wild M. balbisiana to create new ABB varieties that are productive, palatable and tolerant to drought and cold [1]. In nature, M. acuminata and its subspecies are typically delicate and thin, and require favorable environmental conditions for good development, since they originated in the wetlands of Asia. M. balbisiana was diversified and domesticated in regions of drought-prone monsoons in Southeast Asia, which probably contributed to tolerance to abiotic stresses [9].
The most common growth environment were field and in vitro and the main stresses applied were water deficit and osmotic stresses. Drought stresses are given by the suspension of irrigation in plants grown in the greenhouse [39] or in typically dry seasons with commercial plantations [3]. Field and greenhouse studies generally approach the conditions of agricultural cultivation [4]. Mannitol, polyethylene glycol and methyl viologen are organic compounds with osmotic action. As liquid solution, they are commonly used to simulate water deficit conditions [5]. In the selected works, mannitol was used, for example, in a water stress test with five leaves of the cultivars 'BaXi Jiao' and 'Fen Jiao' [6, 10], in different leaf discs Musa spp. [5, 15, 25, 42] and in vitro in the variety ‘Karibale Monthan’ [27]. Polyethyleneglycol (PEG) was used in vitro as it has a high molecular weight and reduces the water potential of the medium, thus inhibiting the growth of bananas, which can not absorb water and nutrients [7, 9, 38]. The methyl viologen was used in leaves of transgenic banana plants to simulate osmotic stress, as it acts by receiving electrons from the primary acceptors of the photosystem-I and transferring them to the oxygen, generating ROS [43]. Sorbitol was considered a neutral inducer of osmotic stress in banana when compared to culture media that use sucrose as the only source of carbon [2]. Therefore, cultures initially acclimated with sucrose in vitro respond better to osmotic stress, but over time, sugar metabolism consumes ATP and boosts respiration, resulting in limiting levels of oxygen, which can eventually lead to anoxia. [10].
In all studies on osmotic stress, the efficiency of at least one of the compounds was determined in the selection of water stress tolerant genotypes. In vitro studies are considered the first step in characterizing the biodiversity of tolerant Musa spp. in a germplasm bank, since they allow a high yield and control of the experiment, however, the artificial conditions are pointed out as disadvantages [18].
More than 20 candidate genes were indicated as potential markers for banana drought tolerance. The MaAGPase, MaAQP, MabZIP, non-redundant DEGs, MaSWEETs and PYL-PP2C-SnRK2 genes showed genome-dependent response in genome "B" compared to genome "A" [26, 30, 38, 44, 45]. The expression of cuticular wax biosynthesis genes (FATB and KCS11) is also a dependent genotype, influencing the water retention capacity of leaves and mitigating the effects of water stress on varieties constituted by the "B" genome, a promising line for future studies [46]. [38] suggests that the degradation of mRNA driven by the enzyme exoribonuclease 5'-3' during abiotic stress may be responsible for sensitivity to drought in cv. 'Grande Naine' (AAA).
In one study, the heterologous expression of the banana aquaporin gene, MaPIP1;1, in Arabidopsis was presented as an alternative to improve the response of species, independent of the genotype, since in Arabidopsis it showed an increase in the primary root length, number of root hairs and survival rates [47]. The same stress acclimation behavior was observed in transgenic plants overexpressing MusaNAC68 [25].
The banana transcriptome and proteome are also altered in response to water stress. The expression of RNAs involved in drought tolerance of the the ‘Saba’ cultivar (“ABB” genome-tolerant) and Grande Naine (“AAA” genome-susceptible) depends on the genetic constitution of the cultivars, whereas the genome “B” is the most efficient [46, 48]. The proteome analysis of banana plants submitted to water stress shows that there is a new equilibrium in stressed plants and that respiration, ROS metabolism, growth and development, especially of plants with the "B" genome, are altered in order to adjust to tolerate stress [16, 18].
The exogenous use of Hormone and Hydroxycinnamic Acids to confer tolerance to Musa spp. triploids (AAA) seems promising. With the exception of Salicylic acid and Jasmonic acid that did not present a significant response in progressive water stress [20], all other acids contributed to the acclimatization of banana varieties to water deficit [21, 33].
In addition to exposure to hormones, water stress tolerant lineages can be obtained from variations induced by ethyl methanesulphonate (EMI) [34] or by maintaining the nitrogen and potassium ratio in the soil, since potassium mitigates the production of dry matter and loss of bunches resulting from water stress in the early stages of development [36].
Of the 47 articles in this review, only 13 cited the use of the banana genome. Until 2009, few complete studies on abiotic stress in bananas had been published, and none on drought [7]. The sequencing of the Musa acuminata genome, whose DNA is in the composition of all edible varieties [13], is an important step for the knowledge of genes with specific functions and of interest for the genetic improvement of the species.
The first epigenetic study relating to the response of in vitro micropropagated banana varieties to mechanisms underlying abiotic stresses was published in 2012. Cytosine hypomethylation of DNA is related to increased leaf stomata density, resulting in excessive water loss and increased foliar senescence [49]. This work was published in a journal with no impact factor. Their results are presented due to the importance of this knowledge for the production of varieties tolerant to water stress.
A series of physiological and biochemical changes were observed in different banana varieties submitted to water stress. In superior plants, a small part of the water absorbed by the roots is used in photosynthesis and biomass production and most of it is returned to the environment in the process and transpiration [49]. The stomatal closure is one of the main strategies used by plants to control the amount of water evaporated into the medium [11]. Under conditions of water deficit, the relative water content in the leaves decreases, consequently leading to the reduction of stomatal conductance, photosynthetic rate [50,51,52] and transpiration rate [53]. Within the cells, the formation of reactive oxygen species (ROS) occurs, leading to the formation of harmful free radicals, lipid peroxidation [47] and denaturing proteins [30]. Species susceptible to water stress are more vulnerable to increased polyphenol oxidase, which is an enzyme which catalyzes the darkening reaction that occurs in many fruits and vegetables [54]. Varieties of bananas tolerant to water deficit are capable of increasing the activity of osmoprotective components, such as Proline, which eliminate free radicals in the cytoplasm and increase the solubility of poorly soluble proteins, free amino acids and total sugars [55, 56]. The activity of antioxidant enzymes, such as Catalase, Superoxide dismutase and Peroxidase of ascorbate [57] is also higher in tolerant species when compared to those susceptible to water stress.
Conclusions
Bananas are a product of export and subsistence in several countries. They are susceptible to a wide range of biotic and abiotic stresses where cultivars with the "B" genome show some superiority over those presenting the "A" genomes, such as increased expression of genes related to plant defense, higher relative content of water, length of primary roots and fresh bunch weight as well as better rates of survival and photosynthesis. Many efforts have been made in studies on the effect of water stress on the expression of genes that are candidates for drought tolerance, but few studies in the last 10 years have addressed the expression of proteins and post-translational mechanisms. We also highlight the need to study the epigenetic inheritance in bananas, elucidating the effects of environmental stresses on DNA and chromatin sequences and possible regulatory mechanisms that are maintained and passed on to the next generations, making them more tolerant through genetic “imprinting”.
Supporting information
(DOCX)
(DOC)
Data Availability
The minimal underlying data set of the study is available at the link: http://doi.org/10.5281/zenodo.1454052.
Funding Statement
This work was supported by a CAPES PhD scholarship to ASS.
References
- 1.Ravi I, Uma S, Vaganan MM, Mustaffa MM. Phenotyping bananas for drought resistance. Frontiers in Physiology. 2013; 4: 1–15. 10.3389/fphys.2013.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Häkkinen M. Reappraisal of sectional taxonomy in Musa (Musaceae). TAXON. 2013; 62: 809–813. 10.12705/624.3 [DOI] [Google Scholar]
- 3.Perrier X, De Langhe E, Donohue M, Lentfer C, Vrydaghs L, Bakryet F, et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proceedings of the National Academy of Sciences. 2011; 108: 11311–11318. 10.1073/pnas.1102001108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Loesecke H. W. Bananas, chemistry, physiology and technology New York: Interscience Publishers, 1949. 189pp. [Google Scholar]
- 5.Hu W, Wang L, Tie W, Yan Y, Ding Z, Liu J, et al. Genome-wide analyses of the bZIP family reveal their involvement in the development, ripening and abiotic stress response in banana. Scientific Reports. 2016; 6: 1–15. 10.1038/s41598-016-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao H, Sun P, Liu Q, Miao Y, Liu J, Xu B, et al. The AGPase Family Proteins in Banana: Genome-Wide Identification, Phylogeny, and Expression Analyses Reveal Their Involvement in the Development, Ripening, and Abiotic/Biotic Stress Responses. International Journal of Molecular Sciences. 2017; 18: 1–17. 10.3390/ijms18081581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davey MW, Graham NS, Vanholme B, Swennen R, May ST, Keulemans J. Heterologous oligonucleotide microarrays for transcriptomics in a non-model species; a proof-of-concept study of drought stress in Musa. BMC Genomics. 2009; 10: 1–19. 10.1186/1471-2164-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisar SYS, Motafakkerazad R, Hossain MM, Rahman IMM. Water Stress in Plants: Causes, Effects and Responses In: Rahman IMM. (Ed.). Water Stress. INTECH Open Access Publisher; ISBN 978-953-307-963-9; 2012. pp 1–14. 10.5772/39363 [Google Scholar]
- 9.Van Asten PJA, Fermont AM, Taulya G. Drought is a major yield loss factor for rainfed East African highland banana. Agricultural Water Management. 2011; 98: 541–552. 10.1016/j.agwat.2010.10.005 [Google Scholar]
- 10.Kissel E, Van Asten P, Swennena R, Lorenzenb J, Carpentiera SC Transpiration efficiency versus growth: Exploring the banana biodiversity for drought tolerance. Scientia Horticulturae. 2015; 185: 175–182. 10.1016/j.scienta.2015.01.035 [DOI] [Google Scholar]
- 11.Miao H, Sun P, Liu Q, Miao Y, Liu J, Zhang K. et al. Genome-wide analyses of SWEET family proteins reveal involvement in fruit development and abiotic/biotic stress responses in banana. Scientific Reports. Scientific Reports. 2017; 7: 1–15. 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Hont A, Denoeud F, Aury J-M, Baurens F-C., Carreel F, Garsmeur O, et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature. 2012; 488: 213–217. 10.1038/nature11241 [DOI] [PubMed] [Google Scholar]
- 13.Gensous N, Aurélie M, Thomas B, Patrick B, Estibaliz L, Julien S,et al. Predictive biological markers of systemic lupus erythematosus flares: a systematic literature review. Arthritis Research & Therapy 2017; 19: 1–12. 10.1186/s13075-017-1442-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Xiaoling W, Xun D, Yanjiao M, Siy H, Yong W. Association of CD14-260 (-159) C/T and Alzheimer’s disease: systematic review and trial sequential analyses. 2018; 125: 1313–1318. Journal of Neural Transmission. 10.1007/s00702-018-1896-y [DOI] [PubMed] [Google Scholar]
- 15.Rustagi A, Jain S, Kumar D, Shekhar S, Jain M, Bhat V, et al. High Efficiency Transformation of Banana [Musa acuminata L. cv. Matti (AA)] for Enhanced Tolerance to Salt and Drought Stress Through Overexpression of a Peanut Salinity-Induced Pathogenesis-Related Class 10 Protein. Molecular Biotechnology. 2015; 57: 27–35. 10.1007/s12033-014-9798-1 [DOI] [PubMed] [Google Scholar]
- 16.Hu W, Hiu X, Huang C, Yan Y, Tie W, Ding Z, et al. Genome-Wide Identification and Expression Analyses of Aquaporin Gene Family during Development and Abiotic Stress in Banana. International Journal of Molecular Sciences. 2015; 16: 19728–19751. 10.3390/ijms160819728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu W, Zuo J, Hou X, Yan Y, Wei Y, Liu J, et al. The auxin response factor gene family in banana: genome-wide identification and expression analyses during development, ripening, and abiotic stress. Frontiers in Plant Science. 2015; 6: 1–16. 10.3389/fpls.2015.00742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng X, Chen F, Liu W, Thu MK, Zhang Z, Chen Y, et al. Molecular Characterization of MaCCS, a Novel Copper Chaperone Gene Involved in Abiotic and Hormonal Stress Responses in Musa acuminata cv. Tianbaojiao. International Journal of Molecular Sciences. 2016; 17: 1–13. 10.3390/ijms17040441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei Y, Hu W, Xia F, Zeng H, Li X, Yan Y, et al. Heat shock transcription factors in banana: genome-wide characterization and expression profile analysis during development and stress response. Scientific Reports. 2016; 6: 1–11. 10.1038/s41598-016-0001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Hu W, Liu J, Zhang J, Jia C, Hu H, et al. A banana aquaporin gene, MaPIP1;1, is involved in tolerance to drought and salt stresses. BMC Plant Biology. 2014; 14: 1–14. 10.1186/1471-2229-14-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng X, Lai Z, Lin Y, Lai G, Lian C. Genome-wide identification and characterization of the superoxide dismutase gene family in Musa acuminata cv. Tianbaojiao (AAA group). BMC Genomics. 2015; 16: 1–16. 10.1186/1471-2164-16-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu H-Y., Dai J-R., Feng D-R., Liu B., Wang H-B., Wang J-F Characterization of a Novel Plantain Asr Gene, MpAsr, that is Regulated in Response to Infection of Fusarium oxysporum f. sp. cubense and Abiotic Stresses. Journal of Integrative Plant Biology. 2010; 52: 315–323. 10.1111/j.1744-7909.2010.00912.x [DOI] [PubMed] [Google Scholar]
- 23.Shekhawat UKS, Srinivas L, Ganapathi TR. MusaDHN-1, a novel multiple stress-inducible SK3-type dehydrin gene, contributes affirmatively to drought- and salt-stress tolerance in banana. Planta. 2011; 234: 915–932. 10.1007/s00425-011-1455-3 [DOI] [PubMed] [Google Scholar]
- 24.Tak H, Negi S, Ganapathi TR. Banana NAC transcription factor MusaNAC042 is positively associated with drought and salinity tolerance. Protoplasma. 2017; 254: 803–816. 10.1007/s00709-016-0991-x [DOI] [PubMed] [Google Scholar]
- 25.Negi S, Tak H, Ganapathi TR. Expression analysis of MusaNAC68 transcription factor and its functional analysis by overexpression in transgenic banana plants. Plant Cell, Tissue and Organ Culture. 2015; 125: 59–70. 10.1007/s11240-015-0929-6 [Google Scholar]
- 26.Sreedharan S, Shekhawat UKS, Ganapathi TR. Transgenic banana plants overexpressing a native plasma membrane aquaporin MusaPIP1;2 display high tolerance levels to different abiotic stresses. Plant Biotechnology Journal. 2013; 11: 942–952. 10.1111/pbi.12086 [DOI] [PubMed] [Google Scholar]
- 27.Sreedharan S, Shekhawat UK.S, Ganapathi TR. MusaSAP1, a A20/AN1 zinc finger gene from banana functions as a positive regulator in different stress responses. Plant Molecular Biology. 2012; 80: 1–15. 10.1007/s11103-012-9947-5 [DOI] [PubMed] [Google Scholar]
- 28.Shekhawat UK, Ganapathi TR. MusaWRKY71 overexpression in banana plants leads to altered abiotic and biotic stress responses. PLoS One. 2013; 8: 1–7. 10.1371/journal.pone.0075506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muthusamy M, Uma S, Backiyarani S, Saraswathi MS, Chandrasekar A. Transcriptomic Changes of Drought-Tolerant and Sensitive Banana Cultivars Exposed to Drought Stress. Frontiers in Plant Science. 2016; 7: 1–14. 10.3389/fpls.2016.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miao H, Sun P, Liu Q Miao Y, Liu J., Zhang K, et al. Genome-wide analyses of SWEET family proteins reveal involvement in fruit development and abiotic/biotic stress responses in banana. Scientific Reports. Scientific Reports. 2017; 7: 1–15. 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goel R, Pandey A, Trivedi PK, Asif MH. Genome-Wide Analysis of the Musa WRKY Gene Family: Evolution and Differential Expression during Development and Stress. Frontiers in Plant Science. 2016; 7: 1–13. 10.3389/fpls.2016.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthusamy M, Uma S, Backiyarani S, Saraswathi MS. Computational prediction, identification, and expression profiling of microRNAs in banana (Musa spp.) during soil moisture deficit stress. Journal of Horticultural Science & Biotechnology. 2014; 89: 208–214. 10.1080/14620316.2014.11513070 [Google Scholar]
- 33.Muthusamy M, Uma S, Backiyarani S, Saraswathi MS. Genome-wide screening for novel, drought stress-responsive long non-coding RNAs in drought-stressed leaf transcriptome of drought-tolerant and -susceptible banana (Musa spp) cultivars using Illumina high-throughput sequencing. Plant Biotechnology Reports. 2015; 9: 279–286. 10.1007/s11816-015-0363-6 [Google Scholar]
- 34.Mattos‑Moreira LA, Ferreira CF, Amorim EP, Pirovani CP, Andrade EM, Coelho Filho MA et al. Differentially expressed proteins associated with drought tolerance in bananas (Musa spp.). Acta Physiologiae Plantarum. 2018; 40: 1–15. 10.1007/s11738-018-2638-3 [DOI] [Google Scholar]
- 35.Vanhove A-C, Vermaelen W, Panis B, Swennen R, Carpentier S. Screening the banana biodiversity for drought tolerance: can an in vitro growth model and proteomics be used as a tool to discover tolerant varieties and understand homeostasis. Frontiers in Plant Science. 2012; 3: 1–10. 10.3389/fpls.2012.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahmood M, Bidabadi SS, Ghobadi C, Gray DJ. Effect of methyl jasmonate treatments on alleviation of polyethylene glycol -mediated water stress in banana (Musa acuminata cv. ‘Berangan’, AAA) shoot tip cultures. Plant Growth Regulation. 2012; 68: 161–169. 10.1007/s10725-012-9702-6 [DOI] [Google Scholar]
- 37.Mahouachi J, Lopez-Climent MF, Gomez-Cadenas A. Hormonal and Hydroxycinnamic Acids Profiles in Banana Leaves in Response to Various Periods of Water Stress. The Scientific World Journal. 2014; 2014: 1–9. 10.1155/2014/540962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bidabadi SS, Mahmood M, Baninasab B, Ghobadi C. Influence of salicylic acid on morphological and physiological responses of banana (Musa acuminata cv. ‘Berangan’, AAA) shoot tips to in vitro water stress induced by polyethylene glycol. Plant Omics Journal. 2012; 5: 33–39. [Google Scholar]
- 39.Li L-F, Wang H-Y, Zhang C, Wang X-F, Shi F-X, Chen W-N, et al. Origins and Domestication of Cultivated Banana Inferred from Chloroplast and Nuclear Genes. PLoS ONE. 2013; 8: 1–11. 10.1371/journal.pone.0080502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.FAO (2017). Banana market review. Food and Agriculture Organization of the United Nations. 2015–2016. Disponível em: http://www.fao.org/economic/est/est-commodities/bananas/en/
- 41.Thill DC, Schimman RD, Appleby AP. Osmotic stability of mannitol and polyethyleneglycol 20000 solutions used as seed germination media. Agronomy Journal. 1979;71:105–108. 10.2134/agronj1979.00021962007100010027x [Google Scholar]
- 42.Hu W, Yan Y, Shi H, Liu J, Miao H, Tie W, et al. The core regulatory network of the abscisic acid pathway in banana: genome-wide identification and expression analyses during development, ripening, and abiotic stress. BMC Plant Biology 2017; 17: 1–16. 10.1186/s12870-016-0951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bidabadi SS, Mahmood M, Meon S, Wahab Z, Ghobadi C. Evaluation of in vitro Water Stress Tolerance among EMS–Induced Variants of Banana (Musa spp., AAA), Using “Morphological, Physiological and Molecular” Traits. Journal of Crop Science and Biotechnology. 2011; 14: 255–263. 10.1007/s12892-011-0073-8 [Google Scholar]
- 44.Bidabadi SS, Meon S, Wahab Z, Subramaniam S, Mahmood M, In'vitro'selection and characterization of water stress tolerant lines among ethyl methanesulphonate (ems) induced variants of banana ('Musa'spp., with AAA genome). Australian Journal of Crop Science. 2012; 6: 567–575. [Google Scholar]
- 45.Placide R, Christian CS, Rony S. Development of in vitro technique to screen for drought tolerant banana varieties by sorbitol induced osmotic stress. African Journal of Plant Science. 2012; 6: 416–425. 10.5897/AJPS12.101 [Google Scholar]
- 46.Sampangi-Ramaiah MH, Ravishankar KV, Seetharamaiah SK, Roy TK, Hunashikatti LR, et al. Barrier against water loss: relationship between epicuticular wax composition, gene expression and leaf water retention capacity in banana. Functional Plant Biology. 2016; 43: 492–501. 10.1071/FP15296 [DOI] [PubMed] [Google Scholar]
- 47.Ochola D, Ocimati W, Tinzaara W, Blomme G, Karamura EB. Effects of water stress on the development of banana xanthomonas wilt disease. Plant Pathology. 2105; 64: 552–558. 10.1111/ppa.12281 [Google Scholar]
- 48.Carpentier SC, Vertommen A, Swennen R, Witters E, Fortes C, Souza MT, et al. Sugar-Mediated Acclimation: The Importance of Sucrose Metabolism in Meristems. Journal of Proteome Research. 2010; 9; 5038–5046. 10.1021/pr100321v [DOI] [PubMed] [Google Scholar]
- 49.Msogoya TJ, Grout BW Cytosine DNA methylation changes drought stress responses in tissue culture derived banana (Musa AAA–East Africa) plants. Journal of Applied Biosciences. 2012; 49: 3383–3387. [Google Scholar]
- 50.Torres-Ruiz JM, Diaz-Espejo A, Perez-Martin A, Hernandez-Santana V. Role of hydraulic and chemical signals in leaves, stems and roots in the stomatal behaviour of olive trees under water stress and recovery conditions. Tree Physiology. 2014; 35: 415–424. 10.1093/treephys/tpu055 [DOI] [PubMed] [Google Scholar]
- 51.Surendar KK, Devi DD, Ravi I, Jeyakuma R P, Velayudham K. Effect of Water Stress on Leaf Temperature, Transpiration Rate, Stomatal Diffusive Resistance and Yield of Banana. Plant Gene and Trait. 2013; 8: 43–47. 10.5376/pgt.2013.04.0008 [Google Scholar]
- 52.Surendar KK, Devi DD, Ravi I, Krishnakumar S, Kumar SR, Velayudham K. Impact of Water Deficit on Photosynthetic Pigments and Yield of Banana Cultivars and Hybrids. Plant Gene and Trait. 2013; 4: 17–24. 10.5376/pgt.2013.04.0004 [Google Scholar]
- 53.Surendar KK, Devi DD, Ravi I, Jeyakumar P, Velayudham K. Water Stress Affects Plant Relative Water Content, Soluble Protein, Total Chlorophyll Content and Yield of Ratoon Banana. International Journal of Horticulture. 2013; 3: 96–103. 10.5376/ijh.2013.03.0017 [Google Scholar]
- 54.Surendar KK, Devi DD, Jeyakumar P, Velayudham K, Ravi I. Changes in Proline and Polyphenol oxidase enzyme activity in some Banana Cultivars and Hybrids under water stress. Genomics and Applied Biology. 2015; 6: 1–6. 10.5376/gab.2015.06.0004 [Google Scholar]
- 55.Chandrashekar N, Ravishankar KV, Laxman RH, Rekha A, Swarupa V. Physiological and biochemical changes during moisture stress in banana. Asian Journal of Bio Science. 2012; 7: 1–4. [Google Scholar]
- 56.Surendar KK, Devi DD, Ravi I, Jeyakumar P, Velayudham K. Physiological and biochemical behavior in banana cultivars and hybrids under water deficit. African Journal of Agricultural Research. 2013; 8: 4198–4208. 10.5897/AJAR2013.7464 [Google Scholar]
- 57.Surendar K.K, Devi DD, Ravi I, Jeyakumar P, Velayudham K. Influence of water stress on antioxidative enzymes and yield of banana cultivars and hybrids. Pakistan Journal of Biological Sciences. 2013; 6; 1997–2002. 10.3923/pjbs.2013.1997.2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
The minimal underlying data set of the study is available at the link: http://doi.org/10.5281/zenodo.1454052.