Abstract
There is a need for a screening tool with capacities of accurate detection of early mild cognitive impairment (MCI) and dementia and is suitable for use in a range of languages and cultural contexts. This research aims to evaluate the psychometric and diagnostic properties of the Taiwan version of Qmci (Qmci-TW) screen and to explore the discriminating ability of the Qmci-TW in differentiating among normal controls (NCs), MCI and dementia. Thirty-one participants with dementia and 36 with MCI and 35 NCs were recruited from a neurology department of regional hospital in Taiwan. Their results on the Qmci-TW, Taiwanese version of the Montreal Cognitive Assessment (MoCA), and Traditional Chinese version of the Mini–Mental State Examination (MMSE) were compared. For analysis, we used Cronbach’s α, intraclass correlation coefficient, Spearman’s ρ, Kruskal–Wallis test, receiver operating characteristic curve analysis, and multivariate analysis, as appropriate. The Qmci-TW exhibited satisfactory test–retest reliability, internal consistency, and interrater reliability as well as a strong positive correlation with results from the MoCA and MMSE. The optimal cut-off score on the Qmci-TW for differentiating MCI from NC was ≤ 51.5/100 and dementia from MCI was ≤ 31/100. The MoCA exhibited the highest accuracy in differentiating MCI from NC, followed by the Qmci-TW and then MMSE; whereas, the Qmci-TW and MMSE exhibited the same accuracy in differentiating dementia from MCI, followed by the MoCA. The Qmci-TW may be a useful clinical screening tool for a spectrum of cognitive impairments.
Introduction
The number of people aged older than 65 years is increasing worldwide [1]. The population of older people with dementia is expected to increase concurrently with global aging [2, 3]. The reported proportion of individuals with mild cognitive impairment (MCI) is expected even higher than of those with dementia [4, 5]; however, in clinical practice, most MCI cases in older adults remain unidentified. Although people with MCI are completely capable of self-care activities, they exhibit slight impairment in memory, attention, orientation to time, visuospatial perception, problem solving, instrumental activities of daily living, and judgment; these impairments may affect community participation and job security [6, 7]. Individuals with MCI involving instrumental activities of daily living limitations are more likely to develop dementia [7]. In general, people with MCI are at a greater risk of developing dementia [8] than other aged-matched individuals [9], and approximately half of progressions into dementia occur within a 5-year period [10]. Differentiation among individuals with MCI and dementia and normal controls (NCs) is critical for appropriate pharmacotherapeutic and nonpharmacotherapeutic treatment courses [11, 12].
In Taiwan, the most widely used cognitive screening instruments are the Traditional Chinese version of the Mini–Mental State Examination (MMSE) [13] and the Taiwanese version of the Montreal Cognitive Assessment (MoCA) [14]. On comparing the MMSE and MoCA, the MoCA can efficiently discriminate MCI, whereas the MMSE can efficiently discriminate dementia [15]. The ceiling effect and lack of dynamic performance range in the MMSE increase the likelihood that individuals in the early stages of MCI or dementia score within the normal range [16]. Moreover, half of the MoCA subtests have shown significant floor effects in assessments of Parkinson’s disease [17]. Floor and ceiling effects must be considered in cognitive function tests. Cognitive screening tools with appropriate diagnostic properties are critical for effective clinical practice.
The Quick Mild Cognitive Impairment (Qmci) screen is a brief cognitive screening instrument, validated for use in Australian, Canadian, Dutch, Irish, and Turkish populations [18–22]. The Qmci, developed through the AB Cognitive Screen 135 [23], added logical memory and reweights the scores of all subtests for maximizing sensitivity and specificity for differentiating NCs from MCI. The Qmci is more accurate in differentiating individuals with MCI from NCs than the standardized MMSE [24]; it also has similar accuracy to and greater specificity than the MoCA [18–20]. The Qmci is strongly associated with the Clinical Dementia Rating scale, Alzheimer’s Disease Assessment Scale-Cognitive Section and Lawton-Brody IADL scale [24]. The overview of the Qmci, MMSE, and MoCA are presented in S1 Table.
In busy clinical settings, the Qmci is superior to the MMSE and MoCA with similar reliability, less administered time and better diagnostic properties for the purpose of early detection and treatment. Thus, because the Qmci is useful for detecting early MCI and variations in cognitive function between NCs and those with severe dementia, a Taiwan version is required. The research questions are (1) whether the Taiwan version of the Qmci (Qmci-TW) screen yields sound psychometric properties (reliability and validity) and diagnostic properties (sensitivity, specificity, and predictive values) in Taiwan population, and (2) whether the Qmci-TW exhibits satisfactory discriminating ability to differentiate NCs from MCI and/or dementia from MCI. This study aims to assess the psychometric and diagnostic properties of the Qmci-TW screen and to explore the discriminating ability of the Qmci-TW in differentiating among NCs, MCI and dementia.
Materials and methods
Participants
A power calculation based on published data of the Qmci related studies was performed using the PAIRSetc.EXE program of the WinPepi software [25, 26]. We used the McNemar’s test to examine the differences of the area under the curve (AUC) between the Qmci and MMSE, and the Qmci and MoCA for matched samples to estimate the required sample sizes. The results indicated that the study would require minimum 80 paired observations to demonstrate significant difference between the instruments and their ability to differentiate MCI from NCs, at a significance level of 0.05 and power of 80% [20, 21].
Participants were categorized into three groups: MCI, dementia, and NCs. Between May 2017 and December 2017, participants were recruited consecutively from the neurology outpatient department of regional hospital in New Taipei, Taiwan. NCs without subjective and objective cognitive problems were recruited from convenience sampling. Only the individuals aged ≥ 65 years and able to follow instructions and understand the content of the assessments through verbal communication were eligible for participation. Dementia (Alzheimer’s disease or vascular or mixed dementia subtypes) and amnestic-type MCI were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [27] and the National Institute of Neurological and Communicative Diseases and Stroke and the Alzheimer's Disease and Related Disorders Association [28] criteria and National Institute on Aging-Alzheimer’s Association workgroup’s diagnostic guidelines for Alzheimer’s disease [29], as applicable. The participants with MCI and dementia were classified using the Clinical Dementia Rating scale global scores of 0.5 and 1–3, respectively [6]. Participants were excluded if they scored > 7 on the Geriatric Depression Scale-Short Form, indicating depression status [30]; or were diagnosed with other MCI or dementia subtypes, including frontotemporal dementia, Parkinson’s disease, or Lewy Body dementia, that present infrequently, typically with exaggerated functional deficits and different MCI syndromes.
The study protocol for the protection of human participants and the consent procedure was approved by the Institutional Review Board of the Taipei Hospital, Ministry of Health and Welfare (TH-IRB-0016-0033). Before the study, the purpose and procedure of the research were explained to all participants. NCs signed informed consents by themselves; whereas, participants with MCI or dementia signed informed consents along with their legal guardians. The participants’ background information was protected as confidential and was used only for research purposes.
Data collection
In this cross-sectional study, the demographic data, including age in years, gender, and years of education, were collected. The same trained rater, blinded to final diagnosis, alternately and randomly administered the Qmci-TW, MoCA, MMSE, Barthel Index [31] and Lawton Instrumental Activities of Daily Living scale [32] on the same day. Additionally, after 2 weeks, the Qmci-TW, with alternative versions [33], was readministered to randomly selected participants by the two trained raters, blinded to final diagnosis.
In total, 119 participants were recruited, but 17 participants who scored > 7 on the Geriatric Depression Scale-Short Form were excluded. The remaining 102 participants (47 men and 55 women) were included for further study. Among the final sampled patients, 35 (34.3%) were NCs, 36 (35.3%) had received a MCI diagnosis, and 31 (30.4%) had received a diagnosis of dementia. Participants with dementia, classified using Clinical Dementia Rating scale global scores, were grouped according to mild (n = 12), moderate (n = 13), and severe dementia (n = 6). The NCs (p < 0.001) and participants with MCI (p = 0.005) were significantly younger than those with dementia. NCs had received significantly more education than those with MCI (p = 0.007) or dementia (p < 0.001). The mean Geriatric Depression Scale-Short Form scores among NCs was significantly lower than that among participants with dementia (p < 0.001). The demographic characteristics of the participants are presented in Table 1.
Table 1. The demographic characteristics of the participants stratified by NC, MCI and dementia.
| Characteristics | Total (n = 102) |
NC (n = 35) |
MCI (n = 36) |
Dementia (n = 31) |
χ2 (df = 2) |
Pairwise comparisona |
|---|---|---|---|---|---|---|
| Age in years | 77.13 ± 7.49 | 73.64 ± 6.39 | 76.22 ± 7.41 | 82.11 ± 6.13 | 22.91** | 3 > 1, 3 > 2 |
| Gender [Male, Number (%)] | 47 (46.1%) | 17 (48.6%) | 14 (38.9%) | 16 (51.6%) | 1.22 | |
| Years in education | 7.26 ± 4.87 | 10.03 ± 3.85 | 6.83 ± 4.87 | 4.61 ± 4.35 | 23.48** | 1 > 2, 1 > 3 |
| GDS-SF | 2.75 ± 1.93 | 1.83 ± 1.72 | 2.69 ± 1.62 | 3.84 ± 1.97 | 17.48** | 3 > 1 |
The listed statistics were mean ± standard deviation (SD) or frequency (percentage).
Abbreviations: NC, Normal control; MCI, Mild cognitive impairment; and GDS-SF, Geriatric Depression Scale-Short Form.
a1. Normal control group; 2. Mild cognitive impairment group; and 3. Dementia group.
*p < 0.05.
**p < 0.001.
Instruments
The Qmci-TW is a performance test, containing six subtests, namely orientation, registration, clock drawing, delayed recall, verbal fluency, and logical memory. The Qmci can be administered and scored with a median time of under 5 minutes, and the alternative word groups and versions of the registration and recall task and the verbal fluency and logical memory subtests in the Qmci have been validated for convenience [33]. We translated the Qmci into the Qmci-TW following the established translation guidelines [34]. The Qmci-TW was administered and scored using the test manual’s instructions. The Qmci-TW scores ranged from 0 to 100, with a higher score indicating greater cognitive function.
The MMSE is also a performance test, standardized and validated to measure cognitive functions in orientation, registration, attention, calculation, recall, language, and copying. MMSE scores range from 0 to 30, with a higher score indicating greater cognitive function [13].
The MoCA is a standardized and validated tool designed to measure cognitive functions in visuospatial and executive tasks, naming, memory, attention, language, abstraction, delayed recall, and orientation. In MoCA scoring, 1 point is added for individuals whose educational level is ≤ 12, with scores ranging from 0 to 30 and a higher score indicating greater cognitive function [14].
The Barthel Index is a validated tool designed to measure activities of daily living independence, specifically regarding feeding, bathing, grooming, dressing, bowels, bladder, toilet use, transfers, mobility, and stairs. Barthel Index scores range from 0 to 20, with a higher score indicating greater independence [31].
The Lawton Instrumental Activities of Daily Living scale is a validated tool designed to measure instrumental activities of daily living independence, specifically with regard to telephone use, shopping, food preparation, housekeeping, laundry, transport mode, medication responsibility, and finance-handling ability. The scores range from 0 to 8, with a higher score indicating greater independence in complex activities of daily living [32].
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics software, version 19.0 (IBM Corporation, Somers, NY, U.S.A.) and the R 3.4.3 software (R Foundation for Statistical Computing, Vienna, Austria). The Shapiro-Wilk test was used to test for normality, and the results indicated that most data were nonparametric. The distributional properties of continuous variables are presented as means ± standard deviations and categorical variables as frequencies and percentages. Missing values were given zero score following the manuals and scoring guidelines of each assessments. For the Qmci-TW, we used Cronbach’s α, intraclass correlation coefficients (ICCs), and Spearman’s ρ for internal consistency, test–retest and interrater reliability, and concurrent and construct validity [35], respectively. Data were analysed using Kruskal–Wallis test and post hoc Dunn’s test, and receiver operating characteristic (ROC) curves for between-group comparisons, and AUC [35], respectively.
Floor and ceiling effects
Frequency was used to calculate the lowest and highest raw scores for a subtest as the floor and ceiling, respectively. Floor and ceiling effects were considered significant if they were exhibited in more than 20% of the sample [36]. The ceiling and floor effects indicated that a subtest was too easy and too difficult for the study population, respectively. A subtest with ceiling or floor effects is considered non-sensitive, and thus, unsuitable for use in discriminating between groups [37].
Internal consistency
Cronbach’s α coefficient was used to examine the internal consistency of the Qmci-TW screen subtests. This coefficient estimates the reliability of an instrument according to the consistency of the subtests, accounting for the number of subtests. Cronbach’s α > 0.7 was considered acceptable for internal consistency [37]. Inter-item correlation was also used to examine the correlations between the subtests of the Qmci-TW screen.
Test–retest reliability and interrater reliability
The ICCs were used to examine the test-retest and interrater reliability of the Qmci-TW screen. The ICCs of 0.75–1.00 indicated an excellent reliability [38].
Concurrent and construct validity
The Spearman’s correlation coefficients were estimated between the Qmci-TW screen and the other validated cognitive screening instruments, MoCA and MMSE, to determine concurrent validity, and between Qmci-TW and the validated activities of daily living and instrumental activities of daily living assessments, Barthel Index and Lawton Instrumental Activities of Daily Living scale, to determine construct validity. The Spearman’s ρ of 0.4–0.8 indicated an acceptable validity [39].
Sensitivity, specificity, and predictive values
The ROC curve analysis was used to calculate diagnostic accuracy based on the AUC. The AUC ≥ 0.8 and ≥ 0.9 represented good and excellent discriminating powers respectively. The optimal cut-off scores were derived using Youden’s Index [40]. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated based on optimal cut-off scores.
To compare the predictive power among the three key instruments, Qmci-TW, MMSE, and MoCA, multivariate analysis was conducted by fitting two logistic regression models of (1) MCI or dementia versus NC in all subjects (n = 102) and (2) dementia versus MCI in the subjects with MCI or dementia (n = 67). The goal of regression analysis was to find one or a few parsimonious regression models that fitted the observed data well for effect estimation and/or outcome prediction. To ensure a good quality of analysis, the model-fitting techniques for (1) variable selection, (2) goodness-of-fit assessment, and (3) regression diagnostics and remedies were used in our logistic regression analyses. Specifically, the stepwise variable selection procedure (with iterations between the forward and backward steps) was applied to obtain the best candidate final logistic regression model using the My.stepwise package of R [41]. As listed in Tables 1 and 2, all the univariate significant and non-significant covariates, including age, gender, education level, and so on, were put on the variable list to be selected. Simple and multiple generalized additive models were fitted to detect nonlinear effects of continuous covariates and identify appropriate cut-off points for discretizing continuous covariates, if necessary, during the stepwise variable selection procedure. The significance levels for entry and for stay were set to 0.15 for being conservative. Then, with the aid of substantive knowledge, the best candidate final logistic regression model was identified manually by dropping the covariates with p value > 0.05 one at a time until all regression coefficients were significantly different from 0. Moreover, the goodness-of-fit measure, the estimated area under the ROC curve (also called the c statistic), and the Hosmer-Lemeshow goodness-of-fit test were examined to assess the goodness-of-fit of the fitted logistic regression model. Finally, the statistical tools of regression diagnostics for residual analysis, detection of influential cases, and check of multicollinearity were applied to discover any model or data problems.
Table 2. The clinical characteristics of the participants stratified by NC, MCI and dementia.
| Characteristics | Total (n = 102) |
NC (n = 35) |
MCI (n = 36) |
Dementia (n = 31) |
χ2 (df = 2) |
Pairwise comparison a |
|---|---|---|---|---|---|---|
| Qmci-TW | 42.80 ± 21.84 | 64.06 ± 8.43 | 43.13 ± 14.71 | 18.44 ± 11.48 | 71.19** | 1 > 2 > 3 |
| Orientation | 7.79 ± 2.83 | 9.97 ± 0.17 | 8.39 ± 1.90 | 4.65 ± 2.54 | 62.32** | 1 > 2 > 3 |
| Registration | 3.58 ± 1.21 | 4.49 ± 0.56 | 3.64 ± 0.72 | 2.48 ± 1.31 | 49.01** | 1 > 2 > 3 |
| Clock drawing | 8.87 ± 5.59 | 13.14 ± 2.80 | 9.17 ± 4.77 | 3.71 ± 4.56 | 47.42** | 1 > 2 > 3 |
| Delayed recall | 10.16 ± 8.01 | 17.49 ± 3.08 | 10.00 ± 7.06 | 2.06 ± 4.11 | 57.58** | 1 > 2 > 3 |
| Verbal fluency | 6.20 ± 2.76 | 8.37 ± 2.07 | 6.38 ± 1.94 | 3.53 ± 1.89 | 53.25** | 1 > 2 > 3 |
| Logical memory | 6.20 ± 5.12 | 10.57 ± 4.27 | 5.56 ± 4.23 | 2.00 ± 2.37 | 52.19** | 1 > 2 > 3 |
| MoCA | 16.75 ± 7.81 | 24.51 ± 2.47 | 16.61 ± 5.05 | 8.13 ± 4.65 | 70.04** | 1 > 2 > 3 |
| Visuospatial and executive function | 2.58 ± 1.60 | 3.91 ± 1.20 | 2.50 ± 1.34 | 1.16 ± 0.86 | 49.10** | 1 > 2 > 3 |
| Naming | 1.90 ± 1.17 | 2.71 ± 0.52 | 1.92 ± 1.08 | 0.97 ± 1.11 | 35.09** | 1 > 2 > 3 |
| Memory | 1.55 ± 0.61 | 1.89 ± 0.32 | 1.61 ± 0.55 | 1.10 ± 0.65 | 28.68** | 1 > 3, 2 > 3 |
| Attention | 0.65 ± 0.48 | 1.00 ± 0.00 | 0.69 ± 0.47 | 0.19 ± 0.40 | 46.90** | 1 > 2 > 3 |
| Calculation | 2.23 ± 0.98 | 2.97 ± 0.17 | 2.33 ± 0.72 | 1.26 ± 1.00 | 50.42** | 1 > 2 > 3 |
| Repetition | 0.77 ± 0.80 | 1.37 ± 0.73 | 0.67 ± 0.72 | 0.23 ± 0.43 | 34.45** | 1 > 2, 1 > 3 |
| Verbal fluency | 0.48 ± 0.50 | 0.94 ± 0.24 | 0.39 ± 0.49 | 0.06 ± 0.25 | 52.16** | 1 > 2 > 3 |
| Abstraction | 0.37 ± 0.60 | 0.83 ± 0.71 | 0.19 ± 0.40 | 0.06 ± 0.25 | 31.95** | 1 > 2, 1 > 3 |
| Delayed recall | 1.06 ± 1.41 | 2.23 ± 1.42 | 0.78 ± 1.17 | 0.06 ± 0.25 | 44.63** | 1 > 2 > 3 |
| Orientation | 4.32 ± 2.05 | 5.91 ± 0.28 | 4.69 ± 1.49 | 2.10 ± 1.78 | 59.16** | 1 > 2, 1 > 3 |
| MMSE | 22.68 ± 6.36 | 28.29 ± 1.18 | 23.61 ± 4.02 | 15.26 ± 4.58 | 75.20** | 1 > 2 > 3 |
| Orientation to time | 3.57 ± 1.73 | 4.91 ± 0.28 | 3.94 ± 1.15 | 1.61 ± 1.48 | 61.08** | 1 > 2 > 3 |
| Orientation to place | 3.83 ± 1.61 | 4.94 ± 0.24 | 4.17 ± 1.21 | 2.19 ± 1.60 | 58.35** | 1 > 2 > 3 |
| Registration | 2.98 ± 0.14 | 3.00 ± 0.00 | 2.97 ± 0.17 | 2.97 ± 0.18 | 1.07 | |
| Calculation | 3.28 ± 1.78 | 4.66 ± 0.54 | 3.42 ± 1.46 | 1.58 ± 1.61 | 45.70** | 1 > 2 > 3 |
| Recall | 1.42 ± 1.14 | 2.03 ± 0.89 | 1.47 ± 1.11 | 0.68 ± 1.01 | 23.49** | 1 > 3, 2 > 3 |
| Naming | 1.98 ± 0.14 | 2.00 ± 0.00 | 2.00 ± 0.00 | 1.94 ± 0.25 | 4.63 | |
| Repetition | 0.91 ± 0.29 | 1.00 ± 0.00 | 0.92 ± 0.28 | 0.81 ± 0.40 | 7.60* | 1 > 3 |
| Reading comprehension | 0.76 ± 0.43 | 1.00 ± 0.00 | 0.81 ± 0.40 | 0.45 ± 0.51 | 27.72** | 1 > 3, 2 > 3 |
| Writing | 0.67 ± 0.47 | 1.00 ± 0.00 | 0.69 ± 0.47 | 0.26 ± 0.45 | 40.51** | 1 > 2 > 3 |
| Verbal comprehension and executive function | 2.80 ± 0.58 | 3.00 ± 0.00 | 2.78 ± 0.59 | 2.61 ± 0.80 | 8.34* | 1 > 3 |
| Construction | 0.48 ± 0.50 | 0.74 ± 0.44 | 0.50 ± 0.51 | 0.16 ± 0.37 | 22.14** | 1 > 3, 2 > 3 |
| Barthel Index | 19.25 ± 2.32 | 19.89 ± 0.47 | 19.94 ± 0.33 | 17.74 ± 3.79 | 27.32** | 1 > 3, 2 > 3 |
| Lawton IADL scale | 5.82 ± 2.41 | 7.54 ± 0.89 | 6.69 ± 1.28 | 2.87 ± 1.84 | 64.90** | 1 > 3, 2 > 3 |
The listed statistics were mean ± standard deviation (SD).
Abbreviations: NC, Normal control; MCI, Mild cognitive impairment; Qmci-TW, Taiwan version of the Quick Mild Cognitive Impairment screen; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; and Lawton IADL scale, Lawton Instrument Activities of Daily Living scale.
a1: Normal control group; 2: Mild cognitive impairment group; and 3: Dementia group.
*p < 0.05.
**p < 0.001.
Results
The total and most subtests of the Qmci-TW, MoCA, and MMSE scores of the NCs were significantly higher than those of participants with MCI and dementia groups, except for memory of the MoCA and registration, recall, naming, repetition, reading comprehension, verbal and executive function, and construction of the MMSE. Moreover, most of these scores were significantly higher in the MCI group than in the dementia group, except for repetition, abstraction, and orientation of the MoCA and registration, naming, repetition, and verbal and executive function of the MMSE. The Barthel Index and Lawton Instrumental Activities of Daily Living scale scores of the NCs and participants with MCI were significantly higher than those of participants with dementia (all p’s < 0.001). The clinical characteristics of the participants are presented in Table 2.
Although some of the demographic and clinical characteristics, including age, educational level, Geriatric Depression Scale-Short Form score, and so on, were significantly different among the subjects of NC, MCI, and dementia (Tables 1 and 2), logistic regression analysis of MCI or dementia versus NC in all subjects (n = 102) revealed that after the keen competitions during the stepwise variable selection procedure, the MoCA score, food preparation score of the Lawton IADL scale in the past, and calculation score of the MMSE stayed in the final logistic regression model as the most important statistically significant predictors (Table 3). Both the estimated area under the ROC curve = 0.99 and the modified Hosmer and Lemeshow goodness-of-fit F test p = 0.7479 (df = 9, 92) indicated an excellent fit.
Table 3. Multivariate analysis of the predictors of MCI or dementia versus NC among all subjects by fitting multiple logistic regression model with the stepwise variable selection method.
| Covariate | Estimated Regression Coefficient | Estimated Standard Error |
z Value | p Value | Estimated Odds Ratio | 95% C.I. of Odds Ratio |
|---|---|---|---|---|---|---|
| Intercept | 30.069 | 9.211 | 3.26 | 0.0011 | – | – |
| MoCA score (0, 1, …, 30) | -0.817 | 0.240 | -3.40 | 0.0007 | 0.4416 | 0.276–0.707 |
| Food preparation score of the Lawton IADL scale in the past (0, 1) | -4.798 | 1.608 | -2.98 | 0.0028 | 0.0082 | < 0.001–0.193 |
| Calculation score of the MMSE (0, 1, …, 5) | -1.986 | 0.953 | -2.08 | 0.0371 | 0.1372 | 0.021–0.888 |
Abbreviations: NC, Normal control; MCI, Mild cognitive impairment; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; Lawton IADL scale, Lawton Instrument Activities of Daily Living scale; and 95% C.I., 95% Confidence Interval.
Goodness-of-fit assessment: n = 102, the estimated area under the Receiver Operating Characteristic (ROC) curve = 0.99 > 0.7, and the modified Hosmer and Lemeshow goodness-of-fit F test p = 0.7479 > 0.05 (df = 9, 92), which indicated an excellent fit.
Next, logistic regression analysis of dementia versus MCI in the subjects with MCI or dementia (n = 67) revealed that after the keen competitions during the stepwise variable selection procedure, the orientation score of the Clinical Dementia Rating scale stayed in the final logistic regression model as the most important statistically significant predictor (Table 4). Both the estimated area under the ROC curve = 0.984 and the modified Hosmer and Lemeshow goodness-of-fit F test p = 0.7378 (df = 9, 57) also indicated an excellent fit.
Table 4. Multivariate analysis of the predictors of dementia versus MCI among the subjects with MCI or dementia by fitting multiple logistic regression model with the stepwise variable selection method.
| Covariate | Estimated Regression Coefficient | Estimated Standard Error |
z Value | p Value | Estimated Odds Ratio | 95% C.I. of Odds Ratio |
|---|---|---|---|---|---|---|
| Intercept | -8.593 | 2.286 | -3.76 | 0.0002 | – | – |
| Orientation score of the CDR (0, 0.5, 1, 2, 3) | 10.993 | 2.911 | 3.78 | 0.0002 | 59483.5178 | 197.852–17883522.326 |
Abbreviations: CDR, Clinical Dementia Rating scale; MCI, Mild cognitive impairment; and 95% C.I., 95% Confidence Interval.
Goodness-of-fit assessment: n = 67, the estimated area under the Receiver Operating Characteristic (ROC) curve = 0.984 > 0.7, and the modified Hosmer and Lemeshow goodness-of-fit F test p = 0.7378 > 0.05 (df = 9, 57), which indicated an excellent fit.
Floor and ceiling effects
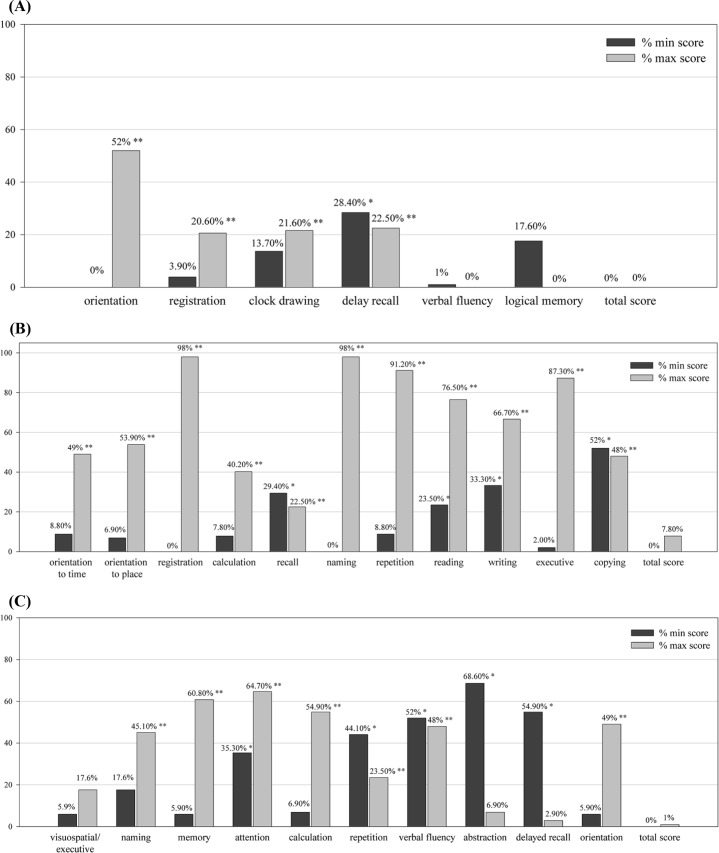
Fig 1 displays percentages of minimum and maximum total and subtest scores on the Qmci-TW, MMSE, and MoCA. The total Qmci-TW, MMSE and MoCA scores exhibited no floor or ceiling effects. Significant floor effects were identified in 1 (16.7%) of 6 Qmci-TW subtests, 4 (36.4%) of 11 MMSE subtests, and 5 (50%) of 10 MoCA subtests, whereas significant ceiling effects were observed in 4 (66.7%) of 6 Qmci-TW subtests, all 11 (100%) MMSE subtests, and 7 (70%) of 10 MoCA subtests.
Fig 1.
Percentage of minimum and maximum scores on total and subtests of the (A) Qmci-TW, (B) MMSE, and (C) MoCA. * ≥ 20% of patients obtained minimum scores. ** ≥ 20% of patients obtained maximum scores.
Psychometric properties
Internal consistency
The internal consistency of the Qmci-TW screen was good, with a Cronbach’s α of 0.85, and the item-to-total correlation were questionable to good, with Cronbach’s α of 0.67–0.80. Significant and positive strong correlations (r = 0.53–0.76, all p’s < 0.001) were found for each of the two Qmci-TW screen subtests. Results of internal consistency and inter-item correlation of the Qmci-TW screen are presented in Table 5.
Table 5. Results of internal consistency and the inter-item correlation of the Qmci-TW.
| Variable | Cronbach’s α | 1. | 2. | 3. | 4. | 5. | 6. |
|---|---|---|---|---|---|---|---|
| Qmci-TW | 0.85 | ||||||
| 1. orientation | 0.79 | ‒ | |||||
| 2. registration | 0.72 | 0.63** | ‒ | ||||
| 3. clock drawing | 0.77 | 0.70** | 0.61** | ‒ | |||
| 4. delayed recall | 0.80 | 0.76** | 0.62** | 0.71** | ‒ | ||
| 5. verbal fluency | 0.80 | 0.69** | 0.71** | 0.67** | 0.71** | ‒ | |
| 6. logical memory | 0.67 | 0.53** | 0.57** | 0.59** | 0.60** | 0.66** | ‒ |
Abbreviations: Qmci-TW, Taiwan version of the Quick Mild Cognitive Impairment screen.
*p < 0.05.
**p < 0.001.
Test–retest reliability and interrater reliability
We observed ICCs [95% Confidence Intervals (CIs)] of 0.87 (0.67–0.95) and 1.00 (1.00–1.00) with 21 participants for test–retest and interrater reliability, respectively (both p < 0.001), suggesting excellent reliability for the Qmci-TW.
Validity
Concurrent and construct validity
The correlation of the Qmci-TW with the MoCA and MMSE for concurrent validity was positive and very strong (ρ = 0.93 and 0.91, respectively; both p < 0.001). The correlation of the Qmci-TW was positive and moderate with the Barthel Index (ρ = 0.46) and strong with the Lawton Instrumental Activities of Daily Living scale (ρ = 0.67) for construct validity, respectively (both p < 0.001).
Diagnostic properties
Sensitivity, specificity, and predictive values
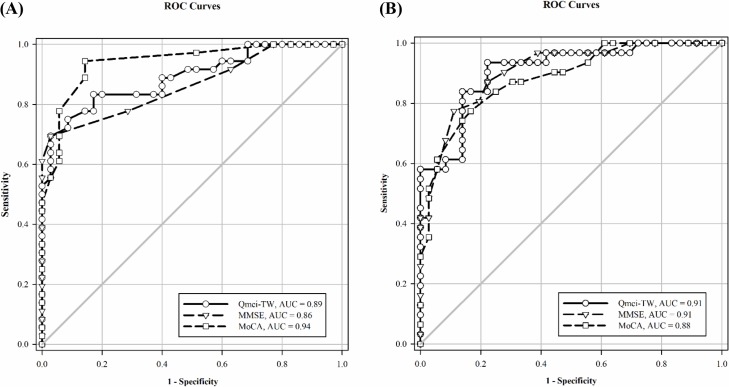
Fig 2 illustrates the ROC curves of the Qmci-TW, MoCA, and MMSE for differentiating MCI from NC and dementia from MCI. The optimal cut-off scores on the Qmci-TW, MoCA, and MMSE for differentiating the participants with MCI from NCs were ≤ 51.5/100 [AUCQmci-TW (95% CI) = 0.89 (0.81–0.97), sensitivityQmci-TW = 69%, specificityQmci-TW = 97%, PPVQmci-TW = 96%, NPVQmci-TW = 76%], ≤ 23/30 [AUCMoCA (95% CI) = 0.94 (0.89–0.99), sensitivityMoCA = 94%, specificityMoCA = 85%, PPVMoCA = 92%, NPVMoCA = 94%], and ≤ 26/30 [AUCMMSE (95% CI) = 0.86 (0.78–0.95), sensitivityMMSE = 69%, specificityMMSE = 97%, PPVMMSE = 96%, NPVMMSE = 76%], respectively. Thus, the Qmci-TW exhibited higher and lower accuracy for differentiating the participants with MCI from NCs than did the MMSE (χ2 = 0.54, p = 0.46), and MoCA (χ2 = -2.78, p = 0.10), respectively. In addition, the MoCA was more accurate in differentiating the participants with MCI from NCs than was the MMSE (χ2 = -3.15, p = 0.08). The optimal cut-off scores on the Qmci-TW, MoCA, and MMSE for differentiating the participants with MCI from those with dementia were ≤ 31/100 [AUCQmci-TW (95% CI) = 0.91 (0.84–0.98), sensitivityQmci-TW = 94%, specificityQmci-TW = 78%, PPVQmci-TW = 78%, NPVQmci-TW = 93%], ≤ 11/30 [AUCMoCA (95% CI) = 0.88 (0.80–0.96), sensitivityMoCA = 77%, specificityMoCA = 84%, PPVMoCA = 80%, NPVMoCA = 81%], and ≤ 18/30 [AUCMMSE (95% CI) = 0.91 (0.84–0.98), sensitivityMMSE = 77%, specificityMMSE = 89%, PPVMMSE = 86%, and NPVMMSE = 82%], respectively. Thus, the accuracy of the Qmci-TW in differentiating the participants with MCI from those with dementia was similar to that of the MMSE (χ2 = -0.0001, p = 0.97) and higher accuracy than that of the MoCA (χ2 = 0.70, p = 0.40). Moreover, the MMSE was more accurate in differentiating the participants with dementia from MCI than was the MoCA (χ2 = 1.90, p = 0.16).
Fig 2.
The ROC curves of the Qmci-TW, MoCA, and MMSE for differentiating (A) MCI from NC, and (B) dementia from MCI.
Discussion
This study demonstrated that the Qmci-TW is a reliable and valid cognitive screening instrument potentially useful for differentiating among NCs and individuals with MCI and dementia. The Qmci-TW exhibited internal consistency, excellent test–retest reliability, and interrater reliability for clinical use. The Qmci-TW also exhibited sound concurrent, and construct validity in comparisons with the MoCA and MMSE and with the Barthel Index and Lawton Instrumental Activities of Daily Living scale respectively. In addition, evaluation criteria for the optimal cognitive screening instruments should also be considered, including bandwidth-fidelity tradeoff, culture fairness measure, economic considerations, and scopes of application. The reliability of the Qmci-TW with slightly narrow band is still satisfactory, and the Taiwan version of the Qmci is also validated without cultural bias in Taiwan population. Moreover, the Qmci-TW may not involve the floor and ceiling effects of the other tests for differentiating among NCs and individuals with MCI and dementia. Hence, the Qmci-TW may be preferable, particularly in patients with varied levels of cognitive function. The Qmci-TW represents the third Qmci screen translation, after the Dutch (Qmci-D) [22] and Turkish (Qmci-TR) versions [20]; the confirmation of its psychometric validity contributes to the growing evidence supporting clinical use of the Qmci.
Our results indicate that the MoCA is the most accurate test for differentiating MCI cases from NCs, followed by the Qmci-TW, and the MMSE. In the MoCA with high sensitivity, a positive test confirms MCI diagnosis, whereas in the Qmci-TW and MMSE with high specificity, a negative test result rules out diagnosis of MCI. In addition, the Qmci-TW and MMSE were found to be more accurate than the MoCA in differentiating dementia cases from MCI cases. In the Qmci-TW with high sensitivity, a positive test confirms the diagnosis of dementia, whereas in the MoCA and MMSE with high specificity, a negative test rules out diagnosis of dementia. According to these results, we recommend the use of the MoCA and Qmci-TW for detecting MCI and dementia, respectively.
As listed in Table 1, our univariate analyses indicated that age, educational level, and the Geriatric Depression Scale-Short Form score differed significantly among the NC, MCI, and dementia groups. Specifically, older age, lower educational level, and higher incidence of depression were more likely observed in the dementia group. These findings are similar to those of a previous study, which demonstrated that depressed mood, illiteracy, and older age were associated with dementia [42]. Next, multivariate analysis was conducted to assess the partial effects of all the relevant covariates in Tables 1 and 2. As shown in Tables 3 and 4, the lower MoCA score, food preparation score of the Lawton IADL scale in the past, and calculation score of the MMSE, the more likely to be MCI or dementia in all subjects, whereas the higher orientation score of the Clinical Dementia Rating scale, the more likely to be dementia in subjects with MCI or dementia. These findings not only could help us make predictions as a screening tool, but also shed light on the complementary roles of the MoCA, Lawton IADL scale, MMSE, and Clinical Dementia Rating scale. Yet, the Qmci-TW did not add much to them in the discriminations among NC, MCI, and dementia.
The current results indicated that the MoCA subtests exhibited the most floor effects, followed by the subtests of the MMSE and Qmci-TW. The MMSE subtests exhibited the most ceiling effects, followed by the subtests of the MoCA and Qmci-TW. The Qmci-TW facilitated accurate evaluation of a wide range of cognition functions with minimal floor and ceiling effects, and thus, was superior to the MoCA and MMSE. In addition, S1 Fig illustrates the ROC curves of the Qmci-TW subtests for differentiating MCI from NC and dementia from MCI. The best indicators of the Qmci-TW subtests for differentiating the participants with MCI from NCs, and participants with dementia from those with MCI were delayed recall, and orientation, respectively. Our results are similar to those of previous studies demonstrating that orientation is a poorer predictor of MCI with significant ceiling effects [43] and logical memory is a highly sensitive and specific for differentiating the participants with MCI from NCs [33].
In this study, 12.7% of all participants (4 and 9 patients with MCI and dementia, respectively) were illiterate. The optimal cut-off score for differentiating participants with MCI from NCs on the Qmci-TW (≤ 51.5/100) was lower than that on the Qmci (≤ 60/100) [18, 19], which is potentially attributable to the low levels of education and high levels of illiteracy in Taiwan’s older population. The optimal cut-off score for discriminating participants with MCI from those with dementia on the Qmci-TW (≤ 31/100) was much lower than that on the Qmci-TR (< 43/100) [20] and the Qmci-D (≤ 42/100) [22], explained by the higher proportion of patients with severe dementia in Taiwan population.
This study revealed that patients’ abilities to execute complex instrumental activities of daily living may be a critical factor for differentiating MCI cases from NCs; however, Lawton Instrumental Activities of Daily Living scale scores in the NC group were not significantly higher than those in the MCI group (p = 0.07). In the MCI group, all participants maintained instrumental activities of daily living functions related to telephone use, housekeeping, transport mode, and finances; however, with regard to instrumental activities of daily living functions of food preparation, medication responsibility, shopping, and laundry, the number of participants with MCI scoring 0 were 16 (44.4%), 12 (33.3%), 11 (30.6%), and 8 (22.2%), respectively. Maintenance of activities of daily living is a critical factor for distinguishing between individuals with dementia and those with MCI and for differentiating between mild and severe dementia. The Barthel Index scores were significantly higher in the MCI group than in the dementia group (p < 0.001), In addition, the Barthel Index scores exhibited a significant negative moderate correlation with Clinical Dementia Rating scale global scores of 1–3 (ρ = −0.64, p < 0.001).
The key contribution of this study provides that the Qmci-TW with satisfactory psychometric and diagnostic properties is a useful and brief cognitive screening instrument to differentiate NCs, MCI and dementia. Notably, logical memory subtest only in the Qmci-TW, not in the MMSE, and MoCA, plays an important role for discriminating MCI from NC. Moreover, the Qmci-TW with time-limited for answering each questions can enhance the discriminating ability to detect patients with MCI and dementia by taking the response speed into consideration [44] and save much time for clinical practitioners. For early detection and treatment of patients with MCI and dementia, we recommend the use of the Qmci-TW in busy clinical settings.
This study has several limitations. First, the potential confounders of age and education may have caused the differences in the AUCs. This limitation is associated with the inherent challenges of a cross-sectional study design, which does not allow for adequate matching between groups. Second, the studied sample size was small and lacked statistical power for use in evaluating accuracy within each participant group. Even though sample size is one of the limitations, the statistically significant findings still deserve our attention, but the inference of the statistically non-significant findings should be conservative due to lack of power. Third, participants with active depression, who may exhibit slower reaction times and processing speeds [45], and patients with dementia subtypes, including frontotemporal dementia, Parkinson’s disease, and Lewy Body dementia, and different MCI syndromes were excluded from this study. These exclusions may have led to some spectrum bias; consequently, our results may not be generalizable to other types of MCI and dementia. Fourth, because of the conceptual constructs of the Qmci-TW, MoCA, and MMSE with non-identical cognitive domains, numbers of items, and scoring criteria, the results of the pure inter-correlation analysis were needed to be carefully interpreted [46].
In conclusion, the Qmci-TW is a reliable and valid cognitive screening instrument with accurate diagnostic properties for detecting MCI and dementia in Taiwanese individuals. Further research including age- and education-matched NCs, larger sample sizes, younger adults, and other settings, such as psychiatry, and general practice clinics, are required. Nevertheless, this study provides evidence that the Qmci and Qmci-TW are useful for cognitive screening in clinical practice.
Supporting information
(DOCX)
The ROC curves of the Qmci-TW subtests for differentiating (A) MCI from NC, and (B) dementia from MCI.
(TIF)
Acknowledgments
The authors like to thank the authors of the Qmci for approving the authorization of developing the Qmci-TW, and also like to thank the neurology outpatient department of the Taipei Hospital, Ministry of Health and Welfare, in New Taipei (Taiwan) for their willingness to participate in this study and their referrals of patients to this study. This manuscript was edited by Wallace Academic Editing.
Data Availability
Data are available from the IRB of the Taipei Hospital, Ministry of Health and Welfare for researchers who meet the criteria for access to confidential data [Address: No.127, Su-Yuan Road, Hsin-Chuang District, New Taipei City, 24213, Taiwan (R.O.C.); E-mail: irb@tph.mohw.gov.tw; Tel: +886-2-22765566].
Funding Statement
This work was supported by Grant Number 201707 from the Taipei Hospital, Ministry of Health and Welfare, Taiwan (R.O.C.), to WYC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rechel B, Grundy E, Robine JM, Cylus J, Mackenbach JP, Knai C, et al. Ageing in the European Union. Lancet. 2013; 381: 1312–1322. 10.1016/S0140-6736(12)62087-X [DOI] [PubMed] [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013; 9: 63–75.e2. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 3.Statistical Yearbook of Interior. 2015 Yearly Bulletin of Interior Statistics. [Cited 30 September 2017]. Available from: http://sowf.moi.gov.tw/stat/year/elist.htm.
- 4.Sachdev PS, Lipnicki DM, Kochan NA, Crawford JD, Thalamuthu A, Andrews G. The Prevalence of Mild Cognitive Impairment in Diverse Geographical and Ethnocultural Regions: The COSMIC Collaboration. PLoS One. 2015; 10: e0142388 10.1371/journal.pone.0142388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Lee HJ, Yang SC, Chen TF, Lin KN, Lin CC, et al. A Nationwide Survey of Mild Cognitive Impairment and Dementia, Including Very Mild Dementia, in Taiwan. PLoS One. 2014; 9: e100303 10.1371/journal.pone.0100303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- 7.Purser JL, Fillenbaum GG, Pieper CF, Wallace RB. Mild cognitive impairment and 10-year trajectories of disability in the Iowa Established Populations for Epidemiologic Studies of the Elderly cohort. J Am Geriatr Soc. 2005; 53: 1966–1972. 10.1111/j.1532-5415.2005.53566.x [DOI] [PubMed] [Google Scholar]
- 8.Shah Y, Tangalos EG, Petersen RC. Mild cognitive impairment. When is it a precursor to Alzheimer's disease? Geriatrics. 2000; 55: 62, 65–68. [PubMed] [Google Scholar]
- 9.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia–meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009; 119: 252–265. 10.1111/j.1600-0447.2008.01326.x [DOI] [PubMed] [Google Scholar]
- 10.Bruscoli M, Lovestone S. Is MCI really just early dementia? a systematic review of conversion studies. Int Psychogeriatr. 2004; 16: 129–140. [DOI] [PubMed] [Google Scholar]
- 11.Fiatarone Singh MA, Gates N, Saigal N, Wilson GC, Meiklejohn J, Brodaty H, et al. The Study of Mental and Resistance Training (SMART) study-resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J Am Med Dir Assoc. 2014; 15: 873–880. 10.1016/j.jamda.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 12.Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM, et al. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ. 2013; 185: 1393–1401. 10.1503/cmaj.130451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo NW, Lui HC, Wong PF, Liao KK, Yan SH, Lin KP, et al. Chinese version and norms of the Mini-Mental State Examination. J Rehabil Med Assoc Taiwan. 1988; 16: 52–59. [Google Scholar]
- 14.Tsai CF, Lee WJ, Wang SJ, Shia BC, Nasreddine Z, Fuh JL. Psychometrics of the Montreal Cognitive Assessment (MoCA) and its subscales: validation of the Taiwanese version of the MoCA and an item response theory analysis. Int Psychogeriatr. 2012; 24: 651–658. 10.1017/S1041610211002298 [DOI] [PubMed] [Google Scholar]
- 15.Tsai JC, Chen CW, Chu H, Yang HL, Chung MH, Liao YM, et al. Comparing the sensitivity, specificity, and predictive values of the Montreal Cognitive Assessment and Mini-Mental State Examination when screening people for mild cognitive impairment and dementia in Chinese population. Arch Psychiatr Nurs. 2016; 30: 486–491. 10.1016/j.apnu.2016.01.015 [DOI] [PubMed] [Google Scholar]
- 16.Spencer RJ, Wendell CR, Giggey PP, Katzel LI, Lefkowitz DM, Siegel EL, et al. Psychometric limitations of the Mini-mental State Examination among nondemented older adults: an evaluation of neurocognitive and magnetic resonance imaging correlates. Exp Aging Res 2013;39(4):382–97. 10.1080/0361073X.2013.808109 [DOI] [PubMed] [Google Scholar]
- 17.Tumas V, Borges V, Ballalai-Ferraz H, Katzel LI, Lefkowitz DM, Siegel EL, et al. Some aspects of the validity of the Montreal Cognitive Assessment (MoCA) for evaluating cognitive impairment in Brazilian patients with Parkinson's disease. Dement Neuropsychol. 2016; 10: 333–338. 10.1590/s1980-5764-2016dn1004013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarnette R, O'Caoimh R, Antony DN, Svendrovski A, Molloy DW. Comparison of the Quick Mild Cognitive Impairment (Qmci) screen to the Montreal Cognitive Assessment (MoCA) in an Australian geriatrics clinic. Int J Geriatr Psychiatry. 2017; 32: 643–649. 10.1002/gps.4505 [DOI] [PubMed] [Google Scholar]
- 19.O'Caoimh R, Timmons S, Molloy DW. Screening for Mild Cognitive Impairment: Comparison of "MCI Specific" Screening Instruments. J Alzheimers Dis. 2016; 51: 619–629. 10.3233/JAD-150881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yavuz BB, Varan HD, O'Caoimh R, Kizilarslanoglu MC, Kilic MK, Molloy DW, et al. Validation of the Turkish Version of the Quick Mild Cognitive Impairment Screen. Am J Alzheimers Dis Other Demen. 2017; 32: 145–156. 10.1177/1533317517691122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Caoimh R, Gao Y, McGlade C, Healy L, Gallagher P, Timmons S, et al. Comparison of the quick mild cognitive impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing. 2012; 41: 624–629. 10.1093/ageing/afs059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunt S, O'Caoimh R, Krijnen WP, Healy L, Gallagher P, Timmons S, et al. Validation of the Dutch version of the quick mild cognitive impairment screen (Qmci-D). BMC Geriatr. 2015; 15: 115 10.1186/s12877-015-0113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molloy DW, Standish TIM, Lewis DL. Screening for mild cognitive impairment: comparing the SMMSE and the ABCS 135. Can J Psychiatry. 2005; 50: 52–58. 10.1177/070674370505000110 [DOI] [PubMed] [Google Scholar]
- 24.O’Caoimh R, Svendrovski A, Johnston BC, Gao Y, McGlade C, Eustace J, et al. The quick mild cognitive impairment screen correlated with the standardized alzheimer’s disease assessment scale-cognitive section in clinical trials. J Clin Epidemiol. 2014; 67: 87–92. 10.1016/j.jclinepi.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 25.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011; 8: 1 10.1186/1742-5573-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fosgate GT. Practical sample size calculations for surveillance and diagnostic investigations. J Vet Diagn Invest. 2009; 21: 3–14. 10.1177/104063870902100102 [DOI] [PubMed] [Google Scholar]
- 27.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984; 34: 939–944. [DOI] [PubMed] [Google Scholar]
- 29.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011; 7: 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HCB, Chiu HFK, Kwok WY, Leung CM, Kwong PK, Chung DWS. Chinese elderly and the GDS short form: A preliminary study. Clinical Gerontologist. 1994; 14: 37–42. [Google Scholar]
- 31.Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J. 1965; 14: 61–65. [PubMed] [Google Scholar]
- 32.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969; 9: 179–186. [PubMed] [Google Scholar]
- 33.Cunje A, Molloy DW, Standish TI, Lewis DL. Alternate forms of logical memory and verbal fluency tasks for repeated testing in early cognitive changes. Int Psychogeriatr. 2007; 19: 65–75. 10.1017/S1041610206003425 [DOI] [PubMed] [Google Scholar]
- 34.Beaton DE, Bombardier C, Guillemin F, Ferraz MB. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976). 2000; 25: 3186–3191. [DOI] [PubMed] [Google Scholar]
- 35.Portney LG, Watkins MP. Foundations of clinical research: Applications to practice. 3rd ed Upper Saddle River, NJ: Pearson Education; 2009. [Google Scholar]
- 36.Holmes W, Shea J. Performance of a new, HIV/AIDS-targeted quality of life (HAT–QoL) instrument in asymptomatic sero-positive individuals. Qual Life Res. 1997; 6: 561–71. [DOI] [PubMed] [Google Scholar]
- 37.Fayers PM, MaChin D. In Quality of life: Assessment, analysis, and interpretation. New York: Wiley; 2000. [Google Scholar]
- 38.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment. 1994; 6(4): 284–90. [Google Scholar]
- 39.Streiner DL, Norman GR. Basic concepts In Health measurement scales: A practical guide to their development and use. 2nd ed Oxford, England: Oxford University Press; 1995. [Google Scholar]
- 40.Youden WJ. Index for rating diagnostic tests. Cancer. 1950; 3: 32–35. [DOI] [PubMed] [Google Scholar]
- 41.Hu FC. My.stepwise: Stepwise variable selection procedures for regression analysis, version 0.1.0; 2017. (URL: https://CRAN.R-project.org/package=My.stepwise).
- 42.Sharifi F, Fakhrzadeh H, Varmaghani M, Arzaghi SM, Alizadeh Khoei M, Farzadfar F, et al. Prevalence of Dementia and Associated Factors among Older Adults in Iran: National Elderly Health Survey (NEHS). Arch Iran Med. 2016; 19: 838–844. [DOI] [PubMed] [Google Scholar]
- 43.O’Caoimh R, Gao Y, Gallagher PF, Eustace J, McGlade C, Molloy DW. Which part of the quick mild cognitive impairment screen (Qmci) discriminates between normal cognition, mild cognitive impairment and dementia? Age Ageing. 2013; 42: 324–330. 10.1093/ageing/aft044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Deursen JA, Vuurman EF, Smits LL, Verhey FR, Riedel WJ. Response speed, contingent negative variation and P300 in Alzheimer's disease and MCI. Brain Cogn. 2009; 69: 592–599. 10.1016/j.bandc.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 45.Iverson GL. Sensitivity of computerized neuropsychological screening in depressed university students. Clin Neuropsychol. 2006; 20: 695–701. 10.1080/138540491005857 [DOI] [PubMed] [Google Scholar]
- 46.Steinborn MB, Langner R, Flehmig HC, Huestegge L. Methodology of performance scoring in the d2 sustained-attention test: Cumulative-reliability functions and practical guidelines. Psychol Assess. 2018; 30: 339–357. 10.1037/pas0000482 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
The ROC curves of the Qmci-TW subtests for differentiating (A) MCI from NC, and (B) dementia from MCI.
(TIF)
Data Availability Statement
Data are available from the IRB of the Taipei Hospital, Ministry of Health and Welfare for researchers who meet the criteria for access to confidential data [Address: No.127, Su-Yuan Road, Hsin-Chuang District, New Taipei City, 24213, Taiwan (R.O.C.); E-mail: irb@tph.mohw.gov.tw; Tel: +886-2-22765566].