Abstract
The widespread emergence of pyrethroid-resistant Anopheles gambiae has intensified the need to find new contact mosquitocides for indoor residual spraying and insecticide treated nets. With the goal of developing new species-selective and resistance-breaking acetylcholinesterase (AChE)-inhibiting mosquitocides, in this report we revisit the effects of carbamate substitution on aryl carbamates, and variation of the 1-alkyl group on pyrazol-4-yl methylcarbamates. Compared to aryl methylcarbamates, aryl dimethylcarbamates were found to have lower selectivity for An. gambiae AChE (AgAChE) over human AChE (hAChE), but improved tarsal contact toxicity to G3 strain An. gambiae. Molecular modeling studies suggest the lower species-selectivity of the aryl dimethylcarbamates can be attributed to a less flexible acyl pocket in AgAChE relative to hAChE. The improved tarsal contact toxicity of the aryl dimethylcarbamates relative to the corresponding methylcarbamates is attributed to a range of complementary phenomena. With respect to the pyrazol-4-yl methylcarbamates, the previously observed low An. gambiae-selectivity of compounds bearing α-branched 1-alkyl groups was improved by employing β- and γ-branched 1-alkyl groups. Compounds 22a (cyclopentylmethyl), 21a (cyclobutylmethyl), and 26a (3-methylbutyl) offer 250-fold, 120-fold, and 96-fold selectivity, respectively, for inhibition of AgAChE vs. hAChE. Molecular modeling studies suggests the high species-selectivity of these compounds can be attributed to the greater mobility of the W84 side chain in the choline-binding site of AgAChE, compared to that of W86 in hAChE. Compound 26a has reasonable contact toxicity to G3 strain An. gambiae (LC50 = 269 μg/mL) and low cross-resistance to Akron strain (LC50 = 948 μg/mL), which bears the G119S resistance mutation.
Graphical Abstract

1. Introduction
Malaria is caused by infection with parasites of the genus Plasmodium, and is transmitted by Anopheline mosquitoes. It presents a terrible disease burden: in 2015 it is estimated to have killed 429,000 and sickened 212,000,000 people [1]. Of these deaths, 90% occurred in sub-Saharan Africa, and 70% of the deceased were children aged 5 years or less [1]. Remarkably, malaria mortality has been reduced 60% from 2000 to 2015 [1], and vector control methods have played an important role in this reduction of mortality. Specifically, the use of insecticide-treated bednets (ITNs) provide a direct physical and chemical barrier to disease transmission [2–5]. However, at present only pyrethroids are authorized by the World Health Organization for deployment on ITNs, and widespread pyrethroid resistance throughout Africa puts these huge gains at risk [6].
Pyrethroids target the voltage-gated sodium channel, so to circumvent resistance, ITN mosquitocides with different mechanisms of action are urgently needed. Acetylcholinesterase (AChE) inhibition is a proven method to control adult malaria mosquitoes, but to date, anticholinesterases have never been approved for use on ITNs, and thus are used only for indoor residual spraying (IRS). These include the carbamates propoxur and bendiocarb, and organophosphates, in one case a longer lasting re-formulation of pyrimiphos- methyl [7]. The potential usefulness of an anticholinesterase for malaria control has prompted several groups to develop new AChE-targeting mosquitocides. Key challenges include high selectivity for inhibition of Anopheles gambiae AChE (AgAChE) vs human AChE (hAChE), and activity against the known resistance mutation (G119S) [8] with consequent high contact toxicity to adult G119S-AgAChE-bearing An. gambiae. These challenges have been met individually, but to date no single compound possesses both qualities.
For example, 2-substituted γ-branched aryl methylcarbamates 1a–3a are quite selective (100- to 500-fold) for inhibition of wild type (WT) AgAChE vs hAChE, unlike 2-substituted α-branched aryl methylcarbamate propoxur 4a (Figure 1) [9, 10].
Fig. 1.
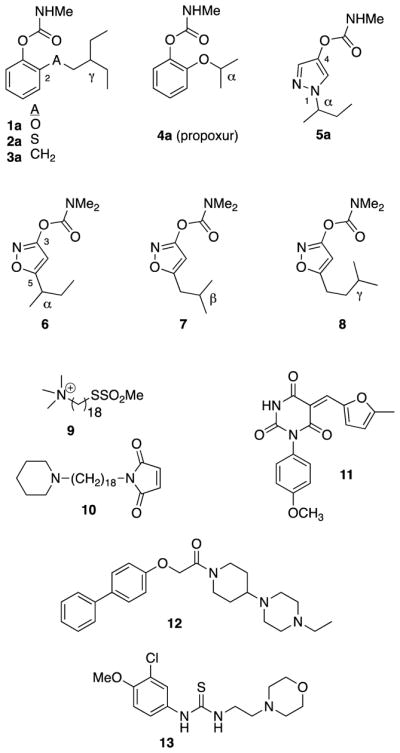
Representative compounds investigated as An. gambiae-selective (1a–3a, 9,10,12,13) or resistance-breaking (5a, 6–8, 11) acetylcholinesterase inhibitors. Propoxur (4a) is approved for IRS but has modest selectivity for An. gambiae [9] and is not resistance-breaking [11].
Consistent with their inhibition of AgAChE, 1a–3a are toxic to adult G3 strain An. gambiae in the tarsal contact assay, though not as toxic as 4a. However, these compounds do not rapidly inactivate G119S AgAChE, and they have very low tarsal contact toxicity to Akron strain An. gambiae, which carry the G119S mutation of AgAChE [10]. Since these compounds target the catalytic serine of AgAChE, and since the G119S mutation was expected to reduce the volume of the active site, we explored carbamates derived from smaller 5-membered ring heterocycles. The 1-alkyl-pyrazol-4-yl methylcarbamate 5a and 5-alkyl-isoxazol-3-yl dimethylcarbamates 6–8 proved to be excellent inhibitors of G119S AgAChE and were contact toxic to both G3 strain and Akron strain adult An. gambiae [11, 12]. However, these heterocyclic carbamates rapidly inactivated hAChE, and thus did not have the desired inhibition selectivity.
Thiol-reactive compounds 9 and 10 were designed by Pang and co-workers to target the free cysteine C286 in the acyl loop of AgAChE, which is a phenylalanine in hAChE, and these compounds and homologs offer exquisite selectivity for inhibition of AgAChE [13, 14]. However, to date, no insecticidal activity of these compounds has been disclosed. In 2012, Weill and co-workers disclosed a series of pyrimidinetrione analogs (e.g. 11) that show preferential inhibition of G119S Culex pipiens AChE relative to the WT enzyme, and enhanced toxicity to An. gambiae larvae bearing that mutation [15]. However, the selectivity of these compounds vs hAChE is not known. Finally, Linusson and co-workers have recently disclosed two series of non-covalent inhibitors of AgAChE; compounds 12 and 13 show greater than 100-fold selectivity for inhibition of AgAChE vs hAChE. In addition, 13 and analogs were demonstrated to be toxic to susceptible adult An. gambiae [16, 17]. However, inhibitory potency of 13 against G119S AgAChE was poor [17].
Reviewing the data from 1a–5a and 6–8, the low inhibition selectivities exhibited by the β-branched isoxazol-3-yl dimethylcarbamate 7 (7-fold) and the γ-branched homolog 8 (2-fold) could be attributed to at least two phenomena. Firstly, it is possible that the putative selectivity pocket in AgAChE engaged by the γ-branched substituent of 1a–3a cannot be reached by β-branched substituent of 7 or the γ-branched substituent of 8, due to the different ring sizes and different placement of the alkyl groups relative to the carbamate in these two series. A second possibility is that the dimethylcarbamoyl functionality of 7–8 also serves to reduce inhibition selectivity. Note that these dimethylcarbamates were investigated as a matter of necessity, because the corresponding methylcarbamates were hydrolytically unstable [12].
To test these hypotheses, we prepared two series of compounds. We synthesized dimethylcarbamate and 1° carbamate derivatives of 1a–2a and 4a–5a, and reinvestigated the 1-alkyl pyrazol-4-yl methylcarbamate series of compounds, exploring analogs that possessed α-branched, β-branched, γ-branched, δ-branched, and unbranched alkyl groups. The overall goal of this study was to determine whether the appropriate 1-alkyl substituent on a pyrazol-4-yl methylcarbamate can confer excellent selectivity for inhibition of AgAChE vs hAChE without reducing engagement of the G119S enzyme and toxicity to Akron strain An. gambiae.
2. Materials and methods
2.1 Insects
Susceptible G3 (MRA-112) and Akron strains (MRA-913) of Anopheles gambiae (African malaria mosquito) were obtained from BEI Resources through the CDC MR4 program. Akron mosquitoes have documented knockdown resistance (kdr) to pyrethroids (L1014F) and the G119S mutation (ace-1R), which confers resistance to carbamates. Mosquito colonies were maintained in the Department of Entomology, Virginia Tech, Blacksburg, VA 24061, USA, or raised up from eggs (obtained from CDC MR4, Atlanta, GA, USA) at the Emerging Pathogens Institute, University of Florida, Gainesville, FL 32610, USA. In all cases, mosquitoes were reared under 75% relative humidity and 28 °C, with a 12/12 dark and light cycle.
2.2 Chemicals
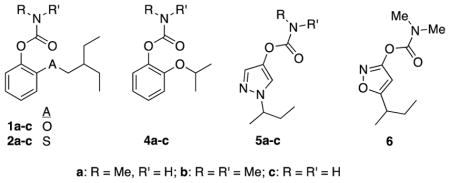
Structural information for the compounds assessed in this work are provided in Tables 1 and 2. Aryl methylcarbamates 1a, 2a, and 4a, pyrazol-4-yl methylcarbamate 5a and isoxaol-3-yl dimethylcarbamate 6 were prepared as described previously [9, 11, 12]. Aryl dimethylcarbamates 1b, 2b, 4b, and pyrazol-4-yl dimethyl carbamate 5b were prepared similarly by reaction of the corresponding phenol/pyrazol-4-ol precursors [9, 11] with N,N-dimethylcarbamoyl chloride. Primary carbamates 1c, 2c, 4c and 5c were prepared by reaction of the corresponding phenol/pyrazol-4-ol precursors [9, 11] with trichloromethylisocyanate (5 equiv), in dichloromethane; followed by passage through a neutral alumina column. Novel 1-alkyl-pyrazol-4-yl methyl carbamates 14a–33a were prepared by deprotonation of the corresponding 1-alkyl-pyrazol-4-ols with potassium t-butoxide in THF/dichloromethane, followed by the addition of N-methylcarbamoyl chloride in dichloromethane, as previously described [11]. The requisite 1-alkyl-pyrazol-4-ols were prepared in three steps from pyrazole and the requisite alkyl halides or tosylates, as previously described [11]. All compounds were purified by column chromatography or preparative TLC on silica gel. Structural identity was confirmed in each case by NMR spectrometry (1H, 13C), and high-resolution mass spectrometry. Greater than 95% purity was confirmed by 1H NMR spectroscopy prior to biological assay.
Table 1.
Enzyme inhibition and tarsal contact toxicity of methylcarbamates (1a–2a, 4a–5a), dimethylcarbamates (1b–2b, 4b–5b, 6), and 1° carbamates (1c–2c, 4c–5c).
| |||||
|---|---|---|---|---|---|
| ki (mM−1 min−1)b | |||||
|
|
|||||
| Compounda | hAChE | WT AgAChE | G119S AgAChE | WT Ag/h selectivityc | G3 LC50 (μg/mL)d |
| 1a [9, 10] | 0.48 ± 0.11e | 255 ± 12e | <0.060 ± 0.015e | 530 ± 130e | 1,388 (1,020–1,751) |
| 1b | 0.19 ± 0.02 | 3.73 ± 0.13 | 0.056 ± 0.15 | 20 ± 2 | 111 (58–177) |
| 1c | 0.416 ± 0.032 | 127 ± 5 | 0.01 ± 0.01 | 310 ± 26 | > 1,000f |
| 2a [9, 10] | 14.5 ± 1.5e | 1,850 ± 100e | <0.047 ± 0.019e | 130 ± 15e | 1,736 (1,120–2,292) |
| 2b | 1.45 ± 0.06 | 85.7 ± 5.5 | 0.040 ± 0.008 | 59 ± 5 | 140 (73–233) |
| 2c | 14.6 ± 0.7 | 713 ± 19 | 0.01 ± 0.01 | 49 ± 3 | > 1,000f |
| 4a [9, 10] | 17.0 ± 0.4e | 266 ± 9e | <0.037 ± 0.007e | 16 ± 1e | 39 (32–45)e |
| (propoxur) | |||||
| 4b | 14.8 ± 0.5 | 22.1 ± 0.5 | <0.025 ± 0.011 | 1.5 ± 0.1 | 59 (44–87) |
| 4c | 13.9 ± 0.7 | 185 ± 5 | 0.01 ± 0.01 | 13 ± 1 | ~500g |
| 5a [11] | 647 ± 24e | 4,130 ± 130e | 137± 4e | 6.4 ± 0.3e | 96 (89–104)e |
| 5b | 5.28 ± 0.59 | 36.7 ± 0.8 | 2.27 ± 0.24 | 7 ± 1 | 124 (117–132) |
| 5c | 1,520 ± 130 | 19,000 ± 600 | 587 ± 37 | 12 ± 1 | 90 (79–100) |
| 6 [12] | 60.1 ± 1.8e | 323 ± 6e | 20.4 ± 0.6e | 5.4 ± 0.2e | 41 (28–58)e |
Compounds 1a, 2a, 4a, 5a, 6 were reported previously.
Apparent bimolecular rate constants for enzyme inactivation, measured as described in Section 2.3 above.
Selectivity ratio is calculated as ki(WT AgAChE)/ki(hAChE), with standard error in the ratio calculated according to propagation of error (see Section 2.3 above).[18]
LC50 values are the concentration of inhibitor used to treat the paper that killed 50% of the insects in the tarsal contact assay (see Section 2.3 above).
Data reported previously.
No mortality at this concentration.
100% mortality @ 1,000 μg/mL and 0% mortality @ 100 μg/mL
Table 2.
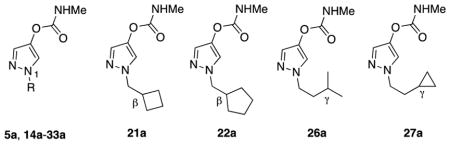
Enzyme inhibition and tarsal contact toxicity of 1-alkylpyrazol-4-yl methylcarbamates 5a, 14a–33a bearing various N1-substituents (R); the structures of An. gambiae-selective compounds 21a, 22a, 26a, and 27a are highlighted for reference.
| |||||||
|---|---|---|---|---|---|---|---|
| ki (mM−1 min−1)b | LC50 (μg/mL) or % mortality at 1000 μg/mL | ||||||
|
|
|
||||||
| Compounda | R | hAChE | WT AgAChE | G119S AgAChE | Ag/h selectivityc | G3 strain | Akron strain |
| 5a | s-Bu | 647 ± 24 | 4,130 ± 130 | 137± 4 | 6.4 ± 0.3 | 96 (89–104) | 81 (78–89) |
| 14a | i-Pr | 79.8 ± 2.4 | 498 ± 13 | 10.7 ± 0.1 | 6.2 ± 2 | 383 (355 – 420) | 650 (488–859) |
| 15a | 2-pentyl | 805 ± 36 | 9,140 ± 260 | 290 ± 7 | 11 ± 1 | 154 (140–167) | 267 (241–289) |
| 16a | 3-pentyl | 168 ± 8 | 2,220 ± 70 | 125 ± 3 | 13 ± 1 | 138 (125–151) | 231 (217–245) |
| 17a | c-C5H9 | 174 ± 7 | 2,380 ± 50 | 36.5 ± 0.8 | 14 ± 1 | 29 (26–32) | 365 (344–384) |
| 18a | 2-heptyl | 585 ± 29 | 2,620 ± 60 | 51.4 ± 3.6 | 4.5 ± 0.2 | 100% | 40% |
| 19a | i-Bu | 6.51 ± 0.35 | 280 ± 5 | 4.51 ± 0.12 | 43 ± 2 | 41 (38 – 44) | 228 (181–286) |
| 20a | -CH2c-C3H5 | 0.53 ± 0.05 | 20.3 ± 0.6 | 1.04 ± 0.04 | 38 ± 4 | 30% | ND |
| 21a | -CH2c-C4H7 | 13.1 ± 0.5 | 1,520 ± 30 | 13.5 ± 0.4 | 120 ± 10 | 100% | 10% |
| 22a | -CH2c-C5H9 | 1.93 ± 0.24 | 477 ± 38 | 6.16 ± 0.24 | 250 ± 40 | 10% | 0% |
| 23a | -CH2c-C6H11 | 0.93 ± 0.03 | 10.8 ± 0.5 | 0.69 ± 0.02 | 12 ± 1 | 30% | 17% |
| 24a | -CH2CH(C2H5)2 | 6.98 ± 0.27 | 33.7 ± 1.0 | 1.15 ± 0.11 | 4.8 ± 2 | 20% | ND |
| 25a | -CH2Ph | 1.29 ± 0.06 | 4.74 ± 0.21 | 0.55 ± 0.05 | 3.7 ± 0.2 | 0% | |
| 26a | -CH2CH2CH-Me2 | 4.87 ± 0.29 | 468 ± 21 | 5.88 ± 0.21 | 96 ± 7 | 269 (253 – 283) | 948 (887 – 1104) |
| 27a | -CH2CH2c-C3H5 | 28.1 ± 1.0 | 1,770 ± 30 | 22.8 ± 1.2 | 63 ± 3 | 30% | 10% |
| 28a | -CH2CH2c-C4H7 | 15.8 ± 1.1 | 463 ± 14 | 9.64 ± 0.45 | 29 ± 2 | 0% | ND |
| 29a | -CH2CH2c-C5H9 | 8.66 ± 0.43 | 25.1 ± 1.0 | 2.58 ± 0.12 | 2.9 ± 0.2 | 0% | ND |
| 30a | -CH2CH2CH-Me(OMe) | 0.60 ± 0.04 | 8.12 ± 0.18 | 0.52 ± 0.06 | 14 ± 1 | 45% | ND |
| 31a | CH2CH2CH2-CHMe2 | 4.33 ± 0.15 | 25.7 ± 1.3 | 1.91 ± 0.05 | 5.9 ± 0.4 | 10% | ND |
| 32a | -CH2CH2CH2Ph | 3.40 ± 0.01 | 18.8 ± 0.6 | 1.80 ± 0.13 | 5.5 ± 0.2 | 10% | ND |
| 33a | Bu | 27.8 ± 1.3 | 1,070 ± 10 | 18.2 ± 0.7 | 38 ± 2 | 100% | 16% |
Data for 5a, 14a–17a were reported previously [11].
Apparent bimolecular rate constants for enzyme inactivation (mM −1 min−1), as described previously.[10, 12]
Selectivity is calculated as ki(WT AgAChE)/ki(hAChE), with standard error in the ratio calculated according to standard propagation of error.[18]
LC50 values are the concentration of inhibitor used to treat the paper that killed 50% of the insects in the tarsal contact assay (see Section 2.3 above).
ND designates ‘not determined’; compounds that did not show significant toxicity to susceptible G3 strain were generally not tested on the resistant Akron strain.
2.3 Enzymes and Enzyme Inhibition Studies
Recombinant WT and G119S AgAChE were prepared as previously described [11]. Recombinant hAChE (C1682) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Inhibition potency of carbamate and carboxamide insecticides was assessed by measuring apparent second-order rate constants ki (mM−1 min−1) for inactivation of the enzymes. We followed our previous progressive inactivation approach [9, 11, 12], in which enzymes were incubated with different concentrations of carbamates or carboxamides for differing times before measuring enzyme residual activity (v/v0). Enzymes were incubated with typically five concentrations of inhibitors (and an inhibitor-free control) for up to 6 minutes at approximately 1 min intervals. Each concentration was present in the microplate in duplicate, and each experiment was repeated. Note that for the G119S enzyme, inhibition was sometimes very low after 10 min at 10 μM. In such cases, incubations with this enzyme were often extended to 60 min (10 min intervals). Residual activities v/v0 are the ratio of the rate in the presence of inhibitor to a time-matched inhibitor-free control and thus correct for slow thermal inactivation of the enzyme. At each inhibitor concentration [I], plots of ln(v/v0) vs. time (t) were constructed; the slopes of these unconstrained linear fits represent the pseudo first-order rate constants (kobs) associated with those inhibitor concentrations. These kobs values (obtained in at least two separate experiments) were then plotted against [I]; in each case the slope of the unconstrained linear fit is the apparent second-order rate constant ki (mM−1 min−1) for inactivation. Note that inhibitor concentrations [I] were chosen to be low enough to remain in the domain where a plot of kobs vs [I] was linear. Enzymatic selectivity for inhibition was calculated from the measured ki values; the error in these ratios was determined using a standard propagation of error method [18].
2.4 Tarsal contact toxicity assay
Adult female non-blood fed An. gambiae (both G3 and Akron strains) 3–5 days old, were used for filter paper assays of tarsal contact toxicity, which were performed in exposure tubes according to the 2006 World Health Organization recommendations [19] with slight modification. In brief, filter papers (15 x 12 cm) were treated with 2.0 mL of various concentrations of the carbamate in ethanol, and allowed to dry overnight. For the G3 strain, batches of 20–25 mosquitoes (in triplicate) were transferred to a holding tube and allowed to adapt for one hour. Due to lower colony numbers, toxicity assays with the Akron strain used batches of 10–15 mosquitoes in duplicate. Mosquitoes were then transferred to the exposure tube (held horizontally) that contained a treated filter paper. Knockdown was noted after 1 h, and all mosquitoes were transferred back to the holding tube (held upright), and given free access to 10% (w/v) sugar water. Mortality was recorded at 24 h. Both during exposure and the 23 h period following, mosquito tubes were kept in an environmental chamber at 24 ± 1 °C and 75% RH. To determine LC50 values, typically 5–8 concentrations were examined, and mortality data were used for probit analysis using PoloPlus [20] or SAS Probit.
3. Results
3.1 Inhibition selectivity and G3 strain An. gambiae tarsal contact toxicity of aryl and pyrazol-4-yl dimethylcarbamates
As can be seen in Table 1, addition of a methyl group to the carbamate nitrogen of An. gambiae-selective methylcarbamates 1a (530-fold) and 2a (130-fold) had a significant effect on the WT AgAChE inactivation rate constants (ki), but less so for hAChE.
WT AgAChE ki values of dimethylcarbamate derivatives 1b, 2b and 4b were significantly reduced to 2–8% of their methylcarbamate analogs, but the corresponding hAChE ki values were only reduced to 10–90% of those of the methylcarbamate analogs. Consequently, the selectivities of 1b, 2b, and 4b were reduced to 20-, 59- and 1.5-fold respectively. Interestingly, for pyrazol-4-yl dimethylcarbamate 5b, both the AgAChE and hAChE ki values were reduced approximately 100-fold relative to the methylcarbamate 5a. Thus the low AgAChE selectivity of 5a (7-fold) remained unchanged in 5b. However, despite the significant decreases in AgAChE ki values for the dimethylcarbamates, their G3 strain tarsal contact toxicities were not markedly reduced. Curiously, the dimethylcarbamates 1b and 2b were appreciably more contact toxic (~12-fold) than the methylcarbamates 1a and 2a. In the case of 4b and 5b, the toxicities were similar to their methylcarbamate analogs, despite the drastic decreases seen in AgAChE ki values. Lastly, for G119S-AgAChE, the dimethylcarbamates 1b–2b, 4b–5b resembled the corresponding methylcarbamates in showing very slow inactivation.
3.2 Inhibition selectivity and G3 tarsal contact toxicity of 1° carbamates
Unsubstituted or 1° carbamates have been little explored as anticholinesterase insecticides since early studies by Metcalf [21], documented their lower insecticidal efficacy relative to the corresponding methylcarbamates. This report noted the hydrolytic instability of these compounds relative to the methylcarbamates, but we found that reproducible ki values could be obtained with freshly prepared dilutions (Table 1). For 1c–2c and 4c, AgAChE, ki values of the 1° carbamates were somewhat lower (40–70%) than those of the corresponding methylcarbamates. At hAChE ki values of 1c–2c and 4c were very similar to those of the methylcarbmates. Thus, high selectivity was seen for 1c (300-fold), but only moderate selectivity for 2c (49-fold) and low selectivity for 4c (13-fold). For 1c, 2c, and 4c, no significant change was seen in ki values at G119S-AgAChE. Interestingly, for the pyrazol-4-yl carbamate 5c, increased ki values were seen at all three enzymes. In fact, the rate constants measured for inactivation of WT AgAChE (19,000 mM−1 min−1) and G119S-AgAChE (587 mM−1 min−1) are the fastest we have measured thus far for any carbamate. However, the tarsal contact toxicity of these compounds to G3 strain of An. gambiae (Table 1) ranged from very low for 1c & 2c (no mortality at 1000 μg/mL) to moderate for 4c (LC50 ~500 μg/mL) to excellent for 5c (LC50 = 90 μg/mL, equitoxic to 5a).
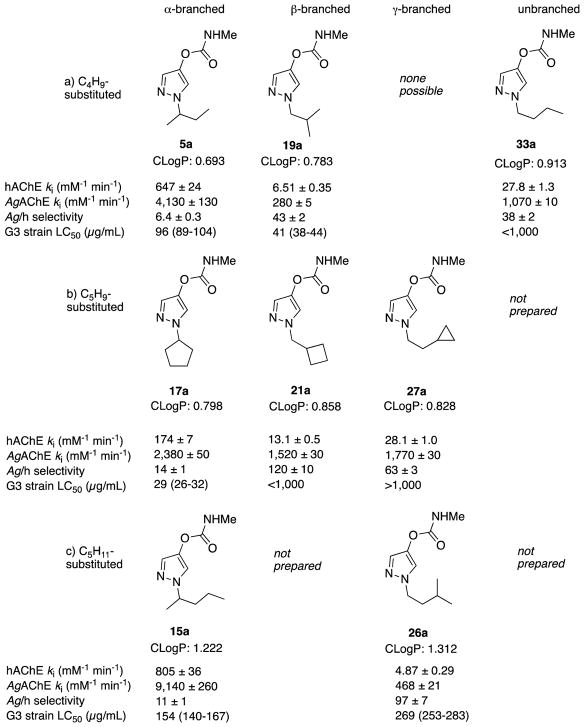
3.3 Inhibition selectivity and G3 tarsal contact toxicity of β-branched and γ-branched 1-alkyl-pyrazol-4-yl methylcarbamates
The comparative study of aryl methyl and dimethylcarbamates above confirmed that dimethylcarbamates can have reduced selectivity for inhibition of WT AgAChE, providing a possible explanation for the low species-selectivity of 5-alkyl-isoxazol-3-yl dimethylcarbamates 7 and 8. Accordingly, a collection of pyrazol-4-yl methylcarbamates bearing α-branched, β-branched, γ-branched, δ-branched and unbranched 1-alkyl groups were prepared and assayed for enzymatic selectivity (Table 2). For hAChE, the highest ki values were seen for the α-branched compounds (5a, 14a–18a, 80–805 mM−1 min−1). Corresponding ki values for β-branched (19a–25a, 0.5–13 mM−1 min−1), γ-branched (26a–30a, 0.6–28 mM−1 min−1), δ-branched (31a–32a, 3–4 mM−1 min−1) and unbranched (33a, 28 mM−1 min−1) compounds were considerably smaller.
In aggregate, compounds bearing α-branched 1-alkyl groups also showed the highest WT AgAChE ki values (500–9,000 mM −1 min−1), but ki values for β-branched (5–1,500 mM−1 min−1) and γ-branched compounds (8–1,770 mM−1 min−1) were not as diminished as they were in the case of hAChE. Consequently, β- and γ-branched compounds showed higher selectivity for inhibition of WT AgAChE than did the α-branched compounds. Compounds 21a (120-fold selective) and 22a (250-fold selective) are β-branched, bearing cyclobutylmethyl and cyclopentylmethyl groups respectively. Compounds 26a (96-fold) and 27a (63-fold) are γ-branched, bearing 3-methylbutyl and 2-cyclopropylethyl groups, respectively. Regarding G119S AgAChE ki values, we would note that the magnitudes and trends seen in hAChE ki values are maintained. α-Branched compound ki values ( 14a–18a, 11–290 mM −1 min−1) were larger than those for β-branched (19a–25a, 0.5–14 mM −1 min−1), γ-branched (26a–30a, 0.5–23 mM−1 min−1), δ-branched (31a–32a, ~2 mM −1 min−1) and unbranched (33a, 18 mM−1 min−1) compounds.
The tarsal contact toxicity data in Table 2 against G3 and Akron strain An. gambiae showed that, in general, the α-branched compounds (14a–18a) have the greatest toxicity, with cross resistance in the Akron strain of less than 2-fold (Table 2). Exceptions were compounds 17a (12.6-fold cross resistance) and 19a, which matched the enzyme inhibition potency and lethality to G3 females of 4a with cross resistance in Akron of 5.6-fold (Table 2). Unfortunately, toxicities of the species-selective carbamates bearing β- and γ-branched alkyl groups (21a, 22a, 26a, 27a) were markedly reduced. Yet, one selective compound 26a (96-fold selectivity) had a G3 strain tarsal contact LC50 value of 269 μg/mL, 5 to 6-fold lower than that of the selective aryl methylcarbamates 1a and 2a, respectively (c.f. Table 1). Furthermore, unlike 1a and 2a, compound 26a rapidly inactivates G119S AgAChE (ki = 5.88 mM−1 min−1), and shows low cross-resistance to Akron strain (LC50 = 948 μg/mL) of 3.5-fold (Table 2).
4. Discussion
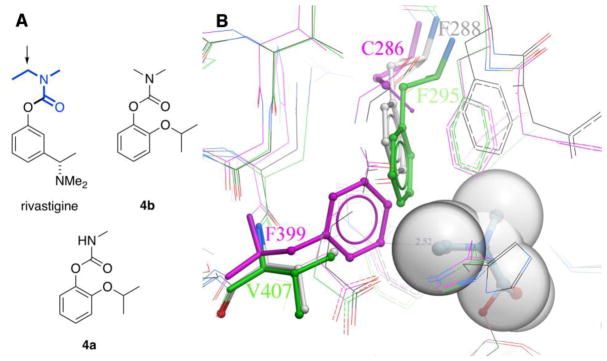
As mentioned previously, in all cases examined thus far, dimethylcarbamates inactivate WT AgAChE much more slowly than the corresponding methylcarbamates (Table 1, cf. 1a, 1b; 2a, 2b; 4a, 4b; 5a, 5b). A decreased rate is also observed for hAChE, as might be expected on the basis of greater steric hindrance of the dimethylcarbamate carbonyl group to nucleophilic attack by the catalytic serine. Yet, dimethylcarbamates show less diminishment in hAChE ki values compared to AgAChE ki values, suggesting that the acyl pocket of hAChE is somehow more accommodating to the additional methyl group. To understand this trend, we examined the 2.2 Å structure (PDB ID: 1GQR) [22] of the ethylmethylcarbamoyl form of Torpedo californica AChE (TcAChE), resulting from its covalent inhibition by rivastigmine (Fig. 2, gray sticks in Fig. 2B).
Fig. 2.
A) Comparison of the structures of rivastigmine, dimethylcarbamate 4b, and methylcarbamate 4a. The transferrable ethylmethylcarbamoyl portion of rivastigmine is shown in bold blue; its CH2 unit is indicated with an arrow. B) Overlay of select residues in ethylmethylcarbamoyl T. californica AChE (gray PDB ID: 1GQR), apo hAChE (green, PDB ID: 4EY4, 2.16 Å) and apo G119S AgAChE (magenta, PDB ID: 6ARX, 2.30 Å). The ethylmethylcarbamoyl group of rivastigmine (gray spheres) is covalently attached to the catalytic serine of TcAChE (S200); the phenolic leaving group is not shown. Note that AgAChE residue numberings begin with the start of the conserved catalytic subunit, and do not include the 161-amino acid N-terminal domain. Thus, D1 is D162 in full- length AgAChE, and F399 is F560 in full- length AgAChE.
On this structure is superimposed the apo form of hAChE (green sticks, PDB ID: 4EY4, 2.16 Å)[23] and the recent apo G119S AgAChE structure (magenta sticks, PDB ID: 6ARX, 2.3 Å).[24] The ethylmethylcarbamoyl group (gray spheres) is bound to the catalytic serine (S200 in TcAChE), and projects into the acyl pocket, which is largely defined by conserved sidechains (Tc/Ag/h respectively: W233/232/236, F290/288/297 and F331/329/338, not shown in Figure 2B). However, as Figure 2B illustrates, the side of the acyl pocket closest to the CH2 unit of the ethylmethylcarbamoyl group is delimited by a phenylalanine sidechain that comes from a different part of the enzyme fold in TcAChE/hAChE vs. AgAChE. In TcAChE and hAChE, the sidechain of F288/295 is in a relatively mobile loop, as suggested by the movement of the F288 sidechain seen in ethylmethylcarbamoyl TcAChE (gray) relative to apo hAChE (green). In contrast, the delimiting phenylalanine (F399) in AgAChE belongs to a deeply buried end of a helix that is likely quite static, and appears too close to the CH2 group of rivastigmine. Thus, we attribute greater expansion plasticity to the acyl pocket of hAChE, allowing adaptation to variously sized moieties. This flexibility reduces the impact of the additional methyl group on hAChE ki values relative to the AgAChE ki values, thus reducing Ag/h inhibition selectivity (Table 1).
Another feature of the dimethylcarbamates that deserves commentary is their greater than expected tarsal contact toxicity, given their low WT AgAChE ki values relative to the corresponding methylcarbamates. For example, 1b and 2b are 10-fold more toxic than 1a and 2a, despite the fact that their WT AgAChE ki values are decreased by more than 95% relative to 1a and 2a. Metabolism is a likely contributing factor, and dimethylcarbamates are known to undergo oxidative metabolism to the methylcarbamates [25], which are more potent inhibitors (Table 1). In contrast, methylcarbamates are expected to undergo oxidative metabolism to the 1° carbamates, which are poorer inhibitors, except in the case of 5c and 5a (Table 1). In this connection, it is interesting to note that 1° carbamates 1c, 2c, and 4c, are less toxic than their methylcarbamate analogs (1a, 2a, 4a), but that 1° carbamate 5c is equitoxic to 5a. However, preliminary piperonyl butoxide synergism studies (data not shown) did not show major effects (i.e. >2-fold) on the toxicity of the methylcarbamates or dimethylcarbamates. For this reason, we propose that additional effects contribute to the high tarsal contact toxicity of dimethylcarbamates. Firstly, decreased rates of hydrolysis of dimethylcarbamates to the phenols, relative to methylcarbamate and 1° carbamates, may contribute to the higher concentrations of the dimethylcarbamates in the CNS following tarsal contact. Secondly, the greater lipophilicity of dimethylcarbamates relative to methylcarbamates (cf. CLogP values of 1.69 and 1.48 for 4b and 4a) may lead to improved transfer efficiency from paper to the mosquito tarsi, and improved transport through the cuticle to the CNS. Further experimentation would be required to test these hypotheses.
With regard to the studies of pyrazol-4-yl methylcarbamates, hAChE and WT AgAChE inactivation rate constants (ki) are significantly affected by the location of the branch in the 1-alkyl group. Compounds bearing α-branched alkyl groups give the highest inactivation rates at WT AgAChE and hAChE, suggesting favorable hydrophobic interactions of the 1-alkyl groups with a pocket close to the catalytic serine in both enzymes. As the branch point is moved from the α-carbon to the β- or γ-carbon, the hAChE ki values are reduced significantly more than are the corresponding WT AgAChE ki values, suggesting that this hydrophobic pocket is more flexible in WT AgAChE than in hAChE. Note that this trend is seen within three isomeric series (Figure 3: a) 5a, 19a, 33a; b) 17a, 21a, 27a; c) 15a, 26a), thus minimizing the likelihood that the trends in ki values are affected by variations in molecular weight or CLogP.
Fig. 3.
Comparison of hAChE ki, AgAChE ki, Ag/h inhibition selectivity, and G3 strain tarsal contact toxicity within three isomeric series a)–c), varying the branch point of the 1-alkyl group from α- to β- to γ-branched to unbranched. Data are taken from Table 2. Note there is minimal variation in CLogP within each isomeric series; calculated topological polar surface area (tPSA) is 53.9 Å2 for all structures (ChemDraw Professional 16.0).
The recent X-ray structure of the covalent complex of G119S AgAChE in complex with the potent 1-alkyl-pyrazol-4-yl difluoromethyl ketone inhibitor 34 [24, 26] does provide support for the aforementioned proposal that the 1-alkyl groups of the pyrazol-4-yl methylcarbamates project into a hydrophobic pocket that is more flexible in AgAChE than in hAChE. In this structure (PDB ID: 6ARY, not shown), the α-branched 3-pentyl group of 34 (see Fig. 4A) projects into a hydrophobic pocket partially defined by the choline-binding site tryptophan W84 [24]. On this basis, we propose that the alkyl groups of 1-alkyl-pyrazol-4-yl methylcarbamates (5a, 14a–33a) project into the same region in their tetrahedral adducts with catalytic serine. We further note that in this X-ray structure of 34 complexed to G119S AgAChE (PDB ID: 6ARY) the 3-pentyl moiety of 34 already fills nearly all the available space between the pyrazole ring and the indole of W84.
Fig. 4.
A) Covalent difluoromethyl ketone inhibitor 34, and An. gambiae-selective inhibitor 22a. B) Superposition of the choline-binding sites of hAChE (PDB ID: 1EY4, gray sticks), and apo G119S AgAChE (PDB ID: 6ARX, magenta ribbon, magenta sticks). Note the canonical orientation of the indole rings of W86 (human) and W84 (apo G119S AgAChE). An alternate conformation of the indole of W84 is shown in aquamarine sticks; this movement expands the hydrophobic binding pocket of AgAChE. C) A view of the tetrahedral intermediate of AgAChE-selective inhibitor 22a with G119S AgAChE, focusing on the cyclopentyl ring of the inhibitor. The surface of the hydrophobic binding pocket of AgAChE is colored gray, except for the surface defined by the indole ring of the W84 in its alternate conformation, which is painted aquamarine. Note that AgAChE residue numberings begin with the start of the conserved catalytic subunit, and do not include the 161-amino acid N-terminal domain. Thus, W84 is W245 in full- length AgAChE.
Based on that structure, it is not clear how AgAChE could accommodate larger groups such as the cyclopentylmethyl group of selective inhibitor 22a, unless the indole unit moved. In hAChE, the position of the indole side chain of W86 is locked by hydrogen-bonding from Y449 (gray sticks, Fig. 4B).
Although the indole side chain of W84 in G119S AgAChE also adopts this canonical orientation (magenta sticks), we propose the replacement of Y449 (hAChE) by D441 (AgAChE) renders the W84 side chain more mobile, since the stabilizing hydrogen bond is lost. Our preliminary modeling of G119S AgAChE identified several low energy rotameric states of W84, including the one depicted in aquamarine sticks in Fig. 4B. In this alternate conformation, the hydrophobic pocket of AgAChE can just accommodate the β-branched methylcyclopentyl group of 22a, but not the larger methylcyclohexyl and CH2CH(Et)2 groups of 23a and 24a respectively (Fig. 4C). In contrast, the smaller hydrophobic pocket in hAChE defined by the canonical orientation of W86 cannot well accommodate 22a. This model thus provides an explanation for the high Ag/h selectivity of 22a, and the lower selectivities of 23a and 24a.
Lastly, as we have noted in our discussion of the dimethylcarbamates above, and in previous studies [10, 12], tarsal contact toxicity of carbamates remains a complex function of several physiochemical and pharmacokinetic parameters, not only AgAChE inhibitory potency. Within each isomeric series, molecular weight and topological polar surface area (tPSA) are identical, and CLogP varies only slightly. Yet within these three series there is no correlation of AgAChE ki values to G3 strain LC50 values, pointing to structure-dependent metabolism and active transport as modulators of toxicity.
Highlights.
Aryl and pyrazol-4-yl carbamates were explored as An. gambiae and human AChE inhibitors
Dimethylcarbamates have lower inhibition selectivity than methylcarbamates
Beta- and gamma-branched alkyl groups confer An. gambiae AChE inhibition selectivity
Comparison of hAChE and G119S AgAChE crystal structures explains these selectivities
Acknowledgments
We thank the National Institutes of Health for funding (AI082581), and acknowledge with gratitude the MR4 as part of the BEI Resources Repository, NIAID, NIH, for providing eggs for the Anopheles gambiae G3 (MRA-112) and Akron (MRA-913) strains.
Footnotes
Conflict of Interest: None of the authors have any conflict to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Malaria Report. The World Health Organization; 2016. [last accessed 8/1/17]. available at http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ [Google Scholar]
- 2.Lindblade KA, Mwandama D, Mzilahowa T, Steinhardt L, Gimnig J, Shah M, Bauleni A, Wong J, Wiegand R, Howell P, Zoya J, Chiphwanya J, Mathanga DP. A cohort study of the effectiveness of insecticide-treated bed nets to prevent malaria in an area of moderate pyrethroid resistance. Malawi, Malaria J. 2015;14:31. doi: 10.1186/s12936-015-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mutuku FM, King CH, Mungai P, Mbogo C, Mwangangi J, Muchiri EM, Walker ED, Kitron U. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malaria J. 2011;10:356. doi: 10.1186/1475-2875-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province. Kenya, Malaria J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, Battle KE, Moyes CL, Henry A, Eckhoff PA, Wenger EA, Briet O, Penny MA, Smith TA, Bennett A, Yukich J, Eisele TP, Griffin JT, Fergus CA, Lynch M, Lindgren F, Cohen JM, Murray CLJ, Smith DL, Hay SI, Cibulskis RE, Gething PW. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranson H, Lissenden N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016;32:187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Rowland M, Boko P, Odjo A, Asidi A, Akogbeto M, N’Guessan R. A New Long-Lasting Indoor Residual Formulation of the Organophosphate Insecticide Pirimiphos Methyl for Prolonged Control of Pyrethroid-Resistant Mosquitoes: An Experimental Hut Trial in Benin. PLOS ONE. 2013;8:e69516. doi: 10.1371/journal.pone.0069516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weill M, Lutfalla G, Mogensen K, Chandre F, Berthomieu A, Berticat C, Pasteur N, Philips A, Fort P, Raymond M. Insecticide resistance in mosquito vectors. Nature. 2003;423:136–137. doi: 10.1038/423136b. [DOI] [PubMed] [Google Scholar]
- 9.Hartsel JA, Wong DM, Mutunga JM, Ma M, Anderson TD, Wysinski A, Islam R, Wong EA, Paulson SL, Li J, Lam PC-H, Totrov MM, Bloomquist JR, Carlier PR. Re-engineering aryl methylcarbamates to confer high selectivity for inhibition of Anopheles gambiae versus human acetylcholinesterase. Bioorg Med Chem Lett. 2012;22:4593–4598. doi: 10.1016/j.bmcl.2012.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong DM, Li J, Lam PCH, Hartsel JA, Mutunga JM, Totrov M, Bloomquist JR, Carlier PR. Aryl methylcarbamates: Potency and selectivity towards wild-type and carbamate-insensitive (G119S) Anopheles gambiae acetylcholinesterase, and toxicity to G3 strain An. gambiae. Chem Biol Interact. 2013;203:314–318. doi: 10.1016/j.cbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong DM, Li J, Chen Q-H, Han Q, Mutunga JM, Wysinski A, Anderson TD, Ding H, Carpenetti TL, Verma A, Islam R, Paulson SL, Lam PC-H, Totrov M, Bloomquist JR, Carlier PR. Select small core structure carbamates exhibit high contact toxicity to “carbamate-resistant” strain malaria mosquitoes, Anopheles gambiae (Akron) PLOS One. 2012;7:e46712. doi: 10.1371/journal.pone.0046712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma A, Wong DM, Islam R, Tong F, Ghavami M, Mutunga JM, Slebodnick C, Li J, Viayna E, Lam PCH, Totrov MM, Bloomquist JR, Carlier PR. 3-Oxoisoxazole-2(3H)-carboxamides and isoxazol-3-yl carbamates: Resistance-breaking acetylcholinesterase inhibitors targeting the malaria mosquito, Anopheles gambiae. Bioorg Med Chem. 2015;23:1321–1340. doi: 10.1016/j.bmc.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang YP, Ekstrom F, Polsinelli GA, Gao Y, Rana S, Hua DH, Andersson B, Andersson PO, Peng L, Singh SK, Mishra RK, Zhu KY, Fallon AM, Ragsdale DW, Brimijoin S. Selective and Irreversible Inhibitors of Mosquito Acetylcholinesterases for Controlling Malaria and Other Mosquito-Borne Diseases. PLOS One. 2009;4:e6851. doi: 10.1371/journal.pone.0006851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou D, Park JG, Rana S, Madden BJ, Jiang H, Pang Y-P. Novel Selective and Irreversible Mosquito Acetylcholinesterase Inhibitors for Controlling Malaria and Other Mosquito-Borne Diseases. Sci Rep. 2013;3 doi: 10.1038/srep01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alout H, Labbé P, Berthomieu A, Djogbénou L, Leonetti J-P, Fort P, Weill M. Novel AChE Inhibitors for Sustainable Insecticide Resistance Management. PLOS One. 2012;7:e47125. doi: 10.1371/journal.pone.0047125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engdahl C, Knutsson S, Ekström F, Linusson A. Discovery of Selective Inhibitors Targeting Acetylcholinesterase 1 from Disease-Transmitting Mosquitoes. J Med Chem. 2016;59:9409–9421. doi: 10.1021/acs.jmedchem.6b00967. [DOI] [PubMed] [Google Scholar]
- 17.Knutsson S, Kindahl T, Engdahl C, Nikjoo D, Forsgren N, Kitur S, Ekström F, Kamau L, Linusson A. N-Aryl-N′-ethyleneaminothioureas effectively inhibit acetylcholinesterase 1 from disease-transmitting mosquitoes. Eur J Med Chem. 2017;134:415–427. doi: 10.1016/j.ejmech.2017.03.050. [DOI] [PubMed] [Google Scholar]
- 18.Andraos J. On the Propagation of Statistical Errors for a Function of Several Variables. J Chem Educ. 1996;73:150–154. [Google Scholar]
- 19.WHO/CDS/NTD/WHOPES/GCDPP/2006.3. World Health Organization; Geneva: 2006. Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets. [Google Scholar]
- 20.Robertson JL, Preisler HK, Russell RM. PoloPlus Probit and Logit Analysis. LeOra Software; 2002. [Google Scholar]
- 21.Fahmy MAH, Metcalf RL, Fukuto TR, Hennessy DJ. Structure and Activity, Effects of Deuteration Fluorination, and Other Structural Modifications of Carbamyl Moiety upon Antichlolinesterase and Insecticidal Activities of Phenyl N-Methylcarbamates. J Agric Food Chem. 1966;14:79–83. [Google Scholar]
- 22.Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine. Biochemistry. 2002;41:3555–3564. doi: 10.1021/bi020016x. [DOI] [PubMed] [Google Scholar]
- 23.Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN, Love J, Franklin MC, Height JJ. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem. 2012;55:10282–10286. doi: 10.1021/jm300871x. [DOI] [PubMed] [Google Scholar]
- 24.Cheung J, Mahmood A, Kalathur R, Liu L, Carlier PR. Structure of the G119S Mutant Acetylcholinesterase of the Malaria Vector Anopheles gambiae Reveals Basis of Insecticide Resistance. Structure. 2018;126:130–136. doi: 10.1016/j.str.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oonnithan ES, Casida JE. Oxidation of methyl- and dimethylcarbamate insecticide chemicals by microsomal enzymes and anticholinesterase activity of the metabolites. J Agric Food Chem. 1968;16:28–44. [Google Scholar]
- 26.Camerino E, Wong DM, Tong F, Körber F, Gross AD, Islam R, Viayna E, Mutunga JM, Li J, Totrov MM, Bloomquist JR, Carlier PR. Difluoromethyl ketones: Potent inhibitors of wild type and carbamate-insensitive G119S mutant Anopheles gambiae acetylcholinesterase. Bioorg Med Chem Lett. 2015;25:4405–4411. doi: 10.1016/j.bmcl.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]