The coining of the concept of cardiovascular risk factors emerged from the Framingham study in the 1960s. Since then we have made major inroads into controlling the traditional risk for atherosclerosis including hypertension, cigarette smoking, and cholesterol, including dramatic recent advances in the management of low-density lipoprotein. Yet, our coronary care units remain full, and we face a considerable burden of residual risk after control of the traditional “Framingham” risk factors. Tardy initiation of preventive strategies likely contributes to this residual risk. For example, considerable evidence shows that lifetime exposure to risk factors such as high LDL due to genetic variants confers a cumulative risk greater than acquired hypercholesterolemia. In addition, risk factors beyond the classic “Framingham” covariates very likely contribute to residual risk.
The recognition that inflammation can drive cardiovascular complications led Atillio Maseri to opine presciently that this insight provided a glimpse of the “hidden side of the moon.” 1 More than two decades later we now have evidence that intervening on inflammation in addition to guideline-directed management of traditional cardiovascular risk factors provides one additional avenue to reducing residual cardiovascular risk.2
If we deepen our exploration of the obscure lunar landscape of risk evoked by Maseri, could we uncover even other heretofore unsuspected contributors to residual cardiovascular risk? Recent findings suggest that, indeed, we have overlooked an important driver of cardiovascular disease. A quest for understanding the fundamental defects that predispose to development of hematologic malignancies led to this realization.
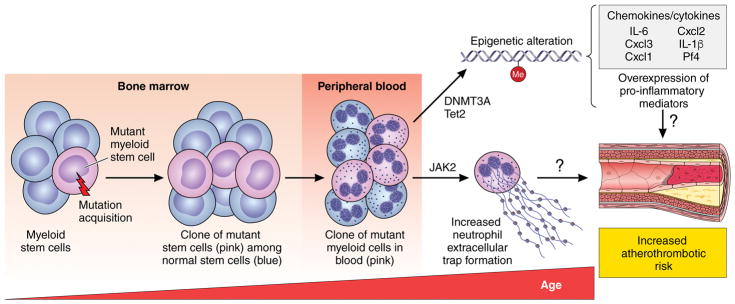
As we age, we accumulate acquired mutations in hematopoietic stem cells in the bone marrow, some of which confer a competitive advantage (Figure). As a consequence, clonal progeny of the mutant stem cells appear in peripheral blood. By age 70, more than 10% of individuals harbor these clones that account on average for about 20% of their peripheral white cells.3 The presence of such clones represents a pre-malignant state, and carriers of these clones have an elevated incidence of hematologic malignancies, with a rate of progression of approximately 0.5% per year. The term clonal hematopoiesis of indeterminate potential (CHIP) describes this condition, as most carriers will never develop a full-blown malignancy, such as acute myeloid leukemia. The diagnosis of CHIP applies to those with mutant clones that exceed 2% of their peripheral leukocyte count. It is not clear yet whether the burden of mutant cells correlates with cardiovascular risk.
Figure 1. Clonal Hematopoiesis and Mechanisms that Might Accelerate Atherothrombotic Risk.
Acquired mutations in bone marrow stem cells accumulate with age and can give rise to a clonal population of mutant leukocytes that circulate in peripheral blood, a condition called CHIP. This figure depicts how the clones arise. In peripheral blood, they account for a around 20% of leukocytes on the average (this diagram shows only this minority of mutant cells). Most individuals with CHIP will never develop a hematologic malignancy, but exhibit a marked increase in the risk of myocardial infarction, stroke, and perhaps likely venous thrombosis and pulmonary embolism. Only four mutations account for most cases of CHIP. Some of them appear to cause increased cardiovascular risk because of changes in methylation of DNA, epigenetic alterations that increase the transcription of pro-inflammatory genes that drive atherogenesis. Another common CHIP mutation in the kinase Jak2 associates with increased thrombotic risk due at least in part to a heightened propensity to develop neutrophil extracellular traps (NETs).
But even individuals with CHIP who never develop a hematologic malignancy have an unexpected and marked increase in mortality. Surprisingly, a number of independent lines of evidence established that this gap in mortality in people with CHIP results from excess cardiovascular disease. Fully adjusted for all traditional risk factors, CHIP carriers have almost a doubling in the relative risk of cardiovascular disease compared to those without these mutant clones.3,4 Although aging associates strongly with CHIP, even those under 50 years old with the condition have an almost fourfold increase in cardiovascular risk. The magnitude of risk enrichment due to CHIP rivals or may even exceed that of some Framingham variables. Studies of experimental atherosclerosis in mice engineered to bear the mutations associated with CHIP indicate that this condition does not merely serve as a marker of aging, but contributes causally to accelerated atherosclerosis.4 Indeed, CHIP may help to explain mechanistically the steep increase in cardiovascular risk with aging.
Diagnosing CHIP requires exome sequencing on peripheral blood, and is not currently a practical routine clinical test, particularly as we have no information about how to mitigate the risk associated with CHIP. Indeed, a key goal for future research would be testing whether interventions can limit this previously unrecognized contributor to cardiovascular risk.
What fundamental mechanisms might account for this striking and hitherto unsuspected increase in cardiovascular risk? Mutations in only four genes account for almost all cases of CHIP. Three of the genes alter either DNA or histone methylation. Such “epigenetic” changes can influence the level of gene expression, although that such mechanisms mediate the increased cardiovascular risk due to CHIP remains speculative at present. In support of dysregulated expression of inflammatory genes, the macrophages in mice that bear Tet2 mutations, one of the common CHIP mutations, have elevated expression of a set of pro-inflammatory genes, including cytokines such as interleukins (IL) 6 and 1, mediators strongly implicated in promoting human cardiovascular events. Another common CHIP mutation occurs in a kinase, Jak2, which is the dominant genetic driver of polycythemia vera, a condition long-associated with a thrombotic diathesis. Neutrophils that carry this specific Jak2 mutation (V617F) exhibit an enhanced ability to form neutrophil extracellular traps (NETs), strands of DNA released from dying granulocytes studded with pro-oxidant, proteolytic, and pro-thrombotic factors, recently implicated in thrombosis and acute coronary events.5 The recognition of the strong link between CHIP and cardiovascular events expands our understanding of residual risk and opens up new avenues of investigation that promise to provide insight into the pathophysiology of atherosclerotic complications and other cardiovascular conditions.
CHIP also provides another link between cancer biology and cardiovascular disease. The burgeoning field of cardio-oncology has focused so far on the adverse effects of anticancer therapies, but should now broaden its scope to include common risk factors and pathophysiologic mechanisms. The finding that anti-inflammatory therapy with a monoclonal antibody that neutralizes IL-1 not only reduces cardiovascular events, but also the incidence of and mortality due to lung cancer in exploratory analyses, provides another unexpected link between cancer biology and cardiovascular disease. We are only at the beginning of an exciting era of convergence between oncology and cardiovascular medicine that promises not only to improve our understanding of disease mechanisms but also to identify new approaches to risk stratification and targets for therapy.
Footnotes
Conflict of Interest Disclosures
B.L.E. has consulted for Celgene.
References
- 1.Maseri A. Inflammation, Atherosclerosis, and Ischemic Events — Exploring the Hidden Side of the Moon. N Engl J Med. 1997;336:1014–1016. doi: 10.1056/NEJM199704033361409. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J Am Coll Cardiol. 2017;70:2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, Silver AJ, Adams D, Castellano CA, Schneider RK, Padera RF, DeAngelo DJ, Wadleigh M, Steensma DP, Galinsky I, Stone RM, Genovese G, McCarroll SA, Iliadou B, Hultman C, Neuberg D, Mullally A, Wagner DD, Ebert BL. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan8292. pii: eaan8292. [DOI] [PMC free article] [PubMed] [Google Scholar]