Abstract
Aims
To study device performance, arrhythmia recurrence characteristics, and methods of outcome assessment using a novel implantable cardiac monitor (ICM) in patients undergoing ablation for atrial fibrillation (AF).
Methods and results
In 419 consecutive patients undergoing first-time catheter ablation for symptomatic paroxysmal (n = 224) or persistent (n = 195) AF an ICM was injected at the end of the procedure. Telemedicine staff ensured full episode transmission coverage and manually evaluated all automatic arrhythmia episodes. Device detection metrics were calculated for ≥2, ≥6, and ≥10 min AF detection durations. Four methods of outcome assessment were studied: continuous recurrence analysis, discontinuous recurrence analysis, AF-burden analysis, and analysis of individual rhythm profiles. A total of 43 673 automatic AF episodes were transmitted over a follow-up of 15 ± 6 months. Episode-based positive predictive values changed significantly with longer AF detection durations (70.5% for ≥2 min, 81.8% for ≥6 min, and 85.9% for ≥10 min). Patients with exclusive short episode recurrences (≥2 to <6 min) were rare and their arrhythmia detection was clinically irrelevant. Different methods of outcome assessment showed a large variation (46–79%) in ablation success. Individual rhythm characteristics and subclinical AF added to this inconsistency. Analysis of AF-burden and individual rhythm profiles were least influenced and showed successful treatment in 60–70% of the patients.
Conclusion
We suggest AF detection duration >6 min and AF burden >0.1% as a standardized outcome definition for AF studies to come in the future.
Keywords: Atrial fibrillation, Ablation, Monitoring, Outcome, Burden, Telemedicine
What’s new?
This is the first complete and continuous post-ablation rhythm monitoring incorporating every single arrhythmia episode in 419 patients over 15 months.
Patients with exclusive short arrhythmia episodes (<6 min) were extremely rare.
Patients exhibit distinctly different post-ablation rhythm profiles. Our first-time observation of extended healing phases (up to 6 months) impacts success rate assessment as well as everyday clinical management.
True outcome differences between paroxysmal and persistent patients were smaller than expected, due to the impact of subclinical atrial fibrillation (AF) recurrences in formerly persistent patients.
Outcome definition significantly impacts success rate. It varied by almost 40% depending on the methodology of rhythm assessment.
We suggest AF detection duration >6 min and AF burden >0.1% as a standardized, objective and easy to obtain outcome definition for AF studies to come in the future.
Introduction
Success rates of atrial fibrillation (AF) ablation remain controversial because many previous studies are based on symptom-reporting and discontinuous monitoring, which underestimate true recurrence rates.1,2 From a clinical perspective it is essential to rely upon appropriate detection of AF to make decisions concerning antiarrhythmic therapy and in the future potentially for oral anticoagulation.3,4 Moreover, reliable diagnosis of AF is scientifically warranted when evaluating new therapeutic approaches.
Implantable cardiac monitors (ICMs) offer the advantage of long-term monitoring. The performance and diagnostic value of older generation ICM have been reported previously.5–11 New generation monitors are smaller, use improved AF detection algorithms and provide remote monitoring for manual electrogram (EGM) analysis.12,13
Our study describes continuous telemedicine-based long-term monitoring with the Reveal LINQ in patients after AF ablation with manual adjudication of every automatically detected arrhythmia episode. The aim was (i) to analyse device detection performance, (ii) to evaluate ablation outcome by different methods of rhythm assessment, and (iii) to study arrhythmia recurrence characteristics.
Methods
Patient population
An investigator-initiated, prospective, non-randomized study, including 419 consecutive patients undergoing first-time catheter ablation for symptomatic, drug refractory paroxysmal, or persistent AF, who agreed to have a Reveal LINQ (Medtronic Inc., Minneapolis, MN, USA) injected for post-ablation rhythm monitoring, was conducted between March 2014 and September 2016. Patients were followed up until the end of the study or the date of re-ablation. The study was approved by the institutional ethical review board (EK 28409202) and conforms to the principles outlined in the Declaration of Helsinki. All data were collected, managed and analysed at Heart Centre Dresden and the Steinbeis Research Institute ‘Electrophysiology and Cardiac Devices’.
Ablation procedure
Ablation was performed with radiofrequency energy using standard irrigated-tip ablation catheter (CARTO THERMOCOOL SF, Biosense Webster, USA; FlexAbility, Abbott, USA). The standard ablation settings included a preselected power of 40 W and a flow rate of 15 mL/min. At the posterior left atrial (LA) wall power delivery was limited to 30 W and 20 s and was terminated at intraoesophageal temperature increases >39°C.
Patients presenting in AF were cardioverted.In sinus rhythm (SR), LA endocardial voltage maps (LASSO NAV, Biosense Webster; Reflexion Spiral, Abbott) were reconstructed in all patients. Bipolar peak-to-peak EGM amplitude <0.5 mV was defined as diseased low-voltage signal (LVA). Lack of local pace capture (10 V and 2 ms) was used to identify scar.14,15
All patients received wide encircling pulmonary vein (PV) isolation with proven entrance and exit block. Additional extra PV ablation was individualized based on the voltage maps where substrates were targeted with (i) homogenization of small LVAs, (ii) linear lesions connecting LVAs to anatomical obstacles (e.g. PVs or mitral annulus), and (iii) linear lesions isolating large LVAs (e.g. isolation of the posterior LA wall). Ablation endpoints were (i) lack of local pace capture and (ii) bidirectional conduction block over linear lesions.14,15
Ablation of the right atrial isthmus was only performed in case of documented/induced typical atrial flutter.
Implantable cardiac monitor implantation procedure
At the end of the procedure, an ICM (Reveal LINQ) was inserted subcutaneously in a left parasternal position. Patients were instructed in the use of the patient assistant, a hand-held activator to activate the ICM in case of acute symptoms. Besides, patients were introduced to the patient monitor, the communication device that remotely forwards monitoring information out of the ICM to the telemedicine platform every night. The ICM is able to detect AF episodes ≥2 min. Besides detecting AF, the device is able to detect other arrhythmias, where the following criteria were used: asystole, defined as an R-R pause of ≥4.5 s; and tachycardia, defined as R-R intervals ≤400 ms (≥150 bpm).
Post-procedural management
Antiarrhythmic medication was routinely discontinued, and patients remained on β-blocker only. In case of recurrences, antiarrhythmic drugs were re-initiated upon individual clinical decision.
Oral anticoagulation was continued for at least 3 months and thereafter according to CHA2DS2-VASc score with deviations upon patient and physician’s discretion.
Implantable cardiac monitor-based follow-up and rhythm assessment
During follow-up telemedicine staff manually evaluated all automatically transmitted episodes and ensured full episode transmission coverage by individual patient contact with add-on manual transmissions if needed. Episodes were manually classified from the EGM as AF, atrial tachycardia, SR, asystole, tachycardia, or no available EGM.
Automatic device reported AF episodes and manually adjudicated episode diagnoses were used to calculate positive predictive value (PPV) of device-detected AF episodes, using the manually adjudicated episode as standard. PPV was compared between three different device-programmable AF detection durations (≥2, ≥6, and ≥10 min).
The date, time, and length of each episode were used to describe AF recurrences, introducing four different methodological approaches of rhythm assessment:
Atrial fibrillation recurrence rates were calculated from a Kaplan–Meier survival analysis implementing a 3 month blanking period using every automatically detected and manually confirmed AF episode as a recurrent event.
On a ‘per month’ analysis the proportion of patients with any AF episode was assessed discontinuously within each post-interventional month.
Total AF-burden was calculated for the entire follow-up period. Burden calculation incorporated all automatically detected AF episodes. Patients were aggregated into the following burden categories: 0%, >0 to ≤0.01%, >0.01 to ≤0.1%, >0.1 to ≤1%, >1% to ≤10%, and >10%. AF-burden ≤0.1% (≤10 min/week) was defined as subclinical AF.
Patients with >18 months of follow-up were divided into 6 groups of individual rhythm profiles: (i) complete freedom from AF, (ii) <3 months healing period, (iii) 3–6 months healing period, (iv) late single cluster recurrence (isolated AF cluster within 1 week >6 months after ablation), (v) late ongoing failures >12 months after ablation, and (vi) immediate and continuous failures.
Statistics
Categorical variables were reported as count and percentages and continuous variables as mean and standard deviation if normally distributed otherwise as median with interquartile range. Comparison of continuous data were performed using a student's t-test if normally distributed otherwise Wilcoxon signed-rank test, and the χ2 test was used to compare categorical data. Recurrence rates of AF were reported with Kaplan–Meier plots for different detection durations (≥2, ≥6, and ≥10 min). All statistical analyses were performed using STATA (12.1). A P-value of <0.05 was considered statistically significant.
Results
Study cohort
We included 419 consecutive patients [224 (53%) with paroxysmal AF] followed up by continuous rhythm monitoring for 15 ± 6 months. Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of all patients and patients with persistent and paroxysmal AF
| Baseline characteristics |
||||
|---|---|---|---|---|
| All (n = 419) | Paroxysmal (n = 224) | Persistent (n = 195) | P-value | |
| Age (years) | 65 ± 10 | 63 ± 11 | 67 ± 10 | <0.001 |
| Male gender | 237 (57%) | 127 (56%) | 111 (57%) | 0.89 |
| BMI | 28.9 ± 5 | 28.6 ± 5 | 29.3 ± 6 | 0.14 |
| Hypertension | 314 (75%) | 157 (70%) | 157 (81%) | 0.014 |
| DM | 78 (19%) | 30 (13%) | 48 (25%) | 0.003 |
| CAD | 70 (17%) | 37 (16%) | 33 (17%) | 0.91 |
| RF | 18 (4%) | 7 (3%) | 11 (6%) | 0.21 |
| ICM | 6 (1%) | 0 (0%) | 6 (1%) | 0.008 |
| DCM | 14 (3%) | 1 (0.5%) | 13 (7%) | <0.001 |
| Stroke | 28 (7%) | 15 (7%) | 13 (7%) | 1.0 |
| CABG | 6 (1%) | 3 (1%) | 3 (2%) | 0.86 |
| Valve surgery | 6 (1%) | 3 (1%) | 3 (2%) | 0.86 |
| CHA2DS2-VASc score | 2.4 ± 1.5 | 2.1 ± 1.5 | 2.7 ± 1.5 | <0.001 |
| LVEF (%) | 57 ± 9 | 59 ± 7 | 55 ± 11 | <0.001 |
| LA diameter (cm) | 44 ± 6 | 43 ± 6 | 46 ± 6 | <0.001 |
| Low voltage zone areas | 105 (26%) | 23 (10%) | 82 (43%) | <0.001 |
BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; DCM, dilative cardiomyopathy; DM, diabetes mellitus; ICM, ischaemic cardiomyopathy; LA, left atrium; LVEF, left ventricular ejection fraction; RF, renal failure.
At 3 months follow-up 21 (5%) patients were on antiarrhythmic medication and 155 (37%) were on β-blockers. At 6 months follow-up and thereafter proportions did not change significantly, 11 (3%) patients on antiarrhythmic drugs and 145 (35%) patients on β-blockers.
During the study period, 70 (17%) patients received electric cardioversion at a median 1 month (interquartile range, 1 to 3) post-ablation. Eighteen (4%) patients had a second and 3 (0.7%) patients a third cardioversion.
Re-ablation was performed in 22 (5%) patients after a median 8 months (interquartile range, 5 to 13) and terminated study follow-up in those patients.
Device detection performance
The devices transmitted a total of 43 673 automatically detected AF episodes. Of these, 16 964 (39%) were without EGM, 7884 (18%) were manually classified as SR, and 18 819 (43%) truly showed AF (Table 2).
Table 2.
All automatically detected episodes by the ICM and the observer adjudicated diagnoses
| Observer adjudication |
||||||
|---|---|---|---|---|---|---|
| ICM diagnose | SR | AF/AT | Pause | Tachycardia | No EGM | Total |
| AF | 7884 | 18 819 | 6 | 0 | 16 964 | 43 673 |
| AT | 7035 | 1830 | 0 | 0 | 7893 | 16 758 |
| Asystole | 4898 | 214 | 328 | 0 | 1351 | 6788 |
| Tachycardia | 707 | 1758 | 0 | 195 | 1009 | 3669 |
| Symptom | 317 | 151 | 0 | 5 | 1 | 474 |
| Total | 20 838 | 22 772 | 334 | 200 | 27 218 | 71 362 |
AF, atrial fibrillation; AT, atrial tachycardia; EGM, electrogram; ICM, implantable cardiac monitor; SR, sinus rhythm.
Increasing detection duration from ≥2 to ≥6 min and ≥10 min led to a >50% decrease in total episode count, episodes without EGM, and episodes with false-positive AF detection (Table 3). The PPV of device-detected AF episodes changed significantly for the different detection durations (70.5% for ≥2 min, 81.8% for ≥6 min, and 85.9% for ≥10 min, Table 3).
Table 3.
Episode-based PPV, proportion of episodes without EGM, and total AF episode count analysed for the three different AF detection durations
| AF detection duration | AF | No AF | PPV (%) | P-value | No EGM | Total |
|---|---|---|---|---|---|---|
| ≥2 min | 18 819 | 7890 | 70.5 | 16 964 | 43 673 | |
| ≥6 min | 11 903 | 2645 | 81.8 | <0.001a | 7076 | 21 624 |
| ≥10 min | 9751 | 1604 | 85.9 | <0.001a | 4292 | 15 647 |
AF, atrial fibrillation; EGM, electrogram; PPV, positive predictive value.
P-value for PPV comparison between ≥2 min and ≥6 to ≥10 min detection durations.
Rhythm assessment I: continuous recurrence analysis
Using a 3 months blanking period, 227 (54%) patients experienced at least one episode of AF recurrence ≥2 min during follow-up. Increasing duration to ≥6 and ≥10 min, proportions decreased to 198 (47%) patients (P = 0.045) and 191 (46%) patients (P = 0.013), respectively.
Twenty-nine patients had exclusive episodes between 2 and 6 min, 17 of these had only one episode, 10 patients had 2–5 episodes and 2 patients had >5 episodes (10 and 12).
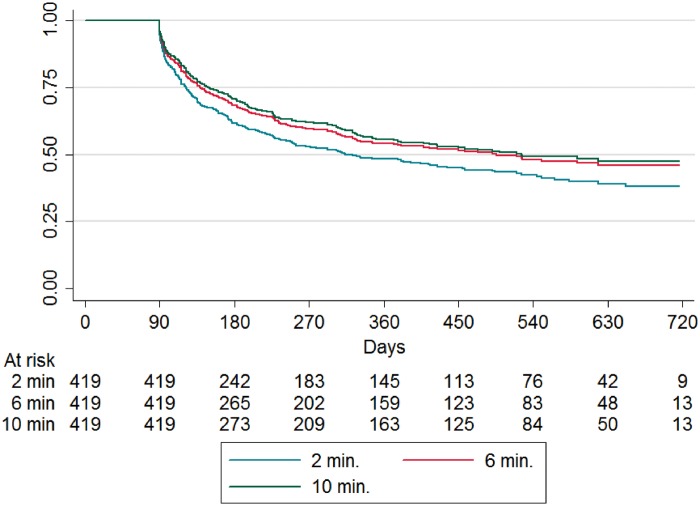
Figure 1 shows time to first AF recurrence with ≥2, ≥6, and ≥10 min detection duration. One-year freedom from AF episode was 48% [95% confidence interval (CI) 43–53] with ≥2 min, 54% (95% CI 49–59) with ≥6 min, and 56% (95% CI 50–60) with ≥10 min detection duration, respectively.
Figure 1.
Continuous recurrence analysis: time to first AF episode ≥2, ≥6, and ≥10 min, after a 3 month blanking period. AF, atrial fibrillation.
Comparing patients with paroxysmal and persistent AF 1 year freedom from AF ≥2 min was 56% (95% CI 49–62) for paroxysmal and 40% (95% CI 32–47) for persistent AF.
Rhythm assessment II: discontinuous recurrence analysis
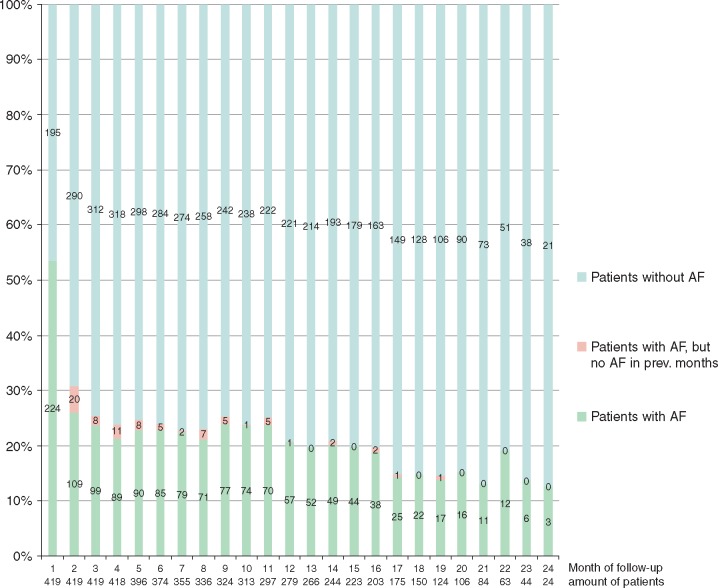
Calculating monthly recurrence rates, 53% experienced one AF episode ≥2 min during the 1st post-procedural month, which declined to 31% and 26% at 2nd and 3rd post-procedural month. During further follow-up, the proportion of patients showing AF episodes within a given month remained stable at around 10–25% (Figure 2).
Figure 2.
Discontinuous monthly recurrence analysis: proportion of patients with any AF episode (≥2 min) assessed within each of the 24 post-interventional months. Patients with ‘de novo recurrences’ are shown in red. AF, atrial fibrillation.
Rhythm assessment III: atrial fibrillation-burden analysis
Atrial fibrillation-burden analysis according to the predefined categories is presented in Table 4. Overall the percentage of patients with no or only subclinical AF burden was 68%, 69%, and 69% for ≥2, ≥6, and ≥10 min detection durations, respectively (P = 0.77 for ≥2 min vs. ≥6 min and P = 0.6 for ≥2 min vs. ≥10 min).
Table 4.
Atrial fibrillation-burden analysis: the proportion of patients within six predefined burden categories is shown for AF detection durations ≥2 min (all patients, paroxysmal AF, and persistent AF) and AF detection durations ≥6 min and ≥10 min (all patients)
| AF burden category | All patients (n = 419) | Paroxysmal AF (n = 224) | Persistent AF (n = 195) | All patients (n= 419) | All patients (n = 419) |
|---|---|---|---|---|---|
| ≥2 min | ≥2 min | ≥2 min | ≥6 min | ≥10 min | |
| 0% | 181 (43.2%) | 112 (50.0%) | 69 (35.4%) | 218 (52.0%) | 227 (54.2%) |
| >0 to <0.01% (<1 min/week) | 56 (13.3%) | 29 (12.9%) | 27 (13.8%) | 25 (6.0%) | 22 (5.3%) |
| >0.01 to <0.1% (<10 min/week) | 47 (11.2%) | 21 (9.4%) | 26 (13.3%) | 45 (10.7%) | 42 (10.0%) |
| No or subclinical AF burden | 284 (68%) | 162 (72%) | 122 (63%) | 288 (69%) | 291 (69%) |
| >0.1 to <1% (<1.7 h/week) | 73 (17.4%) | 36 (16.1%) | 37 (19.0%) | 71 (16.9%) | 69 (16.5%) |
| >1 to <10% (<17 h/week) | 52 (12.4%) | 24 (10.7%) | 28 (14.4%) | 50 (11.9%) | 49 (11.7%) |
| >10% | 10 (2.4%) | 2 (0.9%) | 8 (4.1%) | 10 (2.4%) | 10 (2.4%) |
| Clinical AF burden | 135 (32%) | 62 (28%) | 73 (37%) | 131 (31%) | 128 (31%) |
AF, atrial fibrillation.
Comparing patients with paroxysmal and persistent AF, the analysis shows no or only subclinical AF in 72% and 63%, respectively.
Rhythm assessment IV: individual rhythm profiles
A total of 150 patients (86 paroxysmal, 64 persistent) were followed up for >18 months. According to the 6 individual rhythm profiles, 37 (25%) patients had complete freedom from AF (profile A), 33 (22%) patients experienced a healing period <3 months (profile B), 6 (4%) patients had a prolonged healing phase of 3–6 months (profile C), 13 (9%) patients had a late single cluster recurrence (profile D), 8 (5%) patients were late on-going failures (profile E), and 53 (35%) patients were continuous failures (profile F). From such a clinical perspective, all but the last two patient groups (profile E or F) were successfully treated. Figure 3 provides case examples of the various rhythm profiles.
Figure 3.
Case examples of the six individual rhythm profiles (A–F). AF, atrial fibrillation; AT, atrial tachycardia.
Patients with persistent AF patients were less often successfully treated with 29/64 (45%) showing failure profiles E or F when compared with 28/85 (32%) of the paroxysmal patients. In case of treatment success patients with persistent AF were underrepresented in rhythm profile A (complete freedom from AF) and showed an overrepresentation among more complex rhythm profiles C and D (prolonged healing phase and late single cluster recurrences) (Table 5).
Table 5.
Distribution of all, paroxysmal, and persistent AF patients within the six groups of individual rhythm profiles
| Patients | Individual rhythm profiles |
||||||
|---|---|---|---|---|---|---|---|
| All | A | B | C | D | E | F | |
| All | 150 | 37 | 33 | 6 | 13 | 8 | 53 |
| Paroxysmal AF | 86 | 25 | 20 | 3 | 6 | 4 | 28 |
| Persistent AF | 64 | 12 | 13 | 3 | 7 | 4 | 25 |
| % persistent AF | 43% | 32% | 39% | 50% | 54% | 50% | 47% |
A indicates complete freedom from AF, B indicates healing period <3 months, C indicates prolonged healing period of 3–6 months, D indicates late single cluster recurrences, E indicates late on-going failures, and F indicates immediate and continuous failures.
AF, atrial fibrillation.
Adverse events
Ablation related complications occurred in 26 (6.2%) patients. We experienced a case of telemedicine-detected oesophageal-pericardial fistula (0.2%) that was treated with an intra-oesophageal stent and a single incidence of cardiac tamponade (0.2%). The remaining complications were femoral pseudoaneurysms (n = 17, 4%) and small arteriovenous fistulas (n = 7, 1.7%).
Implantable cardiac monitor related complications occurred in three patients (0.7%), two patients had a mild pocket haematoma and in one patient the device dislodged out of the pocket.
Discussion
Main findings of the study
The present study reports device detection performance, recurrence patterns and ablation outcome using a new generation ICM in a large AF ablation cohort.
Our data question the need to monitor AF episodes <6 min after AF ablation. Longer detection durations improve episode PPV, reduce total episode count, and do not neglect relevant clinical information.
Our data illustrate the dependency of reported success rates from the method of rhythm assessment. With four different approaches, our single procedure success varied between 46% and 79%.
For future AF studies, we suggest AF detection duration >6 min and AF burden >0.1% as a standardized outcome definition.
Device detection performance
Previously Sanders et al.12 investigated the performance of the Reveal LINQ in the LINQ Usability Study. Besides a high sensitivity (98.4%) and specificity (99.5%) they reported an episode-based PPV of 74.8%, which is in line with our data.
This indicates a significant technological advancement compared with previous ICM, including the Reveal XT, and the Confirm ICM, which had episode-based PPVs ranging from 39% to 64%.12,16
We showed that longer AF detection durations (≥6 and ≥10 min) improve the episode-based PPV from 70.5% to 81.8% and 85.9%.
What is the optimal atrial fibrillation detection duration?
Apart from stronger detection performance, longer detection durations reduced (>50%) the overall transmitted episodes and the proportion of episodes without EGM. That reduces workload and the need for manual episode adjudication in a clinical setting.
The clinical relevance of detecting very short AF episodes (≥2 to <6 min) also seems debatable with our data:
Only few patients had exclusively short episodes. Around 90% of patients with AF, also exhibited episodes longer than 6 or 10 min.
Among patients with exclusively short episodes the absolute episode count was low (median of 1), so the relevance in terms of symptoms and thromboembolic risk appears questionable.
Atrial fibrillation burden assessment for different detection durations (≥2, ≥6, and ≥10 min) revealed similar proportions of relevant burden.
Taking into account that until today only atrial high rate episodes ≥6 min, without clinical AF are associated with thromboembolic events,17 our data further questions the relevance of detecting even shorter arrhythmia episodes.
What is the optimal assessment of atrial fibrillation ablation outcome?
The assessment of ablation outcome does not only determine reported success rates, it also triggers clinical decisions—today for antiarrhythmic treatment and re-ablation, in the future potentially for anticoagulation.
Outcome assessment is challenged by the following (i) asymptomatic recurrences, (ii) short episodes, (iii) various follow-up durations due to re-ablation timing, and (iv) individual healing durations and other post-ablation recurrence patterns.
Depending on the method of electrocardiogram (ECG) monitoring, significant differences between discontinuous and continuous monitoring are expected.
In our present study, we used four different approaches to assess ablation outcome from the same data retrieved during continuous monitoring: (i) continuous recurrence analysis, (ii) discontinuous recurrence analysis, (iii) AF-burden analysis and (iv) analysis of individual rhythm profiles.
The continuous recurrence analysis is the most rigid measure of outcome. Every episode beyond the blanking period is counted. In our cohort, 46% of all patients were free from any AF episode ≥2 min. Scientifically this method of outcome assessment may be considered the most accurate one. However, it does not incorporate individual recurrence pattern such as prolonged healing phases, and therefore over-reports failures. In addition, it over-weighs subclinical AF recurrences with rare and short episodes. Especially for persistent AF such an analysis may be misleading.
The monthly recurrence analysis is a maximum version of discontinuous monitoring - similar to serial 30 days Holter-ECG recordings. In our cohort, 79% of the patients were free from any AF during the 12th month after ablation. This method is closest to current clinical practice with serial Holter recordings of various durations. Due to its discontinuous nature patients with rare or cluster episodes may be falsely classified as treatment success.
Burden analysis allows for a continuous assessment over the entire follow-up duration without over-weighing subclinical AF recurrences with rare and short episodes. This outcome assessment resembles quantitative burden analysis and stroke risk calculations used in pacemaker and defibrillator studies in the past.18–20 Moreover, it is not affected by individual recurrence patterns like prolonged healing or single late recurrences and it is not influenced by different detection durations. In our cohort, 68% of all patients were free from a clinical relevant AF burden after a single procedure. Such an assessment requires a definition of clinical and subclinical AF. We decided to use 10 min AF/week (AF burden ≤0.1%) as the clinical/subclinical AF burden cut-off. That number, however, can be debated. Pokushalov et al.8 considered patients with AF ≤50 min/week (AF-burden <0.5%) as ablation success. In a large device cohort Boriani et al.18 reported on 1 h of AF per day (7 h/week) as the AF burden that incrementally increases the annual thromboembolic risk by 3%—for each hour added.
The analysis of different rhythm profiles represents the outcome assessment with the closest link to therapeutic decisions. The definition of the six individual rhythm profiles resulted from the observation, that clinical ablation outcome is not binary, but rather follows a multifactorial clinical course determined by substrate, ablation, lesion recovery, arrhythmia healing, and disease progression. Our study shows that a relevant number of patients had prolonged healing phases up to 6 months or late single cluster recurrences—both, however, overall successfully treated patients.
Outcome in patients with paroxysmal and persistent atrial fibrillation
Outcome of catheter ablation in patients with persistent AF is considered inferior when compared with patients with paroxysmal AF.
Using continuous rhythm assessment from a Kaplan–Meier survival analysis with 3 months blanking our data show freedom from AF episode ≥2 min in 54% of paroxysmal and 37% of persistent AF patients.
However, the inability of a Kaplan–Meier survival analysis to account for prolonged healing phases and subclinical AF recurrences has been discussed. Especially in patients with persistent AF both situations were more frequently observed.
Assessment of ablation outcome through AF-burden analysis is more robust against these two limitations. In our cohort, 72% of paroxysmal and 63% of persistent AF patients were free from a clinically relevant AF burden. That gives a 26% better ablation outcome in patients with persistent AF when compared with the Kaplan–Meier analysis, and the outcome difference between paroxysmal and persistent patients decreased from 17% to only 9%.
These data indicate, that apart from ablation strategies the method of rhythm assessment fundamentally influences the outcome reported in clinical AF ablation trials—with probably an even larger impact in trials on persistent AF.
Limitations
This study is a non-randomised observational study. Our data were collected in a cohort of post-ablation patients treated with a specific ablation concept. Therefore, they cannot be generalized to other groups of patients with AF.
The device only detects AF ≥2 min. Episodes with shorter durations will occur unrecognized. However, exclusively short AF episodes (>30 s to <2 min) seem to be a very rare phenomenon without impact on AF burden or clinical risks. The LINQ Usability Study reported such Holter detected episodes in only 1% of the patients.12
The overall presence of AF episodes without EGM impairs the assessment of detection performance metrics and the AF-burden analysis. Due to the possibility of unrecognized false positive AF detection, the episode-based PPV may differ and the AF burden will be slightly overestimated.
Electrical cardioversions represent a potential bias to assessment of burden. However, the absolute number was low and predominantly performed within the healing phase. Only 5% of the patients needed repeat cardioversions and all of them had documentation of an overall clinical relevant AF-burden.
Furthermore, definitions of recurrence and outcome derived from the present study are only applicable in patients with implant-based continuous monitoring after AF ablation.
Conclusion
Our study supports recently published data on improved detection performance metrics compared with previous ICM device generations.
Analysis of duration, timing and distribution of >43 000 automatic AF episodes showed an incremental improvement of device performance using longer detection durations (≥6 and ≥10 min) and questioned the need to monitor very short post-ablation AF episodes (≥2 min to <6 min).
Applying four different methods of outcome assessment revealed a large variation (46–79%) in treatment outcome depending on the definition of success. Individual post-ablation rhythm characteristics and a relevant amount of only subclinical AF added to this inconsistency.
We suggest AF detection duration >6 min and AF burden >0.1% as a standardized, objective and easy to obtain outcome definition for AF studies to come in the future.
Conflict of interest: C.P. received lecture honoraria from Abbott, Biotronik, Siemens, Medtronic, and Biosense; research support from Abbott, Biotronik, Biosense, and Medtronic; and serves as an advisory board member of Abbott, Siemens, Biosense, Biotronik, and Imricor. All other authors have nothing to disclose.
References
- 1. Hindricks G, Piorkowski C, Tanner H, Kobza R, Gerds-Li J-H, Carbucicchio C. et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation 2005;2:S275–13. [DOI] [PubMed] [Google Scholar]
- 2. Ziegler PD, Koehler JL, Mehra R.. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm 2006;3:1445–52. [DOI] [PubMed] [Google Scholar]
- 3. Lewalter T, Boriani G.. Relevance of monitoring atrial fibrillation in clinical practice. Arrhythm Electrophysiol Rev 2012;1:54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zuern CS, Kilias A, Berlitz P, Seizer P, Gramlich M, Müller K. et al. Anticoagulation after catheter ablation of atrial fibrillation guided by implantable cardiac monitors. Pacing Clin Electrophysiol 2015;38:688–93. [DOI] [PubMed] [Google Scholar]
- 5. Hindricks G, Pokushalov E, Urban L, Taborsky M, Kuck K-H, Lebedev D. et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ Arrhythm Electrophysiol 2010;3:141–7. [DOI] [PubMed] [Google Scholar]
- 6. Eitel C, Husser D, Hindricks G, Frühauf M, Hilbert S, Arya A. et al. Performance of an implantable automatic atrial fibrillation detection device: impact of software adjustments and relevance of manual episode analysis. Europace 2011;13:480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Turov A, Shirokova N. et al. Ablation of paroxysmal and persistent atrial fibrillation: 1-year follow-up through continuous subcutaneous monitoring. J Cardiovasc Electrophysiol 2011;22:369–75. [DOI] [PubMed] [Google Scholar]
- 8. Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Turov A, Shirokova N. et al. Use of an implantable monitor to detect arrhythmia recurrences and select patients for early repeat catheter ablation for atrial fibrillation: a pilot study. Circ Arrhythm Electrophysiol 2011;4:823–31. [DOI] [PubMed] [Google Scholar]
- 9. Camm AJ, Corbucci G, Padeletti L.. Usefulness of continuous electrocardiographic monitoring for atrial fibrillation. Am J Cardiol 2012;110:270–6. [DOI] [PubMed] [Google Scholar]
- 10. Verma A, Champagne J, Sapp J, Essebag V, Novak P, Skanes A. et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med 2013;173:149–56. [DOI] [PubMed] [Google Scholar]
- 11. Gersak B, Pernat A, Robic B, Sinkovec M.. Low rate of atrial fibrillation recurrence verified by implantable loop recorder monitoring following a convergent epicardial and endocardial ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:1059–66. [DOI] [PubMed] [Google Scholar]
- 12. Sanders P, Pürerfellner H, Pokushalov E, Sarkar S, Di Bacco M, Maus B. et al. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: results from the Reveal LINQ Usability Study. Heart Rhythm 2016;13:1425–30. [DOI] [PubMed] [Google Scholar]
- 13. Pürerfellner H, Pokushalov E, Sarkar S, Koehler J, Zhou R, Urban L. et al. P-wave evidence as a method for improving algorithm to detect atrial fibrillation in insertable cardiac monitors. Heart Rhythm 2014;11:1575–83. [DOI] [PubMed] [Google Scholar]
- 14. Huo Y, Christoph M, Forkmann M, Pohl M, Mayer J, Salmas J. et al. Reduction of radiation exposure during atrial fibrillation ablation using a novel fluoroscopy image integrated 3-dimensional electroanatomic mapping system: a prospective, randomized, single-blind, and controlled study. Heart Rhythm 2015;12:1945–55. [DOI] [PubMed] [Google Scholar]
- 15. Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S. et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:825–33. [DOI] [PubMed] [Google Scholar]
- 16. Nölker G, Mayer J, Boldt L-H, Seidl K, VAN Driel V, Massa T. et al. Performance of an implantable cardiac monitor to detect atrial fibrillation: results of the DETECT AF study. J Cardiovasc Electrophysiol 2016;27:1403–10. [DOI] [PubMed] [Google Scholar]
- 17. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A. et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 18. Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M. et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10, 000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 2014;35:508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang S-H, Kang Y-C, Wang C-C, Wen M-S, Hung K-C, Wang C-Y. et al. Annual atrial tachyarrhythmia burden determined by device interrogation in patients with cardiac implanted electronic devices is associated with a risk of ischaemic stroke independent of known risk factors. Eur J Cardiothorac Surg 2015;47:840–6. [DOI] [PubMed] [Google Scholar]
- 20. Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C. et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474–80. [DOI] [PubMed] [Google Scholar]