Abstract
Eukaryotic cells employ distinct means to release specific signals and material. Research within the last decade has identified different types of membrane-enclosed structures collectively called extracellular vesicles (EVs) as one of them. EVs fall into two categories depending on their subcellular origin. Exosomes are generated within the endosomal system and reach the extracellular space upon fusion of multivesicular bodies. Microvesicles or microparticles are generated by shedding of the plasma membrane. Sterols are essential components of eukaryotic membranes and serve as precursors or cofactors of numerous signaling molecules; their content and subcellular distribution are tightly controlled. The prominent roles of sterols in cells raise the question of whether and how these components impact EVs. In this review, we compile evidence for cholesterol accumulation in EVs and discuss its possible contribution to their biogenesis, release, and uptake. We also consider potential implications of EVs in cellular sterol homeostasis and in cholesterol-related diseases.
Keywords: cellular cholesterol, diseases/dyslipidemias, macrophages/monocytes, platelets, cholesterol/efflux, atherosclerosis, endosome, multivesicular bodies
In 1967, Peter Wolf described “minute dust-like material” with coagulant activity in human plasma that was “strongly sudanophilic, and therefore rich in lipid content” (1). Two years later, H. Clarke Anderson described ultrastructurally defined “matrix vesicles” surrounded by an osmiophilic membrane in the cartilage matrix of mouse bones (2). These reports represent probably the first descriptions of extracellular vesicles (EVs) and the first investigations of their lipid content. Meanwhile, the term EV encompasses a diverse group of nanometer- to micrometer-sized structures that are surrounded by a membrane and released by various types of cells through different mechanisms. Reflecting this diversity, these structures have received different names, including nano- and microparticles, microvesicles, ectosomes, exosomes, etc. (3). EVs are thought to serve various functions depending on their origin and molecular composition: exosomes generated by invagination of endosomal membranes and by subsequent release of multivesicular bodies (MVBs) may transfer signaling molecules, such as miRNA, transcripts, proteins, and lipids, to specific target cells and thereby serve intercellular communication. On the other hand, microvesicles generated by outward budding of the plasma membrane (PM) may help cells to dispose of cellular material (4). Within the last years, EVs have come to prominence because of their possible use as biomarkers and therapeutic agents for various pathologic conditions such as inflammation, cancer, and cardiovascular disease (5, 6).
The pioneering studies by Wolf and Anderson revealed early on that lipids are invariable components of EVs and implicated a contribution to their formation and function. Sterols, namely cholesterol, are prominent structural components of membranes regulating their functional properties and subcellular compartmentalization, and they serve as precursors or cofactors for different signaling molecules. In this review, we will summarize how cholesterol contributes to the journey of EVs.
CHOLESTEROL CONTENT OF EVs
Cholesterol levels in EVs have been investigated extensively using a large spectrum of experimental models and methodological approaches (Table 1). Most research has been performed on EVs that are secreted in vitro from cell lines and primary cultures. The cholesterol content of EVs generated in vivo was studied early on in bone cartilage (7), and within the last years in different body fluids, including blood and urine, as well as seminal, lacrimal, and cerebrospinal fluid (Table 1). EVs are frequently isolated by ultracentrifugation, but chromatography- and affinity-based approaches have been used as well. Apart from a few exceptions (8, 9), most studies indicate that EVs are enriched in cholesterol compared with the PM or the total pool of cellular lipids (Table 1). The degree of enrichment varies by the cell of origin, the type or subpopulation of EVs, and by methodological approaches. Raman spectroscopy revealed that exosomes from tumor-derived cell lines contained less cholesterol than those from noncancerous cell lines (10), opening interesting perspectives for cancer diagnosis. Mouse adipocytes secreted two types of EVs that differed in size and in cholesterol content (11). Methods such as chromatography, mass spectrometry, and enzymatic assays can only analyze the average sterol content of EVs in a sample of interest. However, alternative approaches have been used to visualize the cholesterol content of individual vesicles. This includes labeling of cholesterol by specific affinity agents and subsequent electron microscopic inspection (12, 13), as well as Raman spectroscopy (14). These methods indicated that the cholesterol content varies among individual EVs. Indirect evidence for the elevated cholesterol content comes from the observation that EVs from cell line supernatant (15) and from human body fluids (16, 17) contain cholesterol-binding proteins such as prominin-1 (CD133). Membranes of EVs may also contain cholesterol-rich lipid rafts: EVs were bound by an antibody against clustered cholesterol (18) and they contained detergent-resistant membrane fractions featuring the raft marker, stomatin, and enhanced cholesterol levels (19). Treatment of cells with ostreolysin A, a fungal toxin that binds to sphingomyelin/cholesterol-rich membrane domains, induced membrane shedding and the generation of EVs with a 2-fold higher cholesterol content than the cell lysate (20). Notably, EVs may also contain metabolites of cholesterol: 27-hydroxycholesterol was found in exosomes secreted by an estrogen-receptor-positive breast cancer cell line (21). Thus, hydroxysterol-containing EVs may serve as diagnostic markers for specific diseases.
TABLE 1.
Studies revealing the cholesterol content of EVs
| Species | Tissue/Cell Type | Preparation | EV Isolation | Sterol Analysis | EV Type; Cholesterol Content | References |
| Chicken | Epiphyseal cartilage | Acutely isolated | UC | TLC | Matrix vesicles; C/PL 2-fold PM | (7) |
| Guinea pig | Reticulocytes | Primary | UC | TLC | EV; C/PL equal to cells | (8) |
| Rat | Mast cells | Line | UC | GLC | EVs; C/PL equal to cells | (9) |
| Human | Hepatocarcinoma, lung carcinoma, prostate cancer, fibroblasts | Line | UC | Raman spectroscopy | Exosomes; differences between lines | (10) |
| Mouse | Adipocytes | Line, primary | UC | Enzymatic | Exosomes; small versus large | (11) |
| Human | B lymphocytes | Line | — | Perfringolysin O/Immunogold EM | Exosomes; direct staining | (12) |
| Human | Cervical cancer (HeLa) | Line | UC | Theonellamides/Immunogold EM | Exosomes; direct staining; 5-fold PM | (13) |
| Rat | Mesenchymal stromal cells | Lines | UC | Raman spectroscopy | Exosomes; single analysis, CD9+ versus CD9− | (14) |
| Human | Ovarian cancer | Plasma, ascites | UC | Raman spectroscopy | Exosomes; single analysis; CD9+ (low) versus CD9− (high) | (14) |
| Human | Brain | Cerebrospinal fluid | UC | — | Exosomes; prominin-1 | (16) |
| Mouse | Brain (embryonic stage) | Ventricular fluid | UC | — | Exosomes; prominin-1 | (16) |
| Human | Colorectal cancer | Line | UC | — | Exosomes; prominin-1 | (16) |
| Human | Eye | Lacrimal fluid | UC | — | Exosomes; prominin-1 | (16) |
| Human | Kidney | Urine | UC | — | Exosomes; prominin-1 | (16) |
| Human | Mouth | Saliva | UC | — | Exosomes; prominin-1 | (16) |
| Human | Prostate epithelial cells | Seminal fluid | UC | — | Exosomes; prominin-1 | (16) |
| Human | Lymphoma | Lines | UC, FACS | Cholesterol antibody | Exosomes; direct staining | (18) |
| Mouse | Microglia | Line | UC, FACS | Cholesterol antibody | Exosomes; direct staining | (18) |
| Human | Erythrocytes | Plasma | UC | Enzymatic | Exosomes; detergent-resistant fractions | (19) |
| Dog | Kidney cells | Line | FACS | Enzymatic | Toxin-induced EVs; C/L 2-fold cells | (20) |
| Human | Fibroblasts | Primary | FPLC | Isotope-labeled cholesterol | Microparticles; efflux | (64) |
| Human | B lymphocytes | Line | UC, Dynabeads | MS | Exosomes; C/L 2-fold cells | (73) |
| Human | Colorectal cancer | Line | UC | MS | Exosomes; C/P 5-fold cells | (74) |
| Mouse | Epididymis | Fluid | UC, SEC | Epididymosomes; C/PL 2-fold spermatozoa | (75) | |
| Mouse | Leukaemia | Ascites | UC | TLC | EVs; C/PL 3-fold PM | (78) |
| Human | Melanoma | Lines | UC | Enzymatic | Exosomes; C/P 10-fold cells | (79) |
| Human | Mesenchymal stem cells | Line | UC, HPLC | Enzymatic | EVs; C/P 8-fold conditioned medium | (80) |
| Human | Neutrophils | Plasma | Centrifugation | TLC | EVs; C/sphingomyelin 2-fold PM | (81) |
| Mouse | Oligodendrocytes | Line | UC | MS | Exosomes; C/P 2-fold PM | (82) |
| Mouse | Osteoblast | Line | UC | TLC | Matrix vesicles; C/PL 2.5-fold PM | (83) |
| Human | Platelets | Plasma | UC | TLC | Microparticles; C/PL similar to PM | (84) |
| Human | Prostate cancer | Urine | UC | MS | Exosomes; high C/PL | (87) |
| Hamster | Kidney cells | Line | FPLC | Isotope-labeled cholesterol | Microparticles; efflux | (43, 65) |
| Human | Hepatocarcinoma, THP-1-derived macrophages | Line | UC, FACS | GC/MS | Exosomes; increased by curcumin/U18666A | (76, 77) |
| Human | Prostate cancer | Line | UC | MS | Exosomes; C/PL 4-fold cells C/P 3-fold cells | (85, 86) |
| Mouse, human | Macrophages | Line, primary | FPLC | Isotope-labeled cholesterol | Microparticles; efflux | (43, 64, 65) |
| Human | Prostate epithelial cells | Seminal fluid | UC, SEC | LC-MS; TLC | Prostasomes; 55% of lipid C/PL 1.9 | (88, 89) |
UC, ultracentrifugation; C/PL, cholesterol-to-phospholipid ratio; GLC, gas liquid chromatography; FACS, fluorescence-activated cell sorting; C/L, cholesterol-to-lipid ratio; FPLC, fast-performance liquid chromatography; C/P, cholesterol-to-protein ratio; SEC, size exclusion chromatography.
The observation that EVs have a high cholesterol content raises two questions. What causes the cholesterol enrichment and what is its impact on the journey of EVs? In the following, we will discuss the roles of cholesterol in the formation and function of EVs.
CHOLESTEROL FUNCTION DURING EV BIOGENESIS
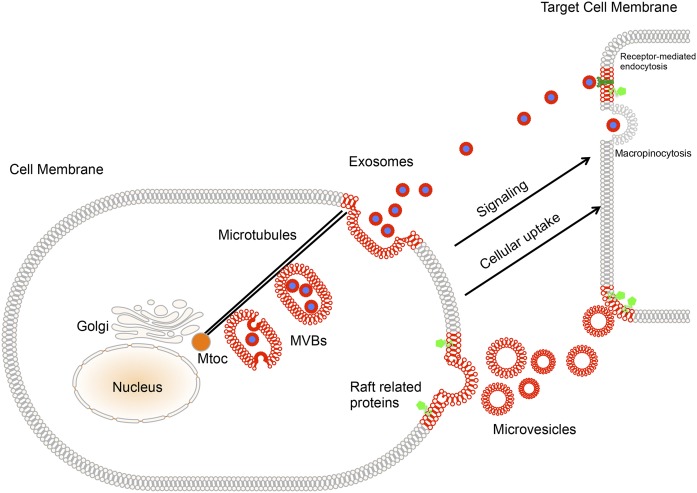
EVs originate from two different cellular membrane compartments, suggesting distinct requirements of cholesterol for their biogenesis (Fig. 1). Microvesicles are shed from the PM, whereas exosomes represent intraluminal vesicles of MVBs that are released after fusion of MVBs with the PM. Recent studies indicate that the molecular machinery that drives the formation of intraluminal vesicles requires lateral segregation of cargo within the limiting membrane of an endosome followed by the inward budding of the membrane and the release of vesicles into the MVB lumen.
Fig. 1.
Contributions of cholesterol to the journey of EVs. Microvesicles are generated by shedding of the PM possibly in domains with elevated cholesterol content. Specific compartments of the endosomal system enriched in cholesterol produce intraluminal vesicles within MVBs. MVBs are actively transported along microtubules to the cell periphery, where their fusion with the PM leads to release of exosomes. EVs are conveyed to target cells by body fluids, where they activate specific signaling pathways or modify the cellular metabolism after selective capture, such as receptor-mediated endocytosis and macropinocytosis for exosomes. Membrane compartments that are enriched in cholesterol and dedicated to the EVs’ journey are highlighted in red. The raft-enriched proteins, such as CD36, are indicated in green. Note that the size of lipids is not to scale in the different membrane compartments.
One first line of evidence for the existence of different subclasses of MVBs with different fates comes from studies using perfringolysin O to label cholesterol. This approach revealed cholesterol-positive and -negative MVBs in cultured B lymphocytes. Interestingly, only those enriched in cholesterol appeared to fuse with the cell surface to release exosomes, suggesting that the cholesterol content of MVBs controls the release of EVs. This study also showed that a subset of MVBs contains up to 63% of the endosomal cholesterol (22). Loading of intraluminal vesicles within MVBs with cholesterol is probably mediated by specific transporters, such as ABCG1, which has been detected in late endosomes (23).
The endosomal sorting complex required for transport (ESCRT) machinery plays a major role in the generation of MVBs (24). In artificial bilayers, this complex forms clusters and induces ordered membrane microdomains in a cholesterol-dependent manner (25). Therefore, the content of cholesterol within endosomal membranes may well represent a driving force behind the formation and release of exosomes through the line-tension between liquid-ordered and disordered domains (26). Interestingly, an interconnection between cholesterol and fusogenic bis(monoacylglycero)phosphate (also called lysobisphosphatidic acid phosphate) levels has been postulated because the bis(monoacylglycero)phosphate-interacting protein, Alix, is critical for the optimal cholesterol content of MVBs (27). Downregulation of Alix decreased cellular cholesterol levels, presumably because the storage capacity of endosomes is reduced and, thus, the clearance of cholesterol is accelerated.
CHOLESTEROL IMPACT ON EV RELEASE
The different types of EVs are released from cells by distinct mechanisms that may differ in their dependence on sterols (Fig. 1). The release of exosomes requires, first, transport of MVBs along microtubules toward the PM. This process is controlled in part by the cholesterol content. The vesicular protein sorting 4 (Vps4), which is an AAA-ATPase present in the ESCRT-III complex, directly interacts with an oxysterol binding protein (OSBP/OSH) (28). OSBPs, like ORP1L, sense the cholesterol content of endosomes and control their movement along microtubules. If cholesterol exceeds a threshold, the transport of endosomes stalls (29). OSBPs may also play a role in the fusion between MVBs and the PM (30). On the other side, cholesterol may favor vesicular fusion with the PM (31) by controlling the content of specific proteins at sites of exocytosis in various secretory cell types (31).
Evidence that the release of EVs is cholesterol dependent comes mainly from studies using cyclodextrin with albeit conflicting results. Treatment of oligodendrocyte-like cells with cyclodextrin-bound cholesterol enhances the release of exosomes, but the effect of cholesterol-free cyclodextrin alone was not assessed (32). In cultured neurons with reduced Niemann-Pick type C (NPC)1 activity, treatment with 50 μM cyclodextrin induced the release of cholesterol-rich lamellar inclusions, which lacked a surrounding membrane and thus were distinct from exosomes (33). Millimolar concentrations of cyclodextrin induced the release of flotillin-positive structures from cell lines that were derived from the livers of NPC1-deficient Balb/c mice (34) and of membrane-bound particles from MDCK cells (35). Moreover, cyclodextrin increased exosomal release of viral proteins (36) and of the lectin-like oxidized LDL receptor (LOX) (37). On the other hand, it was found that cyclodextrin inhibits release of exosomes from a retinal pigment epithelium cell line (32) or chemokine release via exosomes from a tumor cell line (38). An earlier study reported no effect of cyclodextrin treatment on exosome-mediated release from human peripheral blood mononuclear cells (39). It must be pointed out that β-cyclodextrins bind molecules other than cholesterol. Therefore, it is essential to show in “rescue” experiments that addition of cholesterol reverts the cyclodextrin-induced effects on EV release. Moreover, treatment of cells with cyclodextrin at millimolar concentrations may strongly perturb the integrity of the PM, casting further doubt about the specificity of the observed effects.
CHOLESTEROL IMPACT ON THE FATE OF EVs AND THEIR UPTAKE BY TARGET CELLS
The membrane of exosomes is rigid and contains domains with ordered lipid phase, as shown by probes sensing the membrane fluidity, such as diphenylhexatriene (9, 40). This rigidity is conveyed by the enrichment in sphingomyelin and cholesterol and it may account for the long-lasting presence of exosomes in biological fluids and the resistance to attacks by lipolytic enzymes in the blood. It has been shown that exosomes can remain for up to two weeks in the lymph nodes (41).
The biological function of EVs depends on their origin and their content (Fig. 1). Some cells use EVs to dispose of unwanted material. Cells with poor recycling activity may release the material into a drainage system like, for example, epithelial cells facing the tubules of the kidney or the gut. Several lines of evidence suggest that microvesicles shed from the PM help cells to efflux cholesterol in parallel to the release via HDL particles. Loading of cells with cholesterol stimulates microvesicle release from monocyte cell lines and primary human macrophages (42). Moreover, overexpression of the cholesterol transporter, ABCA1, enhances the release of cholesterol-rich EVs from different cell types, including BHK, human THP-1 macrophages, and HepG2 cells (43). Intriguingly, calcineurin inhibition in BHK and RAW 264.7 cells prevented apoA-I binding and reduced JAK2 phosphorylation, which is required for cholesterol efflux to apoA-I. However, neither Ca2+ manipulation nor calcineurin inhibition affected ABCA1-mediated microparticle release (44). EVs may also release specific signals or nutrients for specific target cells and thereby mediate a horizontal transfer of proteins, lipids, and nucleic acids in parallel to other secretory pathways (45). Because of their high cholesterol content and their accumulation in recipient endosomes, exosomes can modify the lipid homeostasis of target cells (26, 46). It has been shown that exosomes isolated from the supernatants of activated human CD4(+) T cells enhance cholesterol accumulation in cultured human monocytes and THP-1 cells (47). This effect was inhibited by antibodies against the phosphatidylserine receptor, suggesting the involvement of these receptors in exosome internalization by monocytes (47). Uptake of mantle cell lymphoma-derived exosomes was reduced by nystatin and simvastatin, which bind and lower cholesterol levels, respectively, but independently from siRNA-mediated knockdown of caveolin and clathrin. Although the selectivity of the treatment with nystatin and simvastatin was not investigated by cholesterol rescue experiments, this finding indicates that exosome uptake is mediated via a nonclassical endocytosis pathway involving dynamin, tyrosine kinase, and cholesterol (48). Moreover, this finding raised hopes for potential therapeutic interventions in B cell malignancies using statins. In line with these observations, the internalization of exosomes derived from glioblastoma cells was negatively regulated by the lipid raft-associated protein, caveolin-1 (49). Furthermore the phosphorylation of several downstream targets known to associate with lipid rafts, such as extracellular signal-regulated kinase-1/2 and heat shock protein 27, was detected after incubation of HUVEC cells with exosomes derived from glioblastoma cells (49). Similar to the release, EV uptake is also impaired by millimolar concentrations of cyclodextrin, as shown for uptake of platelet-derived microparticles in endothelial cells (50) and for uptake of glioblastoma cell-derived exosomes in HUVEC cells (49). Whether these effects were cholesterol-dependent remains to be established. Independent evidence that cholesterol depletion inhibits uptake comes from experiments with artificial HDL-like particles. These particles cluster SR-B1 scavenger receptors and inhibit exosome uptake by melanoma and macrophage cell lines and by human endothelial cells (51).
EVs AND CHOLESTEROL-RELATED DISEASES
Several cholesterol-related diseases affect EVs. Patients with hypercholesterolemia showed elevated plasma concentrations of monocyte- and platelet-derived microparticles compared with age-matched healthy subjects (52–54). Similarly, plasma concentrations of platelet- and endothelial cell-derived microparticles scaled with LDL levels in hyperlipidemic (55) and diabetic patients (56, 57). Moreover, plasma levels of platelet-derived microparticles were strongly elevated in a mouse model of sitosterolemia, where defects in ABCG5 and ABCG8 cause accumulation of plant-derived sterols and hypercholesteremia (58). Together, these findings suggest that EVs may serve as clinical biomarkers for sterol-related diseases (59–61). This attracts particular attention in the field of atherosclerosis where exosomes may be involved in monocyte and macrophage cholesterol metabolism, endothelial cell and platelet activation, and smooth muscle proliferation (61). NPC disease, a lysosomal storage disorder with variable neurovisceral symptoms, entails accumulation of unesterified cholesterol and other lipids in the endosomal-lysosomal system. Previous studies on cell lines revealed secretion of exosomes after treatment with U18666A, which mimics the cellular phenotype of this disease (32), and after treatment with cyclodextrin (34). Whether exosomes can serve as diagnostic and therapeutic tools for NPC patients remains to be determined. EVs may contain cholesterol oxidation products that serve now as validated biomarkers for the disease (62).
At present, it is unclear how cholesterol homeostasis and EV release are linked. In vitro studies revealed that cholesterol enrichment of vascular smooth muscle cells (63) and monocytes (42) enhances the release of microvesicles with coagulation-promoting activity and that overexpression of the cholesterol transporter, ABCA1, enhanced the generation of cholesterol-rich microparticles at the PM (43, 64, 65). As mentioned above, cells may use EVs to mediate cholesterol efflux in parallel to the formation of HDL particles. However, EVs released after cholesterol loading of cells may also have signaling functions. Intake of a cholesterol-rich high-fat diet by LDLR-deficient mice causes the release of microvesicles carrying specific sterol-responsive proteins from macrophages (66). These EVs may inform target cells about the state of cholesterol homeostasis and trigger specific responses.
Notably, cholesterol esters have been detected in exosomes. Given their absence from membranes, their exact location within EVs remains to be determined. Cholesterol esters could be released from cancer cells to promote growth or to modulate gene expression of surrounding cancer cells (67). Sterols other than cholesterol may play roles in cancer progression, but their presence in EVs is still largely unknown (67).
CONCLUSIONS AND OUTLOOK
Taken together, the studies summarized above indicate that cholesterol is essential for the entire journey of EVs: It ensures their biogenesis and release, it guarantees the stability of their membrane, and it is required for their uptake by target cells (Fig. 1). Moreover, there is evidence that EVs form part of the cellular machinery ensuring cholesterol homeostasis, and that they can help to detect and combat pathologic conditions related to cholesterol. Consolidation of these findings and further insight require progress on two frontiers: The first concerns EVs. Most of our knowledge stems from in vitro preparations that have well-known limitations. Therefore, there is a clear need for in vivo studies on the release, composition, and fate of EVs (68–70). Body fluids provide an accessible source of naturally generated EVs, but the procedures to purify them and to characterize their (lipid) content need to be refined and standardized, as has been proposed for therapeutically used EVs (71). Methods to isolate and count exosomes are becoming more refined (72); the analysis of single EVs by nondestructive methods, such as Raman spectrometry, may become a valuable tool to uncover the full spectrum of EV content and size. EVs that travel within tissues and organs without entering body fluids need to be studied with new labeling methods to identify their content and to trace their journey in vivo. A second frontier concerns the visualization and manipulation of cholesterol. Here, new imaging tools and refined agents that allow to detect the compound and to change its concentration, respectively, are required to determine the dependence of EVs on cholesterol and to determine their role in cholesterol homeostasis under normal and pathologic conditions.
Footnotes
Abbreviations:
- ESCRT
- endosomal sorting complex required for transport
- EV
- extracellular vesicle
- MVB
- multivesicular body
- NPC
- Niemann-Pick type C
- OSBP
- oxysterol binding protein
- PM
- plasma membrane
This work was supported by Fondation pour la Recherche Médicale (F.W.P. and N.V.), Niemann-Pick Selbsthilfegruppe e.V. (F.W.P.), Fédération pour la Recherche sur le Cerveau (Rotary Club “Espoir en tête”) (F.W.P.), Vaincre les Maladies Lysosomales (F.W.P.), and Ligue Contre le Cancer (N.V.).
REFERENCES
- 1.Wolf P. 1967. The nature and significance of platelet products in human plasma. Br. J. Haematol. 13: 269–288. [DOI] [PubMed] [Google Scholar]
- 2.Anderson H. C. 1969. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J. Cell Biol. 41: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yánñz-Mó M., Siljander P. R., Andreu Z., Zavec A. B., Borràs F. E., Buzas E. I., Buzas K., Casal E., Cappello F., Carvalho J., et al. 2015. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles. 4: 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Record M., Carayon K., Poirot M., and Silvente-Poirot S.. 2014. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta. 1841: 108–120. [DOI] [PubMed] [Google Scholar]
- 5.Colombo M., Raposo G., and Thery C.. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30: 255–289. [DOI] [PubMed] [Google Scholar]
- 6.Sarko D. K., and McKinney C. E.. 2017. Exosomes: origins and therapeutic potential for neurodegenerative disease. Front. Neurosci. 11: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wuthier R. E. 1975. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochim. Biophys. Acta. 409: 128–143. [DOI] [PubMed] [Google Scholar]
- 8.Vidal M., Sainte-Marie J., Philippot J. R., and Bienvenue A.. 1989. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: evidence precluding a role for “aminophospholipid translocase”. J. Cell. Physiol. 140: 455–462. [DOI] [PubMed] [Google Scholar]
- 9.Laulagnier K., Motta C., Hamdi S., Roy S., Fauvelle F., Pageaux J. F., Kobayashi T., Salles J. P., Perret B., Bonnerot C., et al. 2004. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 380: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith Z. J., Lee C., Rojalin T., Carney R. P., Hazari S., Knudson A., Lam K., Saari H., Ibanez E. L., Viitala T., et al. 2015. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J. Extracell. Vesicles. 4: 28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durcin M., Fleury A., Taillebois E., Hilairet G., Krupova Z., Henry C., Truchet S., Trotzmuller M., Kofeler H., Mabilleau G., et al. 2017. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles. 6: 1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Möbius W., Ohno-Iwashita Y., van Donselaar E. G., Oorschot V. M., Shimada Y., Fujimoto T., Heijnen H. F., Geuze H. J., and Slot J. W.. 2002. Immunoelectron microscopic localization of cholesterol using biotinylated and non-cytolytic perfringolysin O. J. Histochem. Cytochem. 50: 43–55. [DOI] [PubMed] [Google Scholar]
- 13.Edgar J. R., Manna P. T., Nishimura S., Banting G., and Robinson M. S.. 2016. Tetherin is an exosomal tether. eLife. 5: e17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carney R. P., Hazari S., Colquhoun M., Tran D., Hwang B., Mulligan M. S., Bryers J. D., Girda E., Leiserowitz G. S., Smith Z. J., et al. 2017. Multispectral optical tweezers for biochemical fingerprinting of cd9-positive exosome subpopulations. Anal. Chem. 89: 5357–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rappa G., Mercapide J., Anzanello F., Pope R. M., and Lorico A.. 2013. Biochemical and biological characterization of exosomes containing prominin-1/CD133. Mol. Cancer. 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzesco A. M., Janich P., Wilsch-Brauninger M., Dubreuil V., Langenfeld K., Corbeil D., and Huttner W. B.. 2005. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J. Cell Sci. 118: 2849–2858. [DOI] [PubMed] [Google Scholar]
- 17.Huttner H. B., Janich P., Kohrmann M., Jaszai J., Siebzehnrubl F., Blumcke I., Suttorp M., Gahr M., Kuhnt D., Nimsky C., et al. 2008. The stem cell marker prominin-1/CD133 on membrane particles in human cerebrospinal fluid offers novel approaches for studying central nervous system disease. Stem Cells. 26: 698–705. [DOI] [PubMed] [Google Scholar]
- 18.Osteikoetxea X., Balogh A., Szabo-Taylor K., Nemeth A., Szabo T. G., Paloczi K., Sodar B., Kittel A., Gyorgy B., Pallinger E., et al. 2015. Improved characterization of EV preparations based on protein to lipid ratio and lipid properties. PLoS One. 10: e0121184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salzer U., Hinterdorfer P., Hunger U., Borken C., and Prohaska R.. 2002. Ca(++)-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood. 99: 2569–2577. [DOI] [PubMed] [Google Scholar]
- 20.Skočaj M., Yu Y., Grundner M., Resnik N., Bedina Zavec A., Leonardi A., Križaj I., Guella G., Maček P., Kreft M. E., et al. 2016. Characterisation of plasmalemmal shedding of vesicles induced by the cholesterol/sphingomyelin binding protein, ostreolysin A-mCherry. Biochim. Biophys. Acta. 1858: 2882–2893. [DOI] [PubMed] [Google Scholar]
- 21.Roberg-Larsen H., Lund K., Seterdal K. E., Solheim S., Vehus T., Solberg N., Krauss S., Lundanes E., and Wilson S. R.. 2017. Mass spectrometric detection of 27-hydroxycholesterol in breast cancer exosomes. J. Steroid Biochem. Mol. Biol. 169: 22–28. [DOI] [PubMed] [Google Scholar]
- 22.Möbius W., van Donselaar E., Ohno-Iwashita Y., Shimada Y., Heijnen H. F., Slot J. W., and Geuze H. J.. 2003. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 4: 222–231. [DOI] [PubMed] [Google Scholar]
- 23.Tarling E. J., and Edwards P. A.. 2011. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc. Natl. Acad. Sci. USA. 108: 19719–19724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juan T., and Furthauer M.. 2018. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 74: 66–77. [DOI] [PubMed] [Google Scholar]
- 25.Boura E., Ivanov V., Carlson L. A., Mizuuchi K., and Hurley J. H.. 2012. Endosomal sorting complex required for transport (ESCRT) complexes induce phase-separated microdomains in supported lipid bilayers. J. Biol. Chem. 287: 28144–28151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simons M., and Raposo G.. 2009. Exosomes–vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21: 575–581. [DOI] [PubMed] [Google Scholar]
- 27.Chevallier J., Chamoun Z., Jiang G., Prestwich G., Sakai N., Matile S., Parton R. G., and Gruenberg J.. 2008. Lysobisphosphatidic acid controls endosomal cholesterol levels. J. Biol. Chem. 283: 27871–27880. [DOI] [PubMed] [Google Scholar]
- 28.Wang P., Zhang Y., Li H., Chieu H. K., Munn A. L., and Yang H.. 2005. AAA ATPases regulate membrane association of yeast oxysterol binding proteins and sterol metabolism. EMBO J. 24: 2989–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huotari J., and Helenius A.. 2011. Endosome maturation. EMBO J. 30: 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beh C. T., McMaster C. R., Kozminski K. G., and Menon A. K.. 2012. A detour for yeast oxysterol binding proteins. J. Biol. Chem. 287: 11481–11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ammar M. R., Kassas N., Chasserot-Golaz S., Bader M. F., and Vitale N.. 2013. Lipids in regulated exocytosis: what are they doing? Front. Endocrinol. (Lausanne). 4: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strauss K., Goebel C., Runz H., Mobius W., Weiss S., Feussner I., Simons M., and Schneider A.. 2010. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 285: 26279–26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demais V., Barthelemy A., Perraut M., Ungerer N., Keime C., Reibel S., and Pfrieger F. W.. 2016. Reversal of pathologic lipid accumulation in NPC1-deficient neurons by drug-promoted release of LAMP1-coated lamellar inclusions. J. Neurosci. 36: 8012–8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen F. W., Li C., and Ioannou Y. A.. 2010. Cyclodextrin induces calcium-dependent lysosomal exocytosis. PLoS One. 5: e15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casas E., Barron C., Francis S. A., McCormack J. M., McCarthy K. M., Schneeberger E. E., and Lynch R. D.. 2010. Cholesterol efflux stimulates metalloproteinase-mediated cleavage of occludin and release of extracellular membrane particles containing its C-terminal fragments. Exp. Cell Res. 316: 353–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikeda M., and Longnecker R.. 2007. Cholesterol is critical for Epstein-Barr virus latent membrane protein 2A trafficking and protein stability. Virology. 360: 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gioia M., Vindigni G., Testa B., Raniolo S., Fasciglione G. F., Coletta M., and Biocca S.. 2015. Membrane cholesterol modulates LOX-1 shedding in endothelial cells. PLoS One. 10: e0141270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T., Guo J., Yang M., Zhu X., and Cao X.. 2011. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J. Immunol. 186: 2219–2228. [DOI] [PubMed] [Google Scholar]
- 39.Lancaster G. I., and Febbraio M. A.. 2005. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J. Biol. Chem. 280: 23349–23355. [DOI] [PubMed] [Google Scholar]
- 40.Carayon K., Chaoui K., Ronzier E., Lazar I., Bertrand-Michel J., Roques V., Balor S., Terce F., Lopez A., Salome L., et al. 2011. Proteolipidic composition of exosomes changes during reticulocyte maturation. J. Biol. Chem. 286: 34426–34439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luketic L., Delanghe J., Sobol P. T., Yang P., Frotten E., Mossman K. L., Gauldie J., Bramson J., and Wan Y.. 2007. Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J. Immunol. 179: 5024–5032. [DOI] [PubMed] [Google Scholar]
- 42.Liu M. L., Reilly M. P., Casasanto P., McKenzie S. E., and Williams K. J.. 2007. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler. Thromb. Vasc. Biol. 27: 430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hafiane A., and Genest J.. 2017. ATP binding cassette A1 (ABCA1) mediates microparticle formation during high-density lipoprotein (HDL) biogenesis. Atherosclerosis. 257: 90–99. [DOI] [PubMed] [Google Scholar]
- 44.Karwatsky J., Ma L., Dong F., and Zha X.. 2010. Cholesterol efflux to apoA-I in ABCA1-expressing cells is regulated by Ca2+-dependent calcineurin signaling. J. Lipid Res. 51: 1144–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedergang F., Gasman S., Vitale N., Desnos C., and Lamaze C.. 2017. Meeting after meeting: 20 years of discoveries by the members of the Exocytosis-Endocytosis Club. Biol. Cell. 109: 339–353. [DOI] [PubMed] [Google Scholar]
- 46.Müller G., Schneider M., Biemer-Daub G., and Wied S.. 2011. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell. Signal. 23: 1207–1223. [DOI] [PubMed] [Google Scholar]
- 47.Zakharova L., Svetlova M., and Fomina A. F.. 2007. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J. Cell. Physiol. 212: 174–181. [DOI] [PubMed] [Google Scholar]
- 48.Hazan-Halevy I., Rosenblum D., Weinstein S., Bairey O., Raanani P., and Peer D.. 2015. Cell-specific uptake of mantle cell lymphoma-derived exosomes by malignant and non-malignant B-lymphocytes. Cancer Lett. 364: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Svensson K. J., Christianson H. C., Wittrup A., Bourseau-Guilmain E., Lindqvist E., Svensson L. M., Morgelin M., and Belting M.. 2013. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 288: 17713–17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faille D., El-Assaad F., Mitchell A. J., Alessi M. C., Chimini G., Fusai T., Grau G. E., and Combes V.. 2012. Endocytosis and intracellular processing of platelet microparticles by brain endothelial cells. J. Cell. Mol. Med. 16: 1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plebanek M. P., Mutharasan R. K., Volpert O., Matov A., Gatlin J. C., and Thaxton C. S.. 2015. Nanoparticle targeting and cholesterol flux through scavenger receptor type B-1 inhibits cellular exosome uptake. Sci. Rep. 5: 15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pirro M., Schillaci G., Paltriccia R., Bagaglia F., Menecali C., Mannarino M. R., Capanni M., Velardi A., and Mannarino E.. 2006. Increased ratio of CD31+/CD42- microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 26: 2530–2535. [DOI] [PubMed] [Google Scholar]
- 53.Hjuler Nielsen M., Irvine H., Vedel S., Raungaard B., Beck-Nielsen H., and Handberg A.. 2015. Elevated atherosclerosis-related gene expression, monocyte activation and microparticle-release are related to increased lipoprotein-associated oxidative stress in familial hypercholesterolemia. PLoS One. 10: e0121516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suades R., Padro T., Alonso R., Mata P., and Badimon L.. 2015. High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis. Thromb. Haemost. 114: 1310–1321. [DOI] [PubMed] [Google Scholar]
- 55.Pawelczyk M., Chmielewski H., Kaczorowska B., Przybyla M., and Baj Z.. 2015. The influence of statin therapy on platelet activity markers in hyperlipidemic patients after ischemic stroke. Arch. Med. Sci. 11: 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nomura S., Suzuki M., Katsura K., Xie G. L., Miyazaki Y., Miyake T., Kido H., Kagawa H., and Fukuhara S.. 1995. Platelet-derived microparticles may influence the development of atherosclerosis in diabetes mellitus. Atherosclerosis. 116: 235–240. [DOI] [PubMed] [Google Scholar]
- 57.Salem M. A., Adly A. A., Ismail E. A., Darwish Y. W., and Kamel H. A.. 2015. Platelets microparticles as a link between micro- and macro-angiopathy in young patients with type 1 diabetes. Platelets. 26: 682–688. [DOI] [PubMed] [Google Scholar]
- 58.Kanaji T., Kanaji S., Montgomery R. R., Patel S. B., and Newman P. J.. 2013. Platelet hyperreactivity explains the bleeding abnormality and macrothrombocytopenia in a murine model of sitosterolemia. Blood. 122: 2732–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoefer I. E., Steffens S., Ala-Korpela M., Back M., Badimon L., Bochaton-Piallat M. L., Boulanger C. M., Caligiuri G., Dimmeler S., Egido J., et al. 2015. Novel methodologies for biomarker discovery in atherosclerosis. Eur. Heart J. 36: 2635–2642. [DOI] [PubMed] [Google Scholar]
- 60.Martínez M. C., and Andriantsitohaina R.. 2017. Extracellular vesicles in metabolic syndrome. Circ. Res. 120: 1674–1686. [DOI] [PubMed] [Google Scholar]
- 61.Reiss A. B., Vernice N. A., Siegart N. M., DeLeon J., and Kasselman L. J.. 2017. Exosomes in cholesterol metabolism and atherosclerosis. Cardiovasc. Hematol. Disord. Drug Targets. 17: 185–194. [DOI] [PubMed] [Google Scholar]
- 62.Porter F. D., Scherrer D. E., Lanier M. H., Langmade S. J., Molugu V., Gale S. E., Olzeski D., Sidhu R., Dietzen D. J., Fu R., et al. 2010. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med. 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Llorente-Cortés V., Otero-Viñas M., Camino-López S., Llampayas O., and Badimon L.. 2004. Aggregated low-density lipoprotein uptake induces membrane tissue factor procoagulant activity and microparticle release in human vascular smooth muscle cells. Circulation. 110: 452–459. [DOI] [PubMed] [Google Scholar]
- 64.Duong P. T., Collins H. L., Nickel M., Lund-Katz S., Rothblat G. H., and Phillips M. C.. 2006. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J. Lipid Res. 47: 832–843. [DOI] [PubMed] [Google Scholar]
- 65.Nandi S., Ma L., Denis M., Karwatsky J., Li Z., Jiang X. C., and Zha X.. 2009. ABCA1-mediated cholesterol efflux generates microparticles in addition to HDL through processes governed by membrane rigidity. J. Lipid Res. 50: 456–466. [DOI] [PubMed] [Google Scholar]
- 66.Becker L., Gharib S. A., Irwin A. D., Wijsman E., Vaisar T., Oram J. F., and Heinecke J. W.. 2010. A macrophage sterol-responsive network linked to atherogenesis. Cell Metab. 11: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Record M., Poirot M., and Silvente-Poirot S.. 2014. Emerging concepts on the role of exosomes in lipid metabolic diseases. Biochimie. 96: 67–74. [DOI] [PubMed] [Google Scholar]
- 68.Beer K. B., Rivas-Castillo J., Kuhn K., Fazeli G., Karmann B., Nance J. F., Stigloher C., and Wehman A. M.. 2018. Extracellular vesicle budding is inhibited by redundant regulators of TAT-5 flippase localization and phospholipid asymmetry. Proc. Natl. Acad. Sci. USA. 115: E1127–E1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prada I., Gabrielli M., Turola E., Iorio A., D’Arrigo G., Parolisi R., De Luca M., Pacifici M., Bastoni M., Lombardi M., et al. 2018. Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 135: 529–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miranda A. M., Lasiecka Z. M., Xu Y., Neufeld J., Shahriar S., Simoes S., Chan R. B., Oliveira T. G., Small S. A., and Di Paolo G.. 2018. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat. Commun. 9: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reiner A. T., Witwer K. W., van Balkom B. W. M., de Beer J., Brodie C., Corteling R. L., Gabrielsson S., Gimona M., Ibrahim A. G., de Kleijn D., et al. 2017. Concise review: developing best-practice models for the therapeutic use of extracellular vesicles. Stem Cells Transl. Med. 6: 1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He F., Liu H., Guo X., Yin B. C., and Ye B. C.. 2017. Direct exosome quantification via bivalent-cholesterol-labeled dna anchor for signal amplification. Anal. Chem. 89: 12968–12975. [DOI] [PubMed] [Google Scholar]
- 73.Wubbolts R., Leckie R. S., Veenhuizen P. T., Schwarzmann G., Mobius W., Hoernschemeyer J., Slot J. W., Geuze H. J., and Stoorvogel W.. 2003. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J. Biol. Chem. 278: 10963–10972. [DOI] [PubMed] [Google Scholar]
- 74.Lydic T. A., Townsend S., Adda C. G., Collins C., Mathivanan S., and Reid G. E.. 2015. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods. 87: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rejraji H., Sion B., Prensier G., Carreras M., Motta C., Frenoux J. M., Vericel E., Grizard G., Vernet P., and Drevet J. R.. 2006. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol. Reprod. 74: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 76.van Blitterswijk W. J., Emmelot P., Hilkmann H. A., Hilgers J., and Feltkamp C. A.. 1979. Rigid plasma-membrane-derived vesicles, enriched in tumour-associated surface antigens (MLr), occurring in the ascites fluid of a murine leukaemia (GRSL). Int. J. Cancer. 23: 62–70. [DOI] [PubMed] [Google Scholar]
- 77.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A., Coscia C., Iessi E., Logozzi M., Molinari A., et al. 2009. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284: 34211–34222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai R. C., Arslan F., Lee M. M., Sze N. S., Choo A., Chen T. S., Salto-Tellez M., Timmers L., Lee C. N., El Oakley R. M., et al. 2010. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4: 214–222. [DOI] [PubMed] [Google Scholar]
- 79.Stein J. M., and Luzio J. P.. 1991. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem. J. 274: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B., and Simons M.. 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 319: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 81.Sawada N., Taketani Y., Amizuka N., Ichikawa M., Ogawa C., Nomoto K., Nashiki K., Sato T., Arai H., Isshiki M., et al. 2007. Caveolin-1 in extracellular matrix vesicles secreted from osteoblasts. Bone. 41: 52–58. [DOI] [PubMed] [Google Scholar]
- 82.Biró E., Akkerman J. W., Hoek F. J., Gorter G., Pronk L. M., Sturk A., and Nieuwland R.. 2005. The phospholipid composition and cholesterol content of platelet-derived microparticles: a comparison with platelet membrane fractions. J. Thromb. Haemost. 3: 2754–2763. [DOI] [PubMed] [Google Scholar]
- 83.Skotland T., Ekroos K., Kauhanen D., Simolin H., Seierstad T., Berge V., Sandvig K., and Llorente A.. 2017. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur. J. Cancer. 70: 122–132. [DOI] [PubMed] [Google Scholar]
- 84.Canfrán-Duque A., Pastor O., Reina M., Lerma M., Cruz-Jentoft A. J., Lasuncion M. A., and Busto R.. 2015. Curcumin mitigates the intracellular lipid deposit induced by antipsychotics in vitro. PLoS One. 10: e0141829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Canfrán-Duque A., Pastor O., Quintana-Portillo R., Lerma M., de la Pena G., Martin-Hidalgo A., Fernandez-Hernando C., Lasuncion M. A., and Busto R.. 2014. Curcumin promotes exosomes/microvesicles secretion that attenuates lysosomal cholesterol traffic impairment. Mol. Nutr. Food Res. 58: 687–697. [DOI] [PubMed] [Google Scholar]
- 86.Llorente A., Skotland T., Sylvanne T., Kauhanen D., Rog T., Orlowski A., Vattulainen I., Ekroos K., and Sandvig K.. 2013. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta. 1831: 1302–1309. [DOI] [PubMed] [Google Scholar]
- 87.Hosseini-Beheshti E., Pham S., Adomat H., Li N., and Tomlinson Guns E. S.. 2012. Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol. Cell. Proteomics. 11: 863–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brouwers J. F., Aalberts M., Jansen J. W., van Niel G., Wauben M. H., Stout T. A., Helms J. B., and Stoorvogel W.. 2013. Distinct lipid compositions of two types of human prostasomes. Proteomics. 13: 1660–1666. [DOI] [PubMed] [Google Scholar]
- 89.Chiasserini D., Mazzoni M., Bordi F., Sennato S., Susta F., Orvietani P. L., Binaglia L., and Palmerini C. A.. 2015. Identification and partial characterization of two populations of prostasomes by a combination of dynamic light scattering and proteomic analysis. J. Membr. Biol. 248: 991–1004. [DOI] [PubMed] [Google Scholar]