Abstract
NADPH oxidase (NOX) enzymes are one of the major superoxide-generating systems in cells. NOX-generated superoxide has been suggested to promote insulin resistance in the liver. However, the role of NOX enzymes in mediating metabolic dysfunction in other insulin target tissues remains unclear. Here, we show that NOX3 expression is induced in differentiated 3T3-L1 adipocytes upon treatment with proinflammatory cytokines. Superoxide production increased concurrently with NOX3 protein expression in cytokine-treated adipocytes, which was inhibited by the NOX inhibitor diphenyleneiodonium (DPI). Treatment of adipocytes with cytokines increased lipolysis and decreased PPARγ activity. Interestingly, treatment with DPI blunted lipolysis activation by cytokines but failed to restore PPARγ activity. siRNA-mediated NOX3 downregulation also prevented cytokine-induced superoxide generation and lipolysis. In line with increasing lipolysis, cytokines increased the phosphorylation of hormone-sensitive lipase (HSL), which was reversed by treatment with DPI and silencing of NOX3 expression. We conclude that NOX3 is a cytokine-inducible superoxide-generating enzyme in adipocytes, which promotes lipolysis through increasing phosphorylation of HSL. This suggests a key role for NOX3-mediated superoxide production in the increased adipocyte lipolysis in inflammatory settings.
Keywords: adipocytes, inflammation, insulin resistance
Adipose tissue is the major site of lipid turnover in the body. During fasting, triglyceride stores are broken down into FAs and glycerol through lipolysis (1). It has been established that adipose tissue lipolysis is altered in obesity and other inflammatory settings and that release of inflammatory cytokines by enlarged adipocytes or infiltrating immune cells impairs the activity of some key molecules involved in glucose and lipid metabolism (2–4). These include hormone-sensitive lipase (HSL), perilipin, and PPARγ (5).
Reactive oxygen species (ROS) generated during inflammatory settings have been proposed to play a role in mediating insulin resistance and metabolic impairments in adipose tissue (6, 7). It is established that superoxide is overproduced in adipose tissue of obese animals (7, 8). Superoxide can be generated by enzymatic systems such as mitochondrial and peroxisomal pathways in fat cells, but the role of NADPH oxidases (NOXs) in the inflammatory obese state remains elusive. NOX enzymes are widely expressed in mammalian cells and belong to the family of flavocytochrome superoxide-producing enzymes (6, 9, 10). The role of NOX, especially the prototypical NOX2 (also known as CGD91-phox), has been extensively studied in innate immunity (11). Other homologs of NOX2 have been identified, and these include: NOX1, NOX3, NOX4, NOX5, and DUOX1/DUOX2 (9, 11). NOX3, a member of the NOX/dual-domain NOX family, has been first identified in specific portions of the middle ear (12). NOX3 expression was previously reported in liver cells, where it has been shown to mediate TNFα-induced insulin resistance upon JNK activation (13). Also, NOX3 is upregulated in the liver of obese mice, and treatment of hepatic cells with FFAs causes insulin resistance through increasing ROS production by NOX3 and activation of the p38 signaling pathway (14).
Because adipose tissue metabolism is severely impaired in inflammatory settings, we explored the role of NOX3 in adipocytes and its potential implication in cytokine-induced metabolic dysfunction. Our data show that NOX3 is rapidly induced upon cytokine exposure and that the subsequent increase in ROS production promotes lipolysis, which is linked to regulation of HSL phosphorylation. These results demonstrate a new role for NOX3 in regulating lipid metabolism in adipocytes.
METHODS
Materials
Cytokines were used at the following concentrations: TNFα (5 ng/ml; Fitzgerald, Concord, MA), IFN-γ (5,000 U/ml; RD Systems, Minneapolis, MN), and interleukin (IL)-1β (20 ng/ml; Sigma-Aldrich, St. Louis, MO) (15). All cell culture solutions and supplements were purchased from Life Technologies, Inc., except for FBS, which was purchased from Sigma-Aldrich. Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich. Reagents for SDS-PAGE and Western blot were from Bio-Rad (Hercules, CA) and Amersham (GE Healthcare Life Sciences, Mississauga, Canada). Goat polyclonal antibody against NOX3 was purchased from Santa Cruz Biotechnology (Paso Robles, CA). Rabbit monoclonal antibody against NOX4 was purchased from Epitomics (Burlingame, CA). Rabbit polyclonal antibodies against HSL, phospho-HSL (serine 563), and perilipin were purchased from Cell Signaling Technology (Beverly, MA), and FASN Ab was from Sigma-Aldrich. Secondary HRP-coupled antibodies were from Jackson Laboratories (West Grove, PA). NOX inhibitor diphenyleneiodonium (DPI) was used at a concentration of 10 μM (EMDMillipore). Nitroblue tetrazolium (NBT) chloride was purchased from Sigma; deoxy-d-glucose, 2-[1, 2-3H(N)] was from Perkin Elmer (Woodbridge, Canada).
Cell culture and treatments
The 3T3-L1 fibroblasts were grown in α-DMEM (20% calf serum) and differentiated into adipocytes as previously described (5). Only cells showing at least 85% differentiation by morphological examination were used. Adipocytes were treated with a mixture of cytokines TNFα (5 ng/ml), IFN-γ (5,000 U/ml), and IL-1β (20 ng/ml) for either 8 or 24 h. Because transfection was found to affect the cytokine-induced ROS production, we have used a combination of cytokines plus LPS (1 µg/ml) in transfected adipocytes, in order to obtain similar ROS production to that of nontransfected cells. When DPI (10 μM) was used, it was added during the last hour of treatment, and control cells were treated with vehicle (DMSO, 1%).
Adipose explants
The 8- to 12-week-old C57BL/6J mice from our in-house colony were euthanized in accordance with our institutional guidelines. Epididymal fat (epididymal white adipose tissue) depot was removed under sterile conditions, and 50 µg of tissue from each mouse was directly placed in a culture plate with antibiotic-supplemented medium (DMEM low glucose and 10% FBS). Samples were incubated for 24 h before the medium was replaced and treatments performed as described for 3T3-L1 cells. All procedures were previously approved by the Laval University Animal Ethics Committee.
Measurement of superoxide production
Superoxide radicals were measured by the NBT reduction assay as described previously (6, 16). In brief, during the last hour of treatment, NBT (1%) was added to the cells and incubated at 37°C. Then, the incubation medium was removed, the cells were sonicated in 50% solution of acetic acid, and the absorbance of reduced NBT (formazan) was measured at 560 nm.
Western blotting
Cells were lysed in RIPA buffer, and protein content was measured by the BCATM protein assay (Thermo Fisher Scientific, IL). A total of 30 μg of lysates was solubilized in Laemmlli’s electrophoresis sample buffer, loaded on 7.5% acrylamide gel, and transferred to nitrocellulose membrane. Blots were blocked with 5% nonfat dry milk powder/TBS plus Tween 20 (TBST) and incubated overnight at 4°C with primary antibodies in 5% BSA/TBST, followed by incubation for 1 h at room temperature with HRP-coupled secondary antibodies. Bands were visualized using EC3™ Imaging System (UVP Ltd., Cambridge, UK) after incubation with ECL solution (EMD Millipore). Immunoreactive bands were quantified using the ImageJ software.
mRNA extraction and quantitative PCR
Total RNA was TRIzol-extracted as per manufacturer’s instructions (Thermo Fisher Scientific, Burlington, Canada). Reverse transcription was performed using the High-Capacity cDNA reverse transcription kit (ThermoFisher Scientific). cDNA was amplified with advanced quantitative PCR Supergreen master mix (Wisent Bioproducts, St. Bruno, Canada), and real-time PCR quantification was performed on a CFX96 apparatus (Bio-Rad, Mississauga, Canada). Primers were: Nox3 forward: CACAGGCTCAAATGGACGGA, reverse: CTGCCAGCCATAAGAGAGCA: Nox4 forward: CCAAATGTTGGGCGATTGTGT, reverse: GGCTACATGCACACCTGAGA; and 36B4 forward: CGACCTGGAAGTCCAACTAC, 36B4 reverse: ATCTGCTGCATCTGCTTG.
Free glycerol measurement
The 3T3-L1 adipocytes were deprived of serum for 16 h and incubated in phenol-free DMEM with 2% FA-free albumin with or without cytokines. The last hour of treatment, fresh medium was added with or without cytokines and other inhibitors. Free glycerol was measured using the Sigma-Aldrich enzymatic reaction method, as previously described (5).
PPARγ transcription factor binding assay
The 3T3-L1 adipocytes were grown and differentiated as previously described. Fully differentiated adipocytes were treated with cytokines for 24 h. A concentration of 10 μM DPI inhibitor or vehicle was added during the last hour of the treatment. Nuclear extracts were prepared, and 10 μl was analyzed for PPARγ-specific transcription factor binding activity as described by the manufacturer (Cayman, Ann Arbor, MI). In brief, nuclear extracts were loaded on a 96-well plate coated with a double-stranded DNA sequence containing the peroxisome proliferator response element. After 16 h incubation at 4°C, the plates were washed, and a primary antibody directed against PPARγ was added for 1 h at room temperature. A secondary antibody conjugated to HRP was added to provide a sensitive colorimetric readout at 450 nm. The results were corrected for total protein content in 10 μl of nuclear extract.
Glucose uptake
Differentiated 3T3-L1 adipocytes were treated with cytokines for a total of 24 h. Cells were serum-deprived 4 h prior to assaying glucose uptake, and treatments were continued by adding cytokines. Insulin and DPI or vehicle were added for the last hour of treatment at concentrations indicated in the figure legends. Cells were washed three times with HEPES. Glucose uptake was measured by adding 2-deoxy-d-[3H] glucose (1 Ci/ml, 26.2 Ci/mmol in HEPES) for 8 min, and then radioactive medium was aspirated prior to washing adherent cells three times with 0.9% ice-cold saline. Cells were subsequently lysed in 50 mM NaOH, 800 μl, and then transferred to 2 ml of liquid scintillation for quantification using a Tri-Carb2900TR liquid scintillation analyzer (PerkinElmer). Protein concentration in cell lysates was determined using BCATM protein assay (Thermo Scientific).
siRNA and cell transfection
The 3T3-L1 adipocytes were transfected with siRNA using a lipid-based transfection method (17). Briefly, differentiated 3T3-L1 adipocytes were plated at 1.165 × 105 cells/cm2 in a 6-well plate, and 100 nM siRNA {Integrated DNA Technologies (IDT), Coralville, IA] previously mixed with DharmaFecta transfecting agent (Thermo Fisher Scientific) was then added to the cells. Control cells were transfected with scrambled siRNA (IDT). 24 h after transfection, cells where treated with cytokines overnight and then assessed for the knockdown of NOX3 protein expression by Western blot.
Statistical analysis
The effects of the treatments were compared by Student’s t-test or ANOVA analysis using GraphPad Prism 5. All data are presented as means ± SEM. Differences were considered to be statistically significant at P < 0.05.
RESULTS
Cytokine-induced superoxide production in 3T3-L1 adipocytes is NOX-dependent
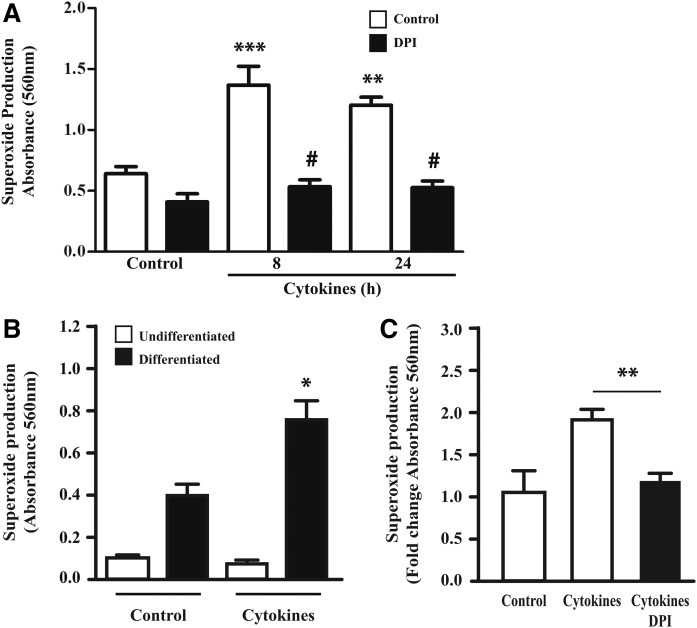
Treatment of differentiated 3T3-L1 adipocytes with proinflammatory cytokines TNFα, IL-1β, and IFNγ led to a 1.9-fold increase in superoxide production (Fig. 1A). This response was not observed in undifferentiated adipocytes (Fig. 1B). Initial studies revealed that IFNγ and TNFα can individually increase superoxide production (data not shown), but we have opted to use a combination of cytokines to better reflect the known proinflammatory characteristics of obesity. To determine the contribution of NOX proteins to cytokine-mediated ROS production in 3T3-L1 adipocytes, we have tested whether DPI, a well-established inhibitor of this group of enzymes (10), could prevent the generation of ROS. As shown in Fig. 1A, DPI fully abrogated cytokine-stimulated superoxide production measured after 8 or 24 h in 3T3-L1 adipocytes. Importantly, cytokine treatment also increased ROS production in adipose explants, which was also inhibited by treatment with DPI (Fig. 1C). These data indicate that NOX enzymes are the principal source of superoxide production in cytokine-treated adipocytes and in adipose tissue.
Fig. 1.
Effect of ROS inhibition on cytokine induced superoxide production in 3T3-L1 adipocytes and adipose explants. Differentiated 3T3-L1 adipocytes (A) and adipose explants (C) were treated with a cytokine mix (IL-1β, IFNγ, and TNFα) for 8 or 24 h. DPI (10 μM) was added the last hour of treatment. B: Undifferentiated fibroblast and differentiated adipocyte 3T3-L1 cells were tested for cytokine-induced superoxide production after 24 h of treatment with cytokine mix. Results are represented as mean ± SEM of three independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001 as compared with control (A, B) or cytokines (C); #P < 0.001 as compared with cytokines.
NOX3, but not NOX4, protein expression is inducible in 3T3-L1 adipocytes treated with cytokines
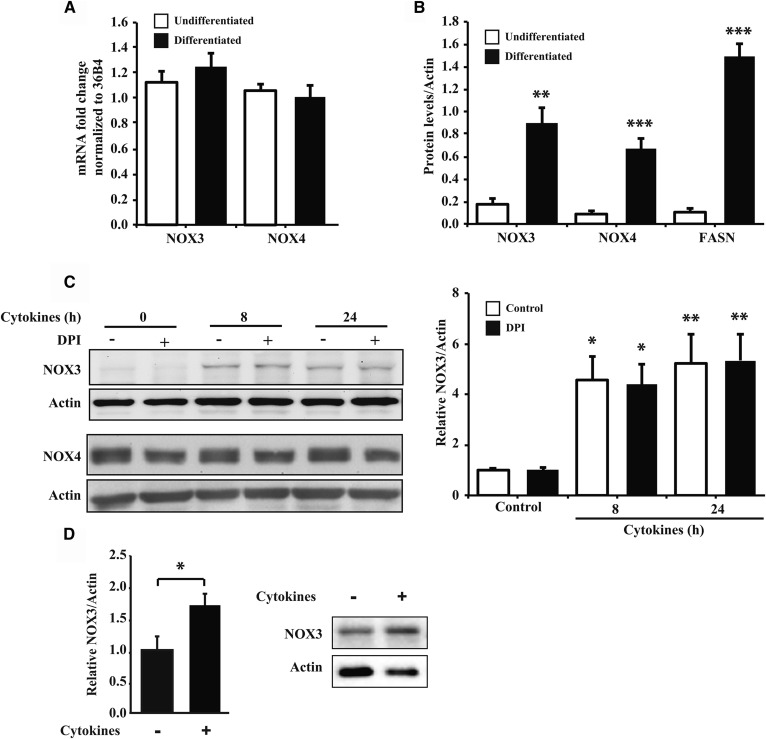
The NOX family consists of seven different members (10), and it has already been shown that NOX4 is expressed in adipocytes, where it plays an important role in adipocyte differentiation (18, 19). Whereas 3T3-L1 fibroblasts and fully differentiated 3T3-L1 adipocytes both express Nox3 and Nox4 mRNAs (Fig. 2A), only differentiated 3T3-L1 cells abundantly expressed NOX3 and NOX4 proteins (Fig. 2B), suggesting that their expression is controlled at the posttranscriptional level. Although NOX4 is constitutively expressed in nontreated adipose cells, its expression was not increased by treatment with cytokines (Fig. 2C). Li et al. (13) have shown that cytokine treatment of hepatocytes led to an increased expression of NOX3. We therefore determined NOX3 protein expression in adipocytes and found that it was strongly induced after 8 h exposure to cytokines and remained elevated up to 24 h after treatment (Fig. 2C). DPI did not affect NOX3 protein expression. We also observed a nearly 2-fold increase of NOX3 expression in adipose explants treated with cytokines for 24 h (Fig. 2D). These results show that NOX3 protein expression is also inducible in mature adipocytes and might contribute significantly to superoxide production in cytokine-treated cells.
Fig. 2.
NOX3 expression in 3T3-L1 cells and adipose explants treated with cytokines. A: RNA of undifferentiated fibroblast and differentiated adipocyte 3T3-L1 cells were analyzed for the expression of NOX3 and NOX4. B: Undifferentiated and differentiated 3T3-L1 cells were evaluated by Western blot for NOX3, NOX4, and FASN expression. C: Differentiated 3T3-L1 adipocytes were treated with a cytokine mix (IL-1β, IFNγ, and TNFα) for different times (8 and 24 h). DPI (10 μM) was added the last hour of treatment. NOX3 and NOX4 protein expression was determined by Western blot as described in Methods. D: Adipose explants were treated for 24 h with the cytokine mix as in C for 24 h. Protein/RNA quantification data are expressed as mean ± SEM of three independent experiments. * P < 0.05; ** P < 0.01; *** P < 0.001, as compared with control or undifferentiated.
NOX3 contributes to superoxide production in cytokine-treated adipocytes
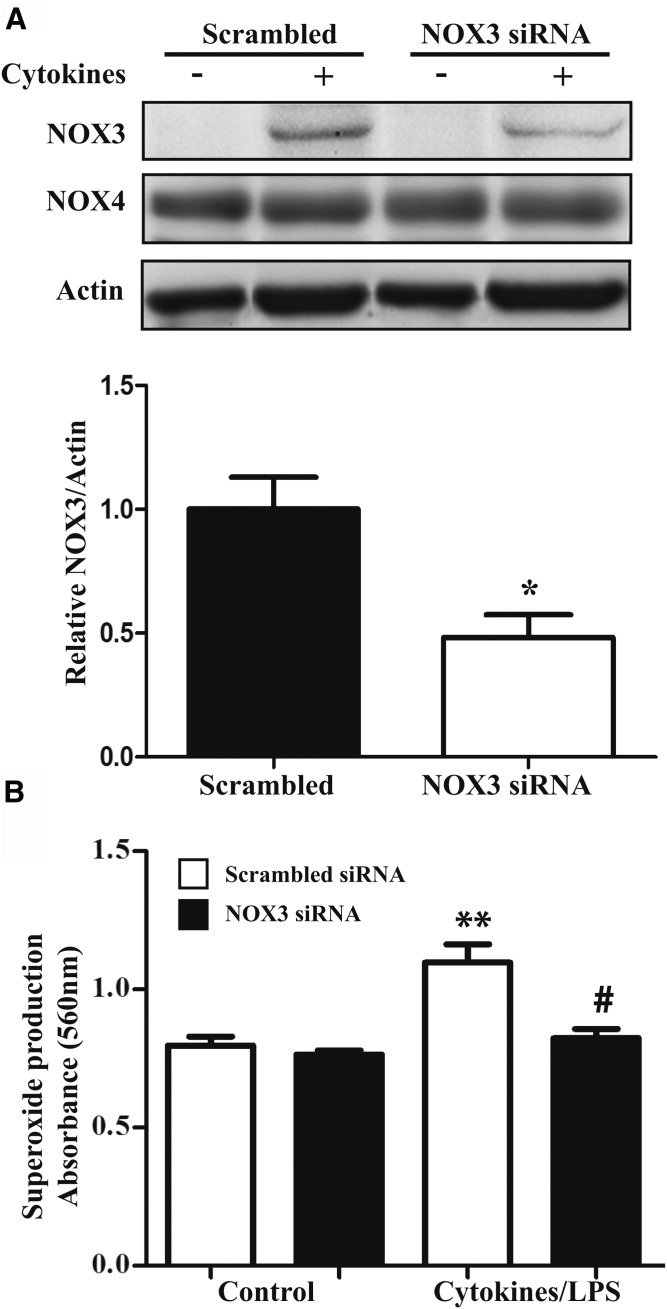
We next evaluated whether NOX3 was involved in superoxide generation. siRNA-mediated knockdown was found to reduce NOX3 expression by about 50% (P < 0.05) in cytokine-treated cells as compared with a scrambled siRNA control (Fig. 3A). NOX4 expression was not affected by NOX3 siRNA. NOX3 knockdown was sufficient to significantly reduce cytokine-induced superoxide generation (Fig. 3B), but did not affect basal ROS production, probably due to a very low expression of NOX3 in untreated cells. These results suggest that NOX3 is required for the increase in superoxide production in cytokine-treated adipocytes.
Fig. 3.
Effect of NOX3 knockdown by siRNA on superoxide production. Differentiated adipocytes were transfected with scrambled siRNA (100 nM) or siRNA (100 nM) targeting the NOX3 gene using lipid-based transfection as described in Methods. A: At 24 h posttransfection, cells were treated with cytokines (IL-1β, IFNγ, and TNFα) for 24 h. NOX3 and NOX4 protein expression was assessed by Western blot. Protein quantification of cytokine-stimulated cells is represented as the mean ± SEM of fold over cytokines of three independent experiments. * P < 0.05, cytokines+scrambled siRNA versus cytokines+NOX3 siRNA. B: Transfected cells were treated with cytokines and LPS for 24 h. DPI (10 μM) was added during the last hour of cytokine treatment. Superoxide production was measured by the NBT assay as described in Methods. Graph represents the mean ± SEM of fold over cytokines of four independent experiments. ** P < 0.01, compared to control; # P < 0.01, cytokines+scrambled siRNA versus cytokines+NOX3siRNA.
Role of NOX3 in modulating adipocyte lipolysis
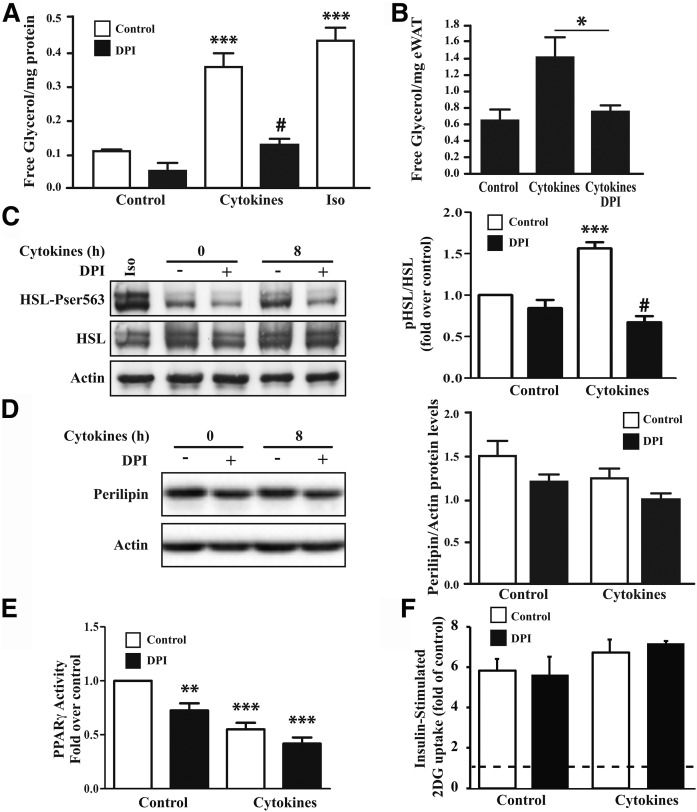
ROS play an important role as signaling molecules in the regulation of cellular growth and differentiation and immunometabolic regulation (20–22). Interestingly, it has been suggested that ROS generated from NOX could increase lipolysis in human adipocytes (21). To assess the contribution of NOX-generated ROS on metabolic functions in cytokine-exposed adipocytes, we tested the effect of blocking superoxide production with DPI on lipolysis by measuring the release of free glycerol. We observed that cytokine treatment significantly increased lipolysis and to levels comparable to a 2 h treatment with isoproterenol (Fig. 4A). This increase in lipolysis by cytokines was also seen in adipose explants (Fig. 4B). In both cases, the induction in lipolysis observed in response to cytokines was almost fully prevented by DPI (Fig. 4A, B). To test the hypothesis that NOX-dependent superoxide production promotes lipolysis through regulating HSL, one of the limiting enzymes controlling triglyceride breakdown in adipocytes, we next assessed the effect of DPI on HSL phosphorylation on serine 563, which is implicated in the lipolytic activation of the enzyme (6, 21, 23–25). We found that cytokine exposure increased phosphorylation of HSL at 8 h and that this effect was abrogated by DPI (Fig. 4C). Interestingly, cytokines did not affect perilipin expression at the same time point (8 h) but did reduce its levels by about half after 24 h (data not shown), suggesting that reduced phosphorylation of HSL precedes the downregulation of perilipin in cytokine-treated adipocytes (Fig. 4D).
Fig. 4.
Effect of DPI on lipolysis, PPARγ activity, and glucose uptake in cytokine-treated adipocytes and adipose explants. A: Differentiated 3T3-L1 adipocytes were treated with a cytokine mix (IL-1β, IFNγ, and TNFα) for 24 h or as a positive control with isoproterenol (Iso; 20 µM) for 2 h in phenol-free DMEM containing 2% BSA. The last hour of treatment, the medium was replaced with fresh medium containing cytokines and 10 μM DPI. The medium was collected, and free glycerol release was measured by enzymatic assay as described in Methods. *** P < 0.001 compared with control; # P < 0.001 compared with control+cytokines. B: Adipose explants were treated for 24 h with the cytokine mix as in A. * P < 0.05. C, D: Protein lysates of cells treated with cytokines for 8 h and nontreated cells were subjected to Western blot as described in Methods. HSL Ser563 phosphorylation and HSL as well as perilipin expression were assessed by Western blot; as a positive control, cells were treated with isoprotenol (Iso; 20 μM) for 20 min. Protein quantification is represented as the mean ± SEM of fold over control of four independent experiments. *** P < 0.001 as compared with control; #P < 0.001 as compared with cytokines. E: Differentiated 3T3-L1 adipocytes were treated with a cytokine mix (IL-1β, IFNγ, and TNFα) for 24 h. DPI (10 μM) was added the last hour of treatment. Nuclei were isolated and used for the measurement of PPARγ activity as described in Methods. Graph represents the mean ± SEM of six independent experiments. ** P < 0.01; *** P < 0.001 as compared with untreated control. F: Differentiated adipocytes were treated with cytokines for a total of 24 h prior to and during glucose uptake assay. Cells were deprived of serum for 4 h before glucose uptake. A concentration of 10 μM DPI was added the last hour of treatment where indicated, and 10 nM insulin was added the last 45 min of treatment. Cells were subjected to glucose uptake assay as described in Methods. Graph represents insulin-stimulated glucose uptake of three independent experiments ± SEM. The dashed line represents the basal glucose transport in untreated cells. 2DG, deoxy-d-glucose; eWAT, epididymal white adipose tissue; pHSL, phosphorylated HSL.
Because ROS have also been shown to block insulin-mediated glucose uptake in adipocytes (26) and to suppress PPARγ activity (6), we next tested whether NOX also modulates these metabolic pathways in cytokine-treated adipocytes. Although we confirmed that cytokine treatment decreased PPARγ activity, this effect was not reversed by DPI (Fig. 4E). Also, insulin-stimulated glucose-uptake was not affected by DPI (Fig. 4F), suggesting that ROS production by NOXs is specifically altering lipolysis in adipocytes.
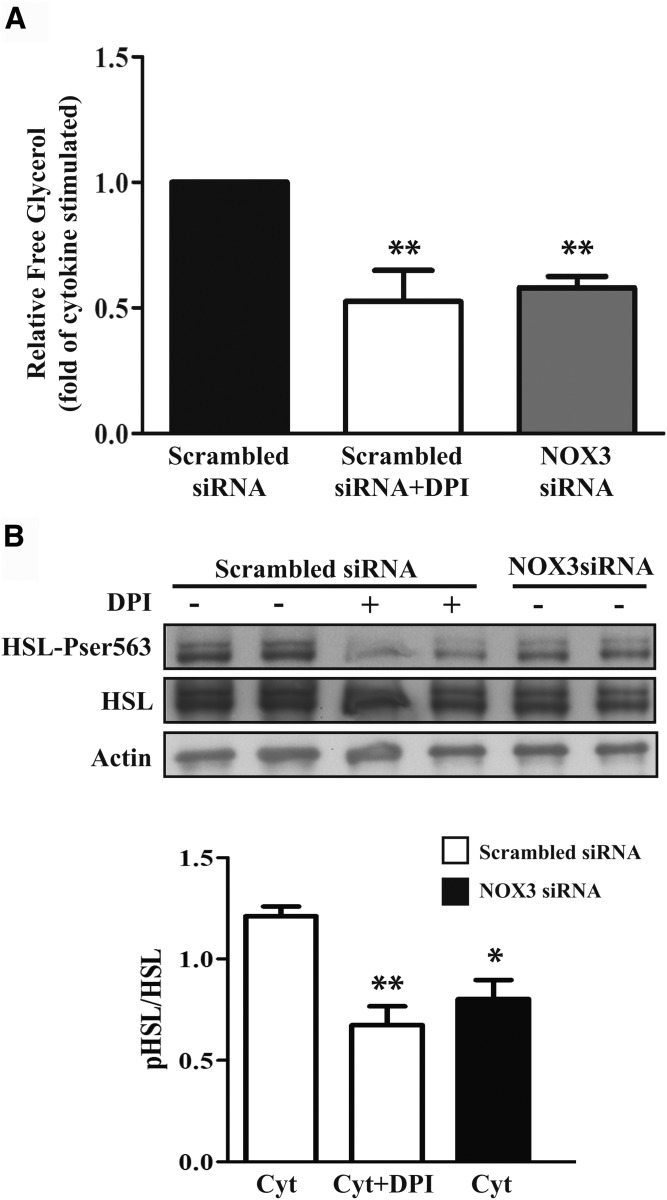
Because DPI reversed the stimulatory effect of cytokines on lipolysis and decreased phosphorylation of HSL on Ser563, we next tested whether NOX3 was involved. Cytokine-induced lipolysis was prevented by siRNA-mediated knockdown of NOX3, similar to the effect of DPI treatment (Fig. 5A). Furthermore, knockdown of NOX3 was found to decrease HSL phosphorylation on Ser563 after 8 h exposure to cytokines, which was again similar to the effect of DPI (Fig. 5B). These results are consistent with a mechanism implicating superoxide generation from NOX3 to activate lipolysis by increasing Ser563 phosphorylation of HSL in cytokine-exposed adipocytes.
Fig. 5.
Effect of NOX3 knockdown on lipolysis and phosphorylation of HSL on Ser563 in cytokine-treated adipocytes. A: Cells transfected with NOX3 siRNA (100 nM) or scrambled siRNA were treated with cytokine mix (Cyt; IL-1β, IFNγ, and TNFα) for 24 h in phenol-red free DMEM containing 2% BSA. The last hour of treatment, the medium was replaced with fresh medium containing cytokines and 10 μM DPI. The medium was collected, and free glycerol release was measured by enzymatic assay as described in Methods. Graph represents the fold decrease of the mean ± SEM of four independent experiments. ** P < 0.05 versus cytokine+scrambled siRNA. B: Transfected cells were treated with a cytokine mix (IL-1β, IFNγ, and TNFα) for 8 h. DPI (10 μM) was added the last hour of treatment. HSL and HSL Ser563 protein expression was determined by Western blot as described in Methods. Protein quantification data are expressed as mean ± SEM of three independent experiments. * P < 0.05; ** P < 0.01, as compared with cytokine+scrambled siRNA. pHSL, phosphorylated HSL.
DISCUSSION
Obesity is a chronic inflammatory disorder associated with insulin resistance and metabolic disturbances that leads to the development T2D. Obesity-linked inflammation is characterized by increased expression of proinflammatory cytokines mainly produced by infiltrating immune cells in adipose tissue (27). Potential mechanisms linking inflammation to the development of insulin resistance have been recognized and involved both ROS and reactive nitrogen species (6, 28–31). We have previously revealed the role of inducible NO synthase-mediated NO production in causing insulin resistance in skeletal muscle and liver through peroxynitrite formation (32–35). Because peroxynitrite is formed from the reaction of NO with superoxide, it is important to identify the major superoxide-producing systems that could potentially regulate metabolic functions under inflammatory conditions.
Furakawa et al. (6) have shown that ROS are increased in adipose tissue of a mouse model of obesity and T2D. This increase in ROS generation was accompanied by an increase in NOX activation and NOX4 expression. They also showed that treatment of adipocytes with FAs increased oxidative stress via NOX activation, correlating with altered adipocyte function and reduced adiponectin and PPARγ expression. Here, we show that under inflammatory settings, superoxide production was increased in adipocytes due to the induction of NOX3 leading to activation of lipolysis, an effect reversed by siRNA-mediated knockdown of NOX3 or by pharmacological inhibition of NOX with DPI.
Interestingly, it has been shown that in differentiated human adipocytes, forskolin-stimulated lipolysis was inhibited in the presence of DPI by preventing the translocation of HSL to the lipid droplets (21). Similarly, Krawczyk et al. (21) have shown that in human adipocytes, DPI decreased the phosphorylation of HSL on Ser552, which corresponds to Ser563 in mice. Consistent with these results, we found that inflammatory cytokines increased the phosphorylation of HSL on Ser563 and that this effect was reversed by DPI treatment, as well as by NOX3 knockdown. These results are consistent with a major role for NOX3 in generating superoxide production upon cytokine treatment, leading to lipolytic activation in adipocytes via the PKA/HSL pathway and thus could also be the molecular link between β-adrenergic activation and lipolysis.
Our work identifies NOX3 as a key modulator of adipocyte metabolism under inflammatory conditions. This was confirmed using both siRNA-mediated knockdown of NOX3 and by using the general NOX inhibitor DPI. Previous studies have focused on NOX4, which is highly expressed in adipocytes and thought to play a role in adipocyte differentiation and in modulating insulin signal transduction (19, 22, 36). Although we have confirmed constitutive NOX4 expression in adipocytes, its expression was not affected by cytokine treatment. Moreover, even partial knockdown of NOX3 was able to significantly abrogate cytokine-induced superoxide production, strongly suggesting that NOX3 is a major superoxide-generating NOX in adipocytes exposed to inflammatory mediators. Moreover, NOX4 is known to mainly generate hydrogen peroxide rather than superoxide when compared with NOX3, which mainly generates superoxide (37). Thus, ROS generated from these two enzymes are likely exerting regulatory effects on signal transduction through different mechanisms. Our results are therefore consistent with a major role of NOX3 in generating superoxide in inflammatory settings.
PPARγ is an important regulator of adipocyte differentiation and lipid storage, and it has been shown that inflammation negatively modulates PPARγ activity in adipose tissue (38). We therefore tested whether NOX3 may also be involved in the regulation of PPARγ. Interestingly, although DPI decreased PPARγ activity in untreated cells, it did not rescue its impaired activity in cytokine-treated adipocytes. These data are in accordance with our previous finding that PPARγ is negatively regulated by NO rather than by peroxynitrite and that superoxide generation is not required for inhibition of PPARγ in the inflammatory setting of obesity (38).
In conclusion, we found that NOX3 is an inducible NOX system that plays a role in modulating lipolysis under inflammatory settings. NOX3 appears to modulate lipolysis through regulating HSL phosphorylation on Ser563 by a mechanism that remains to be fully elucidated. Additional studies are needed to explore the role of NOX3 in mediating perturbations of adipose tissue lipolysis in vivo under chronic inflammatory settings such as obesity. Further understanding of the mechanisms that link NOX3 induction to lipolytic activation in adipocytes may help unravel new potential therapeutic targets to restrain abnormal lipolysis in obesity.
Footnotes
Abbreviations:
- DPI
- diphenyleneiodonium
- HSL
- hormone-sensitive lipase
- IL
- interleukin
- LPS
- lipopolysaccharide
- NBT
- Nitroblue tetrazolium
- NOX
- NADPH oxidase
- ROS
- reactive oxygen species
This work was supported by Canadian Institutes of Health Research (CIHR) Foundation Grant FDN#143247 (to A.M.). A.M. holds a CIHR/Pfizer Research Chair in the pathogenesis of insulin resistance and cardiovascular diseases.
REFERENCES
- 1.Monteiro R., and Azevedo I.. 2010. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010: 289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilherme A., Virbasius J. V., Puri V., and Czech M. P.. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil G. S., Shargill N. S., and Spiegelman B. M.. 1993. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., and Spiegelman B. M.. 1995. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95: 2409–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penfornis P., and Marette A.. 2005. Inducible nitric oxide synthase modulates lipolysis in adipocytes. J. Lipid Res. 46: 135–142. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y., Nakayama O., Makishima M., Matsuda M., and Shimomura I.. 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114: 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houstis N., Rosen E. D., and Lander E. S.. 2006. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 440: 944–948. [DOI] [PubMed] [Google Scholar]
- 8.Talior I., Yarkoni M., Bashan N., and Eldar-Finkelman H.. 2003. Increased glucose uptake promotes oxidative stress and PKC-delta activation in adipocytes of obese, insulin-resistant mice. Am. J. Physiol. Endocrinol. Metab. 285: E295–E302. [DOI] [PubMed] [Google Scholar]
- 9.Lambeth J. D. 2004. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4: 181–189. [DOI] [PubMed] [Google Scholar]
- 10.Ueno N., Takeya R., Miyano K., Kikuchi H., and Sumimoto H.. 2005. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. J. Biol. Chem. 280: 23328–23339. [DOI] [PubMed] [Google Scholar]
- 11.Guichard C., Moreau R., Pessayre D., Epperson T. K., and Krause K. H.. 2008. NOX family NADPH oxidases in liver and in pancreatic islets: a role in the metabolic syndrome and diabetes? Biochem. Soc. Trans. 36: 920–929. [DOI] [PubMed] [Google Scholar]
- 12.Bánfi B., Malgrange B., Knisz J., Steger K., Dubois-Dauphin M., and Krause K. H.. 2004. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 279: 46065–46072. [DOI] [PubMed] [Google Scholar]
- 13.Li L., He Q., Huang X., Man Y., Zhou Y., Wang S., Wang J., and Li J.. 2010. NOX3-derived reactive oxygen species promote TNF-alpha-induced reductions in hepatocyte glycogen levels via a JNK pathway. FEBS Lett. 584: 995–1000. [DOI] [PubMed] [Google Scholar]
- 14.Gao D., Nong S., Huang X., Lu Y., Zhao H., Lin Y., Man Y., Wang S., Yang J., and Li J.. 2010. The effects of palmitate on hepatic insulin resistance are mediated by NADPH oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J. Biol. Chem. 285: 29965–29973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapur S., Marcotte B., and Marette A.. 1999. Mechanism of adipose tissue iNOS induction in endotoxemia. Am. J. Physiol. 276: E635–E641. [DOI] [PubMed] [Google Scholar]
- 16.Vrablic A. S., Albright C. D., Craciunescu C. N., Salganik R. I., and Zeisel S. H.. 2001. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl-rac-glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J. 15: 1739–1744. [DOI] [PubMed] [Google Scholar]
- 17.Kilroy G., Burk D. H., and Floyd Z. E.. 2009. High efficiency lipid-based siRNA transfection of adipocytes in suspension. PLoS One. 4: e6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashan N., Kovsan J., Kachko I., Ovadia H., and Rudich A.. 2009. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev. 89: 27–71. [DOI] [PubMed] [Google Scholar]
- 19.Mouche S., Mkaddem S. B., Wang W., Katic M., Tseng Y. H., Carnesecchi S., Steger K., Foti M., Meier C. A., Muzzin P., et al. . 2007. Reduced expression of the NADPH oxidase NOX4 is a hallmark of adipocyte differentiation. Biochim. Biophys. Acta. 1773: 1015–1027. [DOI] [PubMed] [Google Scholar]
- 20.Brand M. D., Affourtit C., Esteves T. C., Green K., Lambert A. J., Miwa S., Pakay J. L., and Parker N.. 2004. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 37: 755–767. [DOI] [PubMed] [Google Scholar]
- 21.Krawczyk S. A., Haller J. F., Ferrante T., Zoeller R. A., and Corkey B. E.. 2012. Reactive oxygen species facilitate translocation of hormone sensitive lipase to the lipid droplet during lipolysis in human differentiated adipocytes. PLoS One. 7: e34904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schröder K., Wandzioch K., Helmcke I., and Brandes R. P.. 2009. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler. Thromb. Vasc. Biol. 29: 239–245. [DOI] [PubMed] [Google Scholar]
- 23.Anthonsen M. W., Ronnstrand L., Wernstedt C., Degerman E., and Holm C.. 1998. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J. Biol. Chem. 273: 215–221. [DOI] [PubMed] [Google Scholar]
- 24.Anthony N. M., Gaidhu M. P., and Ceddia R. B.. 2009. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity (Silver Spring). 17: 1312–1317. [DOI] [PubMed] [Google Scholar]
- 25.Watt M. J., Holmes A. G., Pinnamaneni S. K., Garnham A. P., Steinberg G. R., Kemp B. E., and Febbraio M. A.. 2006. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 290: E500–E508. [DOI] [PubMed] [Google Scholar]
- 26.Fukuoka H., Iida K., Nishizawa H., Imanaka M., Takeno R., Iguchi G., Takahashi M., Okimura Y., Kaji H., Chihara K., et al. . 2010. IGF-1 stimulates reactive oxygen species (ROS) production and inhibits insulin-dependent glucose uptake via ROS in 3T3–L1 adipocytes. Growth Horm. IGF Res. 20: 212–219. [DOI] [PubMed] [Google Scholar]
- 27.Wellen K. E., and Hotamisligil G. S.. 2003. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 112: 1785–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotamisligil G. S. 2006. Inflammation and metabolic disorders. Nature. 444: 860–867. [DOI] [PubMed] [Google Scholar]
- 29.Pickup J. C., and Crook M. A.. 1998. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 41: 1241–1248. [DOI] [PubMed] [Google Scholar]
- 30.Wellen K. E., and Hotamisligil G. S.. 2005. Inflammation, stress, and diabetes. J. Clin. Invest. 115: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White P. J., Charbonneau A., Cooney G. J., and Marette A.. 2010. Nitrosative modifications of protein and lipid signaling molecules by reactive nitrogen species. Am. J. Physiol. Endocrinol. Metab. 299: E868–E878. [DOI] [PubMed] [Google Scholar]
- 32.Charbonneau A., and Marette A.. 2010. Inducible nitric oxide synthase induction underlies lipid-induced hepatic insulin resistance in mice: potential role of tyrosine nitration of insulin signaling proteins. Diabetes. 59: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapur S., Bedard S., Marcotte B., Cote C. H., and Marette A.. 1997. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes. 46: 1691–1700. [DOI] [PubMed] [Google Scholar]
- 34.Perreault M., and Marette A.. 2001. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat. Med. 7: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 35.Pilon G., Charbonneau A., White P. J., Dallaire P., Perreault M., Kapur S., and Marette A.. 2010. Endotoxin mediated-iNOS induction causes insulin resistance via ONOO(-) induced tyrosine nitration of IRS-1 in skeletal muscle. PLoS One. 5: e15912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahadev K., Motoshima H., Wu X., Ruddy J. M., Arnold R. S., Cheng G., Lambeth J. D., and Goldstein B. J.. 2004. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 24: 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nisimoto Y., Diebold B. A., Cosentino-Gomes D., and Lambeth J. D.. 2014. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 53: 5111–5120. [Erratum. 2014. Biochemistry. 53: 5472.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dallaire P., Bellmann K., Laplante M., Gelinas S., Centeno-Baez C., Penfornis P., Peyot M. L., Latour M. G., Lamontagne J., Trujillo M. E., et al. . 2008. Obese mice lacking inducible nitric oxide synthase are sensitized to the metabolic actions of peroxisome proliferator-activated receptor-gamma agonism. Diabetes. 57: 1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]