Abstract
Dietary fat absorption takes place in the intestine, and the liver mobilizes endogenous fat to other tissues by synthesizing lipoproteins that require apoB and microsomal triglyceride transfer protein (MTP). Dietary fat triggers the synthesis of oleoylethanolamide (OEA), a regulatory fatty acid that signals satiety to reduce food intake mainly by enhancing neural PPARα activity, in enterocytes. We explored OEA’s roles in the assembly of lipoproteins in WT and Ppara−/− mouse enterocytes and hepatocytes, Caco-2 cells, and human liver-derived cells. In differentiated Caco-2 cells, OEA increased synthesis and secretion of triacylglycerols, apoB secretion in chylomicrons, and MTP expression in a dose-dependent manner. OEA also increased MTP activity and triacylglycerol secretion in WT and knockout primary enterocytes. In contrast to its intestinal cell effects, OEA reduced synthesis and secretion of triacylglycerols, apoB secretion, and MTP expression and activity in human hepatoma Huh-7 and HepG2 cells. Also, OEA reduced MTP expression and triacylglycerol secretion in WT, but not knockout, primary hepatocytes. These studies indicate differential effects of OEA on lipid synthesis and lipoprotein assembly: in enterocytes, OEA augments glycerolipid synthesis and lipoprotein assembly independent of PPARα. Conversely, in hepatocytes, OEA reduces MTP expression, glycerolipid synthesis, and lipoprotein secretion through PPARα-dependent mechanisms.
Keywords: microsomal triglyceride transfer protein, apolipoprotein B, oleic acid, chylomicrons, very low density lipoprotein, peroxisome proliferator-activated receptor alpha
Dietary triacylglycerols are hydrolyzed in the intestinal lumen, and hydrolyzed products are taken up by enterocytes for resynthesis of lipids (1, 2). Newly synthesized triacylglycerols are packaged as chylomicrons and delivered to the circulatory system where they undergo lipolysis, and remnant particles are removed from plasma by the liver and catabolized. The liver, in turn, synthesizes triacylglycerols, packages them into VLDLs, and secretes them into the circulation (3). The main purpose of the assembly and secretion of different lipoproteins by the intestine and liver is to deliver dietary and endogenous triacylglycerols to other tissues for energy. Lipoprotein assembly by the intestine and liver is dependent on two proteins; apoB that is a structural protein for these lipoproteins, and microsomal triglyceride transfer protein (MTP) that is a required chaperone for the assembly process (4, 5). A major driver for increased lipoprotein assembly and secretion is the availability of higher concentrations of fatty acid substrates for the synthesis of triacylglycerols. Thus, it is well-known that supplementation of oleic acid (OA) facilitates lipoprotein assembly and secretion by preventing apoB degradation (6). Fatty acids that are in low concentrations have been shown to regulate the assembly and secretion of lipoproteins. For example, fish oil fatty acids reduce secretion of lipoproteins by favoring degradation of apoB (7). In this study, we explored the role of a low abundant regulatory fatty acid, oleoylethanolamide (OEA), on the assembly and secretion of lipoproteins by the intestinal and liver cells.
OEA is a naturally occurring bioactive lipid that reduces food uptake and body weight gain (8, 9). It is present in the diet at very low concentrations. OEA levels are reduced after food deprivation and return to baseline after feeding, indicating a reciprocal regulation by fasting and feeding. Ingestion of OA-rich diets increases plasma OEA levels and is considered a major determinant of cellular OEA levels (10, 11). Similarly, acute administration of different dietary oils increases OEA levels in the intestine, plasma, and liver (12). In humans, OEA concentrations have been reported to be around 7 nM (13). In mice, plasma concentrations of OEA have been shown to range between 10 and 35 nM in two studies (14, 15). Dietary OA is enzymatically converted within enterocytes to OEA, and exerts its effects on satiety by sending neuronal signals involving vagal sensory neurons to the brainstem in a PPARα-dependent fashion (8–10, 16–18). OEA is a high-affinity ligand of PPARα and is also known to activate several genes important in lipid uptake, such as intestinal CD36 (19). OEA acts in liver, adipose tissue, and muscle via PPARα to induce fatty acid β-oxidation, characterized by increased expression of UCP-2 (20).
OEA has been shown to exert its effect on food intake through gut-brain communication (8–10, 17, 18). Schwartz et al. (21) have shown that intra-duodenal infusion of lipids induces satiety involving OEA production in the upper gut. In addition, systemic administration of OEA also decreases food intake in a dose- and time-dependent manner (16). OEA has no effect when administered via intracerebroventricular injections, suggesting that it acts via peripheral sensory neurons. This is supported by the observation that mice treated with the sensory neurotoxin, capsaicin, which destroys peripheral sensory neurons, do not respond to OEA. Thus, it is generally accepted that OEA sends signals via peripheral sensory neurons to the neurons of a solitary tract in the brainstem and paraventricular nucleus in the hypothalamus to limit food intake (8).
Besides food intake, OEA also affects weight gain. OEA may reduce body weight gain by inducing fatty acid oxidation in the adipose tissue and liver (9, 19, 20). However, the cell-autonomous effects of exogenous OEA in meal-related nutrient absorption sites are not well understood. To our knowledge, no study to date has addressed the effect of OEA on lipoprotein assembly and secretion in enterocytes and hepatocytes. The aim of this study was to test the hypothesis that OEA regulates lipid metabolism at the cellular level by modulating lipid synthesis and lipoprotein assembly in enterocytes and hepatocytes independently of its central regulatory mechanisms. Our studies revealed differential regulation of intestinal and hepatic lipoprotein assembly and secretion by OEA.
EXPERIMENTAL PROCEDURES
Cells
Caco-2, HepG2, and Huh-7 cells were cultured as described previously (22, 23). Human apoB and apoA1 secreted from cells were measured by ELISA (22, 24–26).
Materials
OA (catalog O7501-10G), sodium cholic acid (catalog C1254), and taurocholic acid sodium salt hydrate (TC; catalog T4009) were from Sigma. OA:TC (20 × 1.6:0.5 mM) stocks were prepared by weighing 97.4 mg of OA in 10 mM TC solution, mixing by gentle swirling, and incubating at 37°C until a clear solution was achieved. OEA (catalog 1484) from TOCRIS was dissolved in DMSO to prepare 100 mM stock. Trace amounts (∼2.5 μCi/ml) of [3H]glycerol (specific activity 2.00 Ci/mmol, 21.7 mCi/mg; Amersham Biosciences) were used to label newly synthesized glycerolipids. Collagenase type I (catalog M8H2072) was obtained from Worthington Biochemicals, Lakewood, NJ.
Isolation of primary enterocytes
Primary enterocytes were isolated from WT and Ppara−/− mice as described before (27–30). All buffers used for enterocyte isolation were purged with 95% O2 and 5% CO2. Briefly, the whole small intestine was collected from anesthetized mice, and the lumenal contents were emptied, washed with buffer A (115 mM NaCl, 0.96 mM NaH2PO4, 26.19 mM NaHCO3, 5.4 mM KCl, and 5.5 mM glucose; buffer pH 7.4), and incubated in the same buffer for 30 min with 95% O2 and 5% CO2. The intestines were then filled with buffer B (67.5 mM NaCl, 0.96 mM NaH2PO4, 26.19 mM NaHCO3, 1.5 mM KCl, 27 mM sodium citrate, and 5.5 mM glucose, pH 7.4), saturated with 95% O2 and 5% CO2, and incubated in a bath containing oxygenated saline at 37°C with constant shaking. After 15 min, the lumenal solutions were discarded and the intestines were filled with buffer C (115 mM NaCl, 0.96 mM NaH2PO4, 26.19 mM NaHCO3, 5.4 mM KCl, 1.5 mM EDTA, 0.5 mM dithiothreitol, and 5.5 mM glucose, pH 7.4), saturated with 95% O2 and 5% CO2, and bathed in saline as described above. After 15 min, the lumenal contents were collected and centrifuged (433 g, 5 min, room temperature), and the pellets were resuspended in DMEM saturated with 95% O2 and 5% CO2. These cells were used for short-term studies (up to 4 h). Longer incubation studies could not be performed due to the nonviability of these cells in culture.
Isolation of primary hepatocytes
Male 10-week-old C57/BL6J WT (catalog 000664) and Ppara−/− (catalog 008154) mice were purchased from the Jackson Laboratory. They were anesthetized with an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). Each mouse was perfused with buffer I (Hank’s balanced salt solution buffered with HEPES to a final concentration of 10 mM, pH 7.4) for 5 min through the inferior vena cava using an MP II Mini-Peristaltic Pump (Harvard Apparatus, Holliston, MA) at a setting of 8 ml/min until the liver was free of blood. The liver was then perfused with buffer II (0.4 mg/ml collagenase type I in 1× Hank’s solution) and incubated for 18 min. The collagenase-digested liver tissues were transferred into 25 ml of ice-cold William’s Medium E (catalog 12551-032, GibcoBRL). Tissues were filtered through a 70 μm filter (BD Biosciences, Mississauga, ON, Canada) and combined with 25 ml of Percoll solution (2.5 ml of 10× Hank’s solution plus 22.5 ml of Percoll). The cell suspension solution was centrifuged at 193 g for 10 min to pellet liver cells. The pellet was washed three times with 25 ml of William’s Medium E solution to remove traces of Percoll and hepatocytes were resuspended in DMEM containing 10% FBS and plated in 6-well collagen-coated plates (BD Biosciences, San Jose, CA). Cells were allowed to adhere to plates by incubating the plates for 16 h at 37°C in humidified chambers equilibrated with 5% CO2. The culture medium was changed and fresh DMEM with 10% FBS added. For experiments, cells were supplemented with different lipids and isotopes for 4 or 16 h, as described in different experimental conditions.
Treatment of cells with OEA
Caco-2 cells were plated on Transwells and allowed to differentiate for 3 weeks. Cells were washed and supplemented with fresh medium containing different amounts of OEA dissolved in DMSO. For control, cells received DMSO only. Isolated enterocytes do not adhere and do not survive long-term cultures. Hence, they were immediately supplemented with or without OEA. Isolated primary hepatocytes were allowed to adhere overnight, washed, and then treated with OEA/DMSO or DMSO in fresh medium. Hepatoma cells were plated. After 1–2 days of culturing, cells were washed and supplemented with fresh medium containing indicated concentrations of OEA in DMSO. Control cells received DMSO only.
Lipid uptake and secretion studies
For cellular uptake experiments, enterocytes were incubated with 2.5 μCi/ml [3H]glycerol in DMEM containing high glucose (4.5 g/l) at 37°C for different times with constant shaking. Cell suspensions were gassed at 15 min intervals with 95% O2 and 5% CO2. After washing with DMEM, lipids were extracted with 2 ml of isopropanol and total radioactivity was counted in a scintillation counter and normalized for protein.
For secretion studies, enterocytes were labeled with 2.5 μCi/ml of [3H]glycerol in DMEM for 1 h at 37°C, as described for the uptake experiments. After 1 h, enterocytes were centrifuged (3,000 rpm, 5 min), and pellets were washed with excess DMEM to remove external [3H]glycerol and then chased for 2 h with DMEM with high glucose at 37°C (oxygenated at 15 min intervals) containing lipid/bile salt micelles as described before (27, 28, 31).
Glycerol uptake by hepatocytes has a km value of 100–400 μM (32, 33). Most of the glycerol taken up by cells is used for gluconeogenesis. We have used trace amounts of 3H-glycerol to study newly synthesized glycerolipids. These trace amounts of glycerol are unlikely to affect any interpretations concerning the effects of OEA on lipid synthesis, as the same amounts of 3H-glycerol were provided to control cells.
FPLC assay
Conditioned media from Huh-7 cells were subjected to fast-performance LC (FPLC) using a Superose 6 column (GE Healthcare). Samples were chromatographed at a 0.2 ml/min flow rate, and 250 μl fractions were collected. Radioactivity was measured using a scintillation counter. apoB and apoA1 levels were also measured in these fractions using ELISA kits (34).
Density gradient ultracentrifugation
Caco-2 cell culture media were used for sequential density gradient ultracentrifugation to isolate large chylomicrons, small chylomicrons, and VLDLs as described (23, 27–29). This method is suitable for separating large triacylglycerol-rich lipoproteins based on their buoyant density (35–37). After ultracentrifugation, samples were collected from the top, lipids were separated on TLC, and triglyceride bands were counted for radioactivity using a scintillation counter. The density in each fraction was measured using a refractometer (Fisher Scientific) as described (29, 35).
Analyses of MTP activity
Cells, primary enterocytes, and hepatocytes were collected in 1 ml of ice-cold 1 mM Tris-HCl (pH 7.6) buffer containing 1 mM EGTA and 1 mM MgCl2 and homogenized in a glass homogenizer. The homogenates were centrifuged (SW55 Ti rotor; 303,800 g, 10°C, 1 h), and the supernatants were used for MTP transfer assay as described (22, 24, 28, 38, 39).
Gene expression
Total RNA was isolated from cells or primary cells using Trizol™ (Invitrogen). RNA (1 μg) was used to synthesize cDNA using RT-kits (catalog 4368813, Thermo Fisher Scientific) as described (22, 24, 31, 40). Real-time PCR measurements of individual cDNAs were performed using different primers and SYBR Green (qPCR Core Kit for SYBR Green I #RT-SN10-05, Eurogentec Headquarters) in a StepOnePlus real-time PCR system (catalog 4376600) as described before (22, 24, 27, 31, 41). GAPDH was used as control.
Statistical analyses
Data are presented as mean ± SD; n = 3–6 animals were used to isolate enterocytes and hepatocytes. Each treatment was performed in triplicate wells. Animal use was approved by the animal care and use committees at the State University of New York Downstate Medical Center and New York University Winthrop Hospital. Statistical testing was performed by the unpaired Student’s t-test. For studies of three or more groups, one-way ANOVA was performed followed by Dunnett’s post hoc comparison to the control group. All statistical analyses were conducted using GraphPad Prism, and differences were considered significant if P < 0.05.
RESULTS
OEA increases triacylglycerol synthesis and secretion in differentiated human colon carcinoma Caco-2 cells
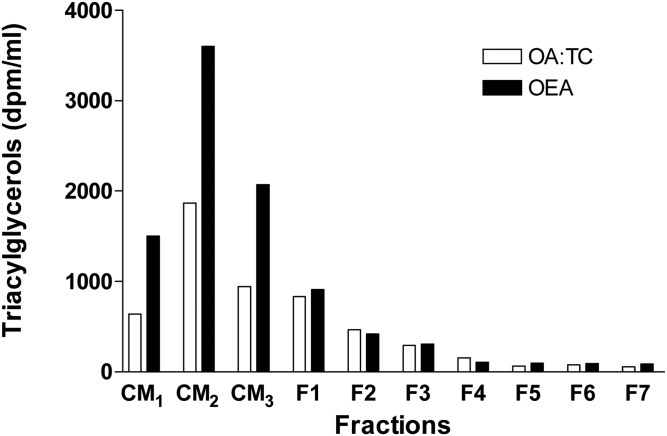
Fatty acids, such as OA, are substrates for triacylglycerol synthesis. They have been known to increase triacylglycerol synthesis and support synthesis and secretion of larger lipoproteins by enterocytes and hepatocytes (42, 43). However, recent studies have suggested that other fatty acids, such as OEA, play regulatory roles in lipid absorption by acting as neuronal signals in the hypothalamus (8, 10). We have previously shown that OA increases assembly and secretion of chylomicrons by differentiated Caco-2 cells (23, 36, 37). Here, we studied the effects of OEA and OA on the secretion of lipoproteins in differentiated Caco-2 cells (Fig. 1). As reported earlier (23), OA increased secretion of apoB and triacylglycerols in chylomicrons. Surprisingly, OEA increased secretion of triacylglycerols more than OA in differentiated Caco-2 cells (Fig. 1).
Fig. 1.
Effects of OA and OEA on secretion of lipoproteins by differentiated Caco-2 cells. Caco-2 cells differentiated on Transwells in triplicate for 3 weeks were supplemented on the apical side with OA:TC micelles (1.6:0.5 mM) or OEA (200 μM) in DMSO for 16 h with 2.5 μCi/ml of [3H]glycerol. Basolateral media were pooled and subjected to density gradient ultracentrifugation and fractions were collected from the top of the tube. Lipids were extracted from each fraction and separated by TLC; triacylglycerol bands were scraped and quantified. CM1, chylomicrons Sf <400; CM2, chylomicrons Sf <60–400; CM3, chylomicrons Sf <20–60, d <1.006 g/ml; F1–F3, intermediate and low density lipoproteins (d = 1.02–1.063 g/ml); F4–F7, high density lipoproteins (d = 1.63–1.1 g/ml).
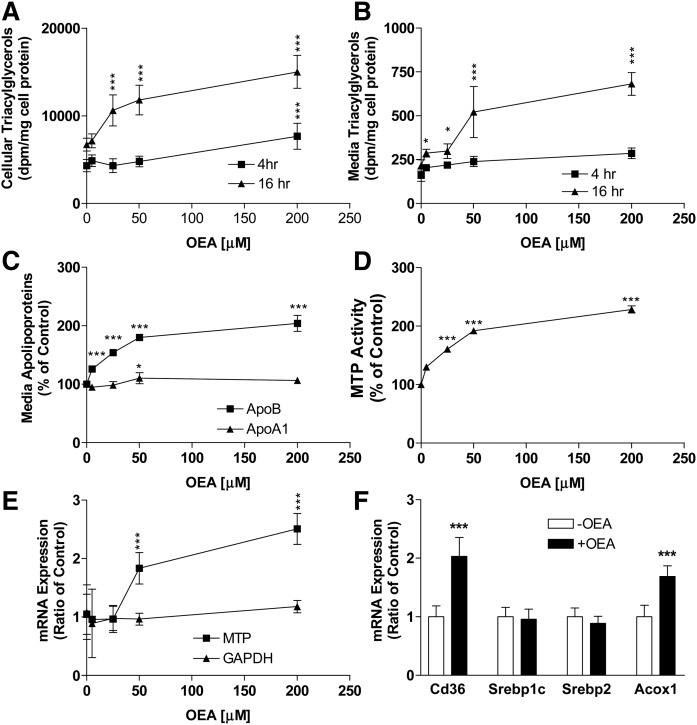
To evaluate the biochemical mechanisms by which OEA could increase lipoprotein secretion, we studied the effects of various concentrations of OEA on lipid synthesis in differentiated Caco-2 cells for 4 or 16 h in the presence of trace amounts of [3H]glycerol to monitor newly synthesized lipids. At 4 h, various amounts of OEA had no significant effect on newly synthesized cellular (Fig. 2A) and media (Fig. 2B) triacylglycerol levels. However, at 16 h, OEA dose-dependently increased cellular (2.2-fold at 200 μM) and media (1.8-fold at 200 μM) triacylglycerols (Fig. 2A, B). These studies show that OEA has no effect on triacylglycerol synthesis and secretion at 4 h, but significantly increases cellular and media levels of newly synthesized triacylglycerol at 16 h. Next, we studied secretion of apolipoproteins. At 16 h, OEA enhanced apoB secretion, but had no effect on apoA1 secretion (Fig. 2C). These studies indicated that OEA induces increased synthesis and secretion of triacylglycerols and apoB in differentiated Caco-2 cells.
Fig. 2.
Effects of different concentrations of OEA on triacylglycerol synthesis and secretion in differentiated Caco-2 cells. A, B: Differentiated Caco-2 cells were incubated with different indicated concentrations of OEA and 2.5 μCi/ml of [3H]glycerol on the apical side for 4 or 16 h. At indicated times, cells (A) and media (B) were collected and lipids were extracted and subjected to TLC. Individual bands corresponding to triacylglycerols were scraped and counted in a scintillation counter. C, D: Differentiated Caco-2 cells were supplemented with different indicated concentrations of OEA on the apical side for 16 h. Media (C) were used to quantify apoB and apoA1 via ELISA, and cells (D) were used to measure MTP activity. E: Differentiated Caco-2 cells were supplemented with different indicated concentrations of OEA on the apical side. Cells collected at 16 h were used to measure mRNA levels of MTP and GAPDH. F: In a separate experiment, differentiated Caco-2 cells (n = 3) were treated with or without OEA (200 μM) for 16 h and cells were used to quantify mRNA levels of indicated genes. The data are plotted as mean ± SD, n = 3. *P < 0.05; and ***P < 0.001 as compared with the no OEA group. In some cases, error bars are smaller than the symbol sizes.
To understand the molecular mechanisms for enhanced chylomicron secretion, we measured activity and mRNA levels of MTP, an essential chaperone for lipoprotein assembly and secretion. Caco-2 cells treated with different concentrations of OEA had significantly increased amounts of MTP activity and mRNA levels at 16 h (Fig. 2D, E). OEA is known to activate PPARα. Hence, we measured PPARα target genes and observed that OEA induced CD36 and ACOX1 expression (Fig. 2F). In contrast, OEA had no effect on Srebp1c and Srebp2 gene expression (Fig. 2F). These studies in differentiated Caco-2 cells indicated that OEA dose-dependently enhances lipid secretion as part of chylomicrons by inducing the expression of MTP.
OEA increases triacylglycerol secretion and MTP activity in mouse primary enterocytes
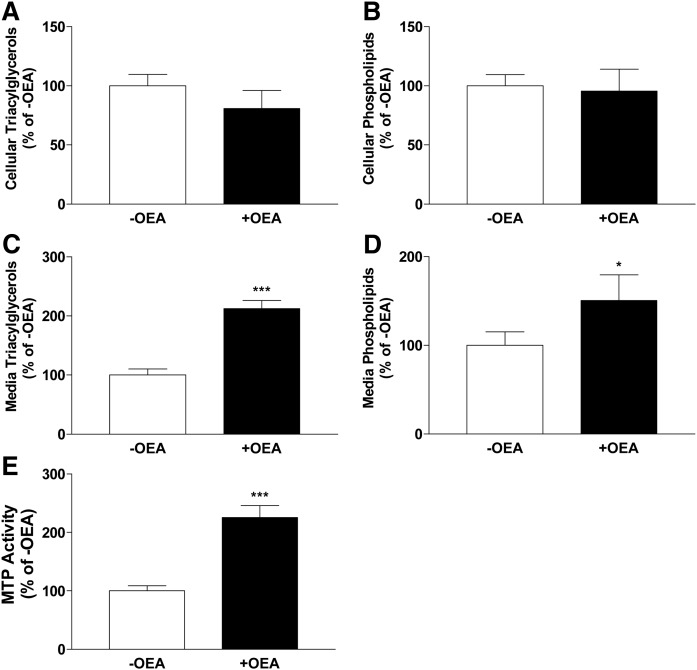
To determine whether observations made in Caco-2 cells were not specific to transformed cells, mouse primary enterocytes from WT mice were incubated with OEA (+OEA) or without OEA (−OEA) for 4 h in the presence of [3H]glycerol (Fig. 3). There were no significant differences in the amounts of triacylglycerols and phospholipids in cells (Fig. 3A, B). However, amounts of triacylglycerols and phospholipids secreted into the media were significantly higher in OEA-treated cells than in control DMSO-treated cells (Fig. 3C, D). Further, MTP activity was significantly higher in OEA-treated cells (Fig. 3E). These studies suggested that OEA increases MTP activity and triacylglycerol secretion in primary enterocytes as it does in Caco-2 cells.
Fig. 3.
Effect of OEA on cellular and media triacylglycerol and phospholipid levels and cellular MTP activity in mouse primary enterocytes. Primary enterocytes were isolated from the proximal intestines of WT C57BL6J mice and incubated in triplicate without (−OEA) or with 200 μM OEA (+OEA) in DMSO for 4 h in the presence of 1.0 μCi/ml of [3H]glycerol. Lipids were extracted from cells (A, B) and media (C, D) and separated on TLC, and triacylglycerol (A, C) and phospholipid (B, D) bands were quantified. In a separate experiment, MTP activity was measured in cells treated or not treated with OEA 200 μM (E). The data are plotted as mean ± SD, n = 3; *P < 0.05; ***P < 0.001 as compared with no OEA group.
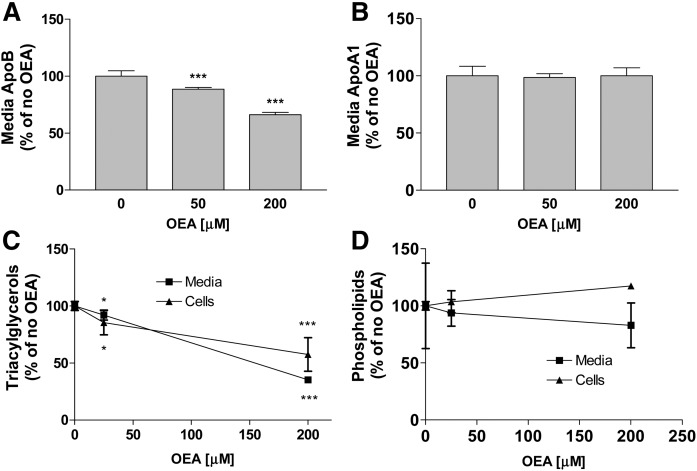
OEA decreases apoB and triacylglycerol secretion in human hepatoma cells
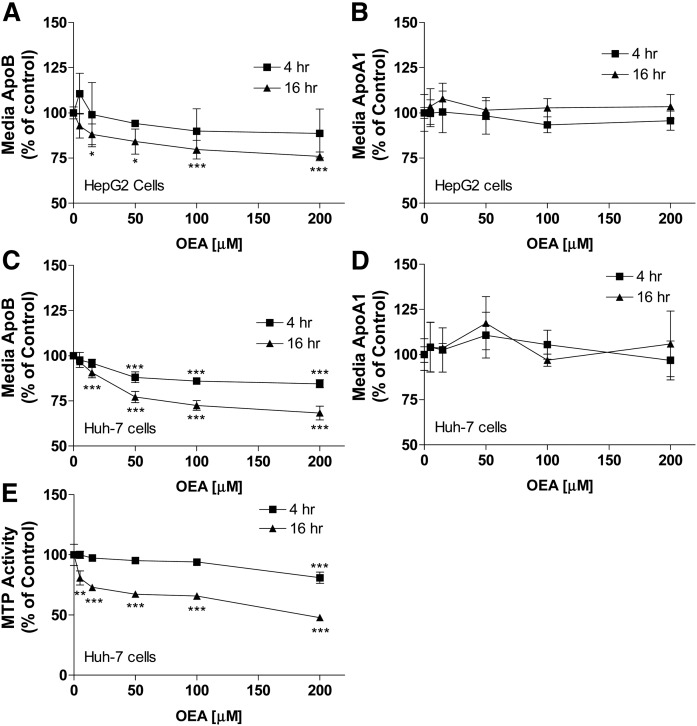
Next, we studied the effects of OEA on the secretion of apoB and apoA1 in human hepatoma cells (Fig. 4). In HepG2 cells, increasing amounts of OEA significantly reduced apoB secretion at 16 h (Fig. 4A). At 4 h, a trend toward reduced apoB secretion was observed (Fig. 4A). OEA had no significant effect on apoA1 secretion at either 4 or 16 h in these cells (Fig. 4B). In Huh-7 cells, OEA dose-dependently reduced apoB secretion at both 4 and 16 h (Fig. 4C), but had no effect on apoA1 secretion at both 4 and 16 h time points examined (Fig. 4D). Furthermore, OEA, dose-dependently reduced MTP activity after 16 h incubations in Huh-7 cells. These studies showed that OEA decreases apoB secretion in hepatoma cells.
Fig. 4.
Effect of OEA on media apoB and apoA1 as well as cellular MTP activity in human hepatoma cells. A, B: Human hepatoma HepG2 cells were incubated in triplicate with different indicated concentrations of OEA, and media were collected at 4 or 16 h to measure apoB (A) and apoA1 (B) by ELISA. Cells treated with DMSO without OEA served as controls. C–E: Human hepatoma Huh-7 cells were incubated in triplicate with different indicated concentrations of OEA for 4 or 16 h. Media were used to quantify apoB (C) and apoA1 (D). Cells were used to measure MTP activity (E). Cells treated with DMSO without OEA served as controls. The data from three independent experiments were combined and plotted as mean ± SD, n = 3. *P < 0.05; **P < 0.01, and ***P < 0.001 as compared with no OEA group. In some cases, error bars are smaller than the symbol sizes.
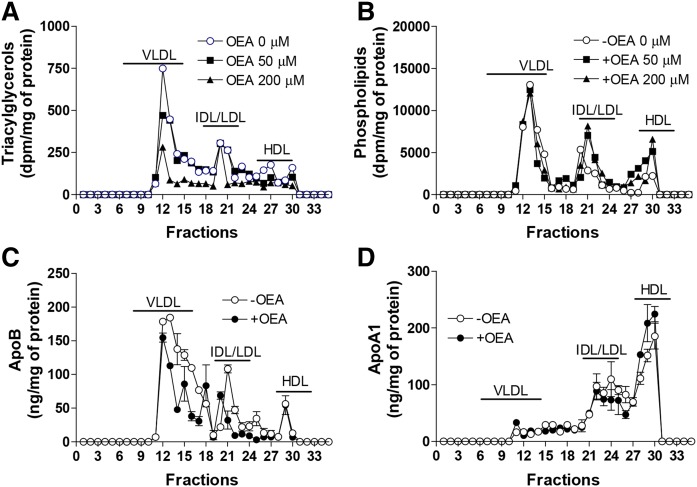
In a separate experiment, OEA again significantly reduced apoB secretion, but had no effect on apoA1 secretion (Fig. 5A, B). In addition, we observed that OEA dose-dependently reduced cellular and secreted triacylglycerols after 16 h incubations in Huh-7 cells (Fig. 5C). Cellular and media phospholipid levels were not significantly reduced (Fig. 5D). We then studied the effects of OEA on the secretion of different lipids as part of lipoproteins (Fig. 6). OEA dose-dependently reduced triacylglycerols in VLDLs, but had no effect on phospholipids in these fractions (Fig. 6A, B). Quantifications of apolipoproteins revealed that OEA reduced apoB in VLDLs, but had no effect on apoA1 secretion in different lipoproteins (Fig. 6C, D). These studies showed that OEA reduces secretion of triacylglycerols as apoB-containing VLDLs, indicating for a possible decrease in the secretion of number of VLDL particles.
Fig. 5.
OEA decreases triacylglycerol synthesis and secretion as well as media apoB in Huh-7 cells. Huh-7 cells were incubated in triplicate with different indicated amounts of OEA for 16 h in the presence of 2.5 μCi/ml of [3H]glycerol. A, B: Media were also used to quantify apoB (A) and apoA1 (B) by ELISA. C, D: Media and cellular lipids were extracted and separated on TLC. Bands corresponding to triacylglycerols (C) and phospholipids (D) were scraped and radioactivities in these bands were measured. The data are plotted as mean ± SD. *P < 0.05; ***P < 0.001 as compared with the no OEA group.
Fig. 6.
OEA decreases secretion of triacylglycerol and apoB with VLDLs. A, B: Huh-7 cells were incubated in triplicate with different concentrations of OEA for 16 h in the presence of 2.5 μCi/ml [3H]glycerol. Media were pooled and subjected to FPLC. Lipids were extracted from fractions and separated on TLC; bands corresponding to triacylglycerols (A) and phospholipids (B) were scraped and measured. C, D: Huh-7 cells were incubated in triplicate with or without OEA [200 μM] for 16 h. Media were pooled and subjected to FPLC. Fractions were collected to measure apoB (C) and apoA1 (D) in triplicate by ELISA.
OEA decreases triacylglycerol synthesis and secretion in mouse primary hepatocytes
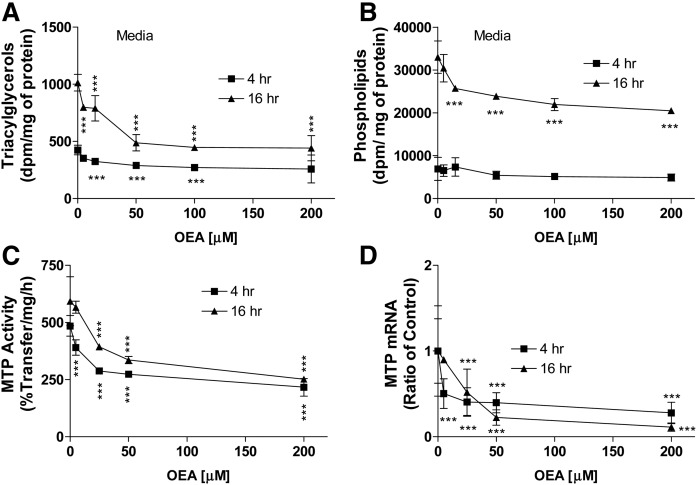
We then studied the effects of OEA on the secretion of newly synthesized lipids in primary hepatocytes. OEA dose-dependently decreased media triacylglycerols (Fig. 7A). OEA significantly reduced media phospholipids at 16 h, but not at 4 h (Fig. 7B). OEA dose-dependently reduced MTP activity and mRNA levels at 4 h and these reductions were persistent until 16 h incubations (Fig. 7C, D). These studies showed that OEA significantly diminishes MTP activity and triacylglycerol secretion in mouse primary hepatocytes.
Fig. 7.
Effect of OEA on triacylglycerol and phospholipid secretion, and MTP expression in mouse primary hepatocytes. A, B: Primary hepatocytes were isolated from WT chow-fed C57BL6J mice. They were incubated in triplicate with different indicated amounts of OEA for 4 or 16 h in the presence of 2.5 μCi/ml of [3H]glycerol. Lipids were extracted from media and separated on TLC to quantify triacylglycerols (A) and phospholipids (B). C, D: Primary hepatocytes were isolated from WT chow-fed C57BL6J mice, and then cells were incubated in triplicate with different indicated concentrations of OEA in DMSO. Cells treated with DMSO only without OEA served as controls. The cells were also used to measure MTP activity (C) and mRNA levels (D). The data are plotted as mean ± SD. ***P < 0.001 as compared with the no OEA group in each time course.
OEA increases triacylglycerol secretion and MTP activity in mouse primary enterocytes independent of PPARα activity
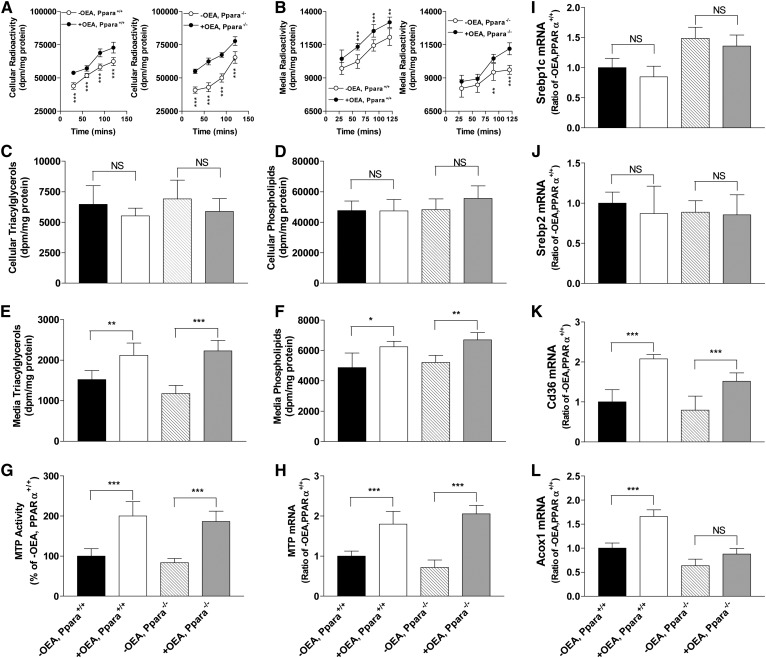
Because it is generally accepted that OEA mainly exerts its effect by binding and activating PPARα (8–10, 16–18), we asked whether OEA requires PPARα to exert its effects on lipid synthesis and lipoprotein production in the intestinal and liver cells. Primary enterocytes were isolated from WT (Ppara+/+) and Ppara−/− mice and treated with or without OEA (Fig. 8). Uptake studies showed that OEA-treated WT and Ppara−/− enterocytes took up more label than untreated cells (Fig. 8A). Pulse-chase secretion studies revealed that OEA-treated enterocytes from both WT and Ppara−/− mice secreted more lipids (Fig. 8B). At the end of the pulse-chase study, there were no significant differences in cellular triacylglycerol (Fig. 8C) and phospholipid (Fig. 8D) levels in WT and Ppara−/− enterocytes treated or not treated with OEA. In contrast, OEA-treated WT and Ppara−/− enterocytes secreted more triacylglycerols (Fig. 8E) and phospholipids (Fig. 8F). Further, OEA increased MTP activity (Fig. 8G) and mRNA levels (Fig. 8H) in WT as well as in Ppara−/− enterocytes. Expression of Srebp1c and Srebp2 mRNA levels were unaffected by OEA treatment (Fig. 8 I, J). CD36 mRNA levels were increased in both WT and Ppara−/− enterocytes; however, increases in Ppara−/− enterocytes were lower than in WT enterocytes (Fig. 8K). OEA significantly increased Acox1 mRNA levels in WT enterocytes, but not in Ppara−/− enterocytes (Fig. 8L). These studies showed that OEA increases lipid secretion and MTP expression to similar levels in WT and Ppara−/− enterocytes, indicating that OEA modulates MTP expression and lipoprotein secretion independently of PPARα activity.
Fig. 8.
Effect of OEA on cellular and media triacylglycerol and phospholipid levels and cellular MTP activity and mRNA levels in PPARα-deficient primary enterocytes. Primary enterocytes were isolated from the proximal intestines of WT and Pparα knockout mice and incubated in triplicate without (−OEA) or with 200 μM OEA (+OEA) in DMSO in the presence of 2.5 μCi/ml of [3H]glycerol. Comparisons were made between the +OEA- and −OEA-treated groups. A: For uptake studies, cells were collected at indicated times and radioactivity was measured. B: To determine secretion rates, cells were pulse labeled with [3H]glycerol for 2 h, washed, and incubated in label-free media. At indicated times, radioactivities in media were quantified. C–F: Cells were treated with or without 200 μM OEA in DMSO in the presence of 2.5 μCi/ml of [3H]glycerol for 2 h, washed, and incubated in label-free media for 2 h. Lipids were extracted from cells and media, separated on TLC, and triacylglycerol (C, E) and phospholipid (D, F) bands were quantified. G–L: Enterocytes were incubated in triplicate with or without OEA for 4 h. MTP activity (G) and different mRNA levels (H–L) were measured in cells. The data are plotted as mean ± SD, n = 3. ***P < 0.001 as compared with the no OEA group.
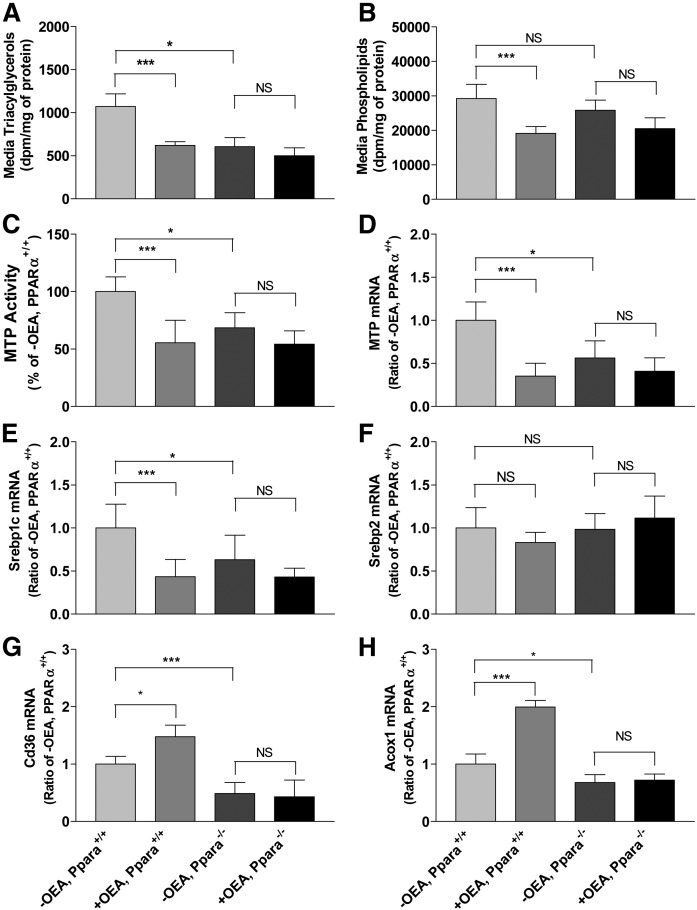
Decreases in triacylglycerol secretion and MTP activity in mouse primary hepatocytes requires PPARα activity
Next, we studied the effect of OEA on lipid secretion and MTP activity in WT and Ppara−/− hepatocytes (Fig. 9). OEA significantly reduced triacylglycerol secretion in WT hepatocytes (Fig. 9A). Ppara−/− hepatocytes secreted significantly lower levels of triacylglycerols, and these reduced levels were not further reduced by OEA treatment (Fig. 9A). Similarly, secreted phospholipids in media were significantly reduced after OEA treatment in WT hepatocytes, but not in Ppara−/− hepatocytes (Fig. 9B). Next, we studied the effects of OEA in WT and Ppara−/− hepatocytes on MTP activity and mRNA (Fig. 9C, D). OEA reduced MTP activity and mRNA levels in WT hepatocytes (Fig. 9C, D) consistent with earlier studies in Fig. 7C, D. Compared with WT hepatocytes, Ppara−/− hepatocytes had significantly lower MTP activity and mRNA levels, and the activity and mRNA levels were not changed by OEA treatment in Ppara−/− hepatocytes (Fig. 9C, D). OEA reduced Srebp1c mRNA levels, but had no effect on Srebp2 mRNA levels in WT hepatocytes (Fig. 9E, F). In Ppara−/− hepatocytes, OEA had no effect on the expression of Srebp1 and Srebp2 (Fig. 9 E, F). OEA increased CD36 and Acox1 mRNA levels in WT hepatocytes, but not in Ppara−/− hepatocytes (Fig. 9 G, H). These studies indicated that PPARα is required for OEA to reduce lipid secretion and MTP expression in hepatocytes.
Fig. 9.
Effect of OEA on media triacylglycerol and phospholipid levels, and cellular MTP activity and mRNA levels in Pparα-deficient primary hepatocytes. A, B: Primary hepatocytes were isolated from the livers of WT and Pparα knockout mice. After overnight culture, hepatocytes were incubated in triplicate with or without 200 μM OEA in DMSO in the presence of 2.5 μCi/ml of [ 3H]glycerol for 16 h. Lipids were extracted from media and separated on TLC to quantify triacylglycerols (A) and phospholipids (B). C, D: Primary hepatocytes were incubated in triplicate with or without OEA for 16 h. MTP activity (C) and mRNA levels (D–H) were measured in cells. The data are plotted as mean ± SD, n = 3. ***P < 0.001 as compared with the no OEA mice.
DISCUSSION
A novel observation of this study is that OEA has differential effects on glycerolipid synthesis and lipoprotein secretion in intestine- and liver-derived cells. We show that OEA increases glycerolipid synthesis and lipoprotein secretion in intestine-derived cells. In contrast, it reduces glycerolipid synthesis and lipoprotein secretion in liver-derived cells. It is well known that OEA mainly exerts its effects by binding to PPARα (10, 11). Studies in PPARα-deficient enterocytes revealed that increases in intestinal glycerolipid synthesis and lipoprotein secretion by OEA are independent of PPARα (Fig. 8). Contrary to this, reductions in lipid synthesis and lipoprotein secretion by OEA in hepatocytes depend on PPARα, as PPARα-deficient hepatocytes do not respond to OEA (Fig. 9). These studies show, for the first time, that OEA can act at cellular level to modulate glycerolipid synthesis and lipoprotein assembly in enterocytes and hepatocytes independently of its role in food intake and satiety involving intestinal-neuronal signaling communications.
At a molecular level, OEA increased mRNA levels of MTP in intestinal cells. OEA may enhance lipoprotein assembly and secretion by increasing MTP expression. We hypothesized that OEA may increase MTP expression by activating PPARα. Surprisingly, our studies showed that PPARα is not required for the increased expression of MTP by enterocytes. It remains to be determined whether OEA or its metabolite(s) activate alternative transcription factors to regulate MTP expression and lipoprotein secretion. Identification of such transcription factors may reveal novel regulatory mechanisms involved in intestinal lipid synthesis and mobilization.
Although it is well established that OEA induces satiety, suppresses food intake, inhibits body weight gain, and reduces plasma cholesterol levels via PPARα activation (10, 11), there are other effects of OEA that do not require PPARα. For example, OEA suppresses intestinal motility (44) and short-term food intake (45) in rodents and prevents cytokine-induced permeability increases in Caco-2 cells via PPARα-independent mechanisms (46). In fact, it has been shown that OEA can interact with transient receptor potential ion channel V1 (TRPV1) to modulate permeability in Caco-2 cells (41). Further, it is known that OEA acts as an agonist of the G protein-coupled receptors, GPR119 and GPR55 (47, 48). Thus, any one of these receptors or other as yet unidentified proteins might be involved in OEA’s ability to increase lipid absorption and/or lipoprotein assembly.
We considered the possibility that OEA may serve as a substrate for glycerolipid synthesis and promote lipoprotein assembly. This possibility was discounted, as the amounts of OEA used were low (200 μM) compared with the amounts of OA (1.6 mM) needed to promote assembly and secretion of chylomicrons (Fig. 1). We have previously shown that 200 μM of OA does not support increased assembly and secretion of lipoproteins in intestinal cells (23, 29). Thus, OEA-derived substrates might not be sufficient for increased lipoprotein assembly. Our studies showed that OEA can increase lipid synthesis (Figs. 2A, 8A). Thus, newly synthesized glycerolipid could be a major source for lipoprotein secretion in OEA-stimulated enterocytes. Besides glycerolipid synthesis, intracellular lipid droplets are also a source of glycerolipid for lipoprotein assembly and secretion (49). It remains to be determined whether OEA can induce hydrolysis of lipid droplets present in intestinal cells and mobilize them for secretion.
In contrast to the intestinal cells, liver-derived cells did not respond to OEA in the absence of PPARα (Fig. 9). Thus, OEA most likely represses hepatic lipid synthesis and lipoprotein assembly and secretion by activating PPARα. This was unanticipated. When bound to ligands, PPARα usually acts as a transcriptional enhancer (50). For example, it is known that MTP is upregulated by Pparα agonists in rodent livers (51). Thus, reduced MTP expression may involve a novel mechanism whereby OEA and PPARα activate a repressor involved in hepatic MTP expression.
We speculate that OEA may exert differential regulatory effects on glycerolipid synthesis and lipoprotein assembly and secretion in the intestine and liver by interacting with different transcription factors to optimize dietary fat absorption. We hypothesize that under postprandial conditions OEA acutely optimizes dietary lipid absorption by enterocytes and their utilization by other tissues. A major reason for lipid absorption is to provide dietary fat as an energy source to other tissues. By inhibiting hepatic lipoprotein assembly, OEA may divert fatty acids for oxidation. OEA is known to enhance fatty acid oxidation in the liver (11, 18). Thus, in hepatocytes, OEA may channel fatty acids for oxidation instead of lipoprotein assembly. Several hours after a meal, when plasma OEA levels decrease, hepatic lipoprotein assembly may increase to facilitate mobilization of endogenous fat as an energy source for other tissues. Thus, OEA may serve as a lipid mediator to communicate and control lipid mobilization between intestine and liver.
A major caveat of these studies is that they were restricted to cells in culture. This was to test the hypothesis that OEA may exert cell-specific effects that are independent of gut-brain communication. Because these studies were performed in isolated cells, it can be concluded that the observed effects are cell-specific and are independent of gut-brain neuronal signaling. Future animal studies are required to permit the integration of the present diverse PPAR-independent and -dependent effects on lipid mobilization and oxidation with the systemic effects of OEA on feeding and body weight regulation. Another caveat is the possible use of pharmacological concentrations of OEA in our cell culture studies. Although plasma concentrations of OEA are available in some studies (13–15), to our knowledge, no studies have measured OEA levels in the intestinal and liver microenvironments.
In summary, these studies provide evidence that OEA may act cell autonomously to modulate lipid synthesis and lipoprotein secretion in the intestine and liver. OEA has differential effects on lipid synthesis and lipoprotein assembly in enterocytes and hepatocytes. In enterocytes, it augments glycerolipid synthesis and lipoprotein secretion, while it inhibits these processes in hepatocytes. OEA appears to exert these differential modulatory effects by engaging distinct transcription factors. In the liver, it most likely activates PPARα to reduce lipoprotein secretion. The receptor(s) engaged by OEA in enterocytes to augment glycerolipid synthesis and secretion remains elusive. Overall, the present data suggest that OEA acts as a lipid messenger between intestine and liver to optimize dietary fat delivery to peripheral tissues.
Footnotes
Abbreviations:
- FPLC
- fast-performance LC
- OA
- oleic acid
- OEA
- oleoylethanolamide
- MTP
- microsomal triglyceride transfer protein
- TC
- taurocholic acid sodium salt hydrate (taurocholate)
This work was supported in part by National Institutes of Health Grants HL95924, DK046900, and HL137202, and Veterans Affairs Merit Award BX001728 (to M.M.H.). Additional support was provided by National Institutes of Health Grant HL137912 and American Heart Association Grant-in-Aid 16GRNT30960027 (to X.P.) and the New York Obesity Research Center [National Institutes of Health Grant DK026687 (to G.J.S.)]. The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Pan X., and Hussain M. M.. 2012. Gut triglyceride production. Biochim. Biophys. Acta. 1821: 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iqbal J., and Hussain M. M.. 2009. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 296: E1183–E1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain M. M., and Pan X.. 2009. Clock genes, intestinal transport and plasma lipid homeostasis. Trends Endocrinol. Metab. 20: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussain M. M., Shi J., and Dreizen P.. 2003. Microsomal triglyceride transfer protein and its role in apolipoprotein B-lipoprotein assembly. J. Lipid Res. 44: 22–32. [DOI] [PubMed] [Google Scholar]
- 5.Hussain M. M., Rava P., Walsh M., Rana M., and Iqbal J.. 2012. Multiple functions of microsomal triglyceride transfer protein. Nutr. Metab. (Lond.). 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon J. L., Furukawa S., and Ginsberg H. N.. 1991. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J. Biol. Chem. 266: 5080–5086. [PubMed] [Google Scholar]
- 7.Wang H., Chen X., and Fisher E. A.. 1993. N-3 fatty acids stimulate intracellular degradation of apolipoprotein B in rat hepatocytes. J. Clin. Invest. 91: 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiPatrizio N. V., and Piomelli D.. 2015. Intestinal lipid-derived signals that sense dietary fat. J. Clin. Invest. 125: 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown J. D., Karimian A. E., and Ayala J. E.. 2017. Oleoylethanolamide: a fat ally in the fight against obesity. Physiol. Behav. 176: 50–58. [DOI] [PubMed] [Google Scholar]
- 10.Piomelli D. 2013. A fatty gut feeling. Trends Endocrinol. Metab. 24: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowen K. J., Kris-Etherton P. M., Shearer G. C., West S. G., Reddivari L., and Jones P. J. H.. 2017. Oleic acid-derived oleoylethanolamide: a nutritional science perspective. Prog. Lipid Res. 67: 1–15. [DOI] [PubMed] [Google Scholar]
- 12.Olatinsu A. O., Sihag J., and Jones P. J. H.. 2017. Relationship between circulating fatty acids and fatty acid ethanolamide levels after a single 2-h dietary fat feeding in male Sprague-Dawley rats: elevated levels of oleoylethanolamide, palmitoylethanolamide, linoleoylethanolamide, arachidonoylethanolamide and docosahexanoylethanolamide after a single 2 h dietary fat feeding in male Sprague Dawley rats. Lipids. 52: 901–906. [DOI] [PubMed] [Google Scholar]
- 13.Little T. J., Cvijanovic N., Dipatrizio N. V., Argueta D. A., Rayner C. K., Feinle-Bisset C., and Young R. L.. 2018. Plasma endocannabinoid levels in lean, overweight, and obese humans: relationships to intestinal permeability markers, inflammation, and incretin secretion. Am. J. Physiol. Endocrinol. Metab. 315: E489–E495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newberry E. P., Kennedy S. M., Xie Y., Luo J., Crooke R. M., Graham M. J., Fu J., Piomelli D., and Davidson N. O.. 2012. Decreased body weight and hepatic steatosis with altered fatty acid ethanolamide metabolism in aged L-Fabp -/- mice. J. Lipid Res. 53: 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Igarashi M., Narayanaswami V., Kimonis V., Galassetti P. M., Oveisi F., Jung K. M., and Piomelli D.. 2017. Dysfunctional oleoylethanolamide signaling in a mouse model of Prader-Willi syndrome. Pharmacol. Res. 117: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez de Fonseca F., Navarro M., Gómez R., Escuredo L., Nava F., Fu J., Murillo-Rodríguez E., Giuffrida A., LoVerme J., Gaetani S., et al. 2001. An anorexic lipid mediator regulated by feeding. Nature. 414: 209–212. [DOI] [PubMed] [Google Scholar]
- 17.Fu J., Gaetani S., Oveisi F., Lo V. J., Serrano A., Rodriguez De F. F., Rosengarth A., Luecke H., Di G. B., Tarzia G., et al. 2003. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 425: 90–93. [DOI] [PubMed] [Google Scholar]
- 18.Sihag J., and Jones P. J. H.. 2018. Oleoylethanolamide: The role of a bioactive lipid amide in modulating eating behaviour. Obes. Rev. 19: 178–197. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y., Chen M., Georgeson K. E., and Harmon C. M.. 2007. Mechanism of oleoylethanolamide on fatty acid uptake in small intestine after food intake and body weight reduction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292: R235–R241. [DOI] [PubMed] [Google Scholar]
- 20.Guzmán M., Lo Verme J., Fu J., Oveisi F., Blázquez C., and Piomelli D.. 2004. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha). J. Biol. Chem. 279: 27849–27854. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz G. J., Fu J., Astarita G., Li X., Gaetani S., Campolongo P., Cuomo V., and Piomelli D.. 2008. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 8: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan X., and Hussain M. M.. 2007. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J. Biol. Chem. 282: 24707–24719. [DOI] [PubMed] [Google Scholar]
- 23.Luchoomun J., and Hussain M. M.. 1999. Assembly and secretion of chylomicrons by differentiated Caco-2 cells: Nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J. Biol. Chem. 274: 19565–19572. [DOI] [PubMed] [Google Scholar]
- 24.Pan X., Zhang Y., Wang L., and Hussain M. M.. 2010. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 12: 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakillah A., Zhou Z., Luchoomun J., and Hussain M. M.. 1997. Measurement of apolipoprotein B in various cell lines: correlation between intracellular levels and rates of secretion. Lipids. 32: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 26.Hussain M. M., Zhao Y., Kancha R. K., Blackhart B. D., and Yao Z.. 1995. Characterization of recombinant human apoB-48-containing lipoproteins in rat hepatoma McA-RH7777 cells transfected with apoB48 cDNA: Overexpression of apoB-48 decreases synthesis of endogenous apoB-100. Arterioscler. Thromb. Vasc. Biol. 15: 485–494. [DOI] [PubMed] [Google Scholar]
- 27.Pan X., and Hussain M. M.. 2009. Clock is important for food and circadian regulation of macronutrient absorption in mice. J. Lipid Res. 50: 1800–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan X., Munshi M. K., Iqbal J., Queiroz J., Sirwi A. A., Shah S., Younus A., and Hussain M. M.. 2013. Circadian regulation of intestinal lipid absorption by apolipoprotein AIV involves forkhead transcription factors A2 and O1 and microsomal triglyceride transfer protein. J. Biol. Chem. 288: 20464–20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anwar K., Iqbal J., and Hussain M. M.. 2007. Mechanisms involved in vitamin E transport by primary enterocytes and in vivo absorption. J. Lipid Res. 48: 2028–2038. [DOI] [PubMed] [Google Scholar]
- 30.Iqbal J., and Hussain M. M.. 2005. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 46: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 31.Pan X., Jiang X. C., and Hussain M. M.. 2013. Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. Circulation. 128: 1758–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C. C., and Lin E. C.. 1983. Glycerol transport and phosphorylation by rat hepatocytes. J. Cell. Physiol. 117: 230–234. [DOI] [PubMed] [Google Scholar]
- 33.Westergaard N., Madsen P., and Lundgren K.. 1998. Characterization of glycerol uptake and glycerol kinase activity in rat hepatocytes cultured under different hormonal conditions. Biochim. Biophys. Acta. 1402: 261–268. [DOI] [PubMed] [Google Scholar]
- 34.Pan X., Hussain F. N., Iqbal J., Feuerman M. H., and Hussain M. M.. 2007. Inhibiting proteasomal degradation of microsomal triglyceride transfer protein prevents CCl4 induced steatosis. J. Biol. Chem. 282: 17078–17089. [DOI] [PubMed] [Google Scholar]
- 35.Luchoomun J., Zhou Z., Bakillah A., Jamil H., and Hussain M. M.. 1997. Assembly and secretion of VLDL in nondifferentiated Caco-2 cells stably transfected with human recombinant apolipoprotein B48 cDNA. Arterioscler. Thromb. Vasc. Biol. 17: 2955–2963. [DOI] [PubMed] [Google Scholar]
- 36.Iqbal J., Anwar K., and Hussain M. M.. 2003. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J. Biol. Chem. 278: 31610–31620. [DOI] [PubMed] [Google Scholar]
- 37.Anwar K., Kayden H. J., and Hussain M. M.. 2006. Transport of vitamin E by differentiated Caco-2 cells. J. Lipid Res. 47: 1261–1273. [DOI] [PubMed] [Google Scholar]
- 38.Athar H., Iqbal J., Jiang X. C., and Hussain M. M.. 2004. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45: 764–772. [DOI] [PubMed] [Google Scholar]
- 39.Rava P., Athar H., Johnson C., and Hussain M. M.. 2005. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J. Lipid Res. 46: 1779–1785. [DOI] [PubMed] [Google Scholar]
- 40.Hussain M. M., and Zannis V. I.. 1990. Intracellular modification of human apolipoprotein AII (apoAII) and sites of apoAII mRNA synthesis: comparison of apoAII with apoCII and apoCIII isoproteins. Biochemistry. 29: 209–217. [DOI] [PubMed] [Google Scholar]
- 41.Pan X., Bradfield C. A., and Hussain M. M.. 2016. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidemia and enhances atherosclerosis. Nat. Commun. 7: 13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olofsson S. O., and Boren J.. 2005. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J. Intern. Med. 258: 395–410. [DOI] [PubMed] [Google Scholar]
- 43.Hussain M. M., Kancha R. K., Zhou Z., Luchoomun J., Zu H., and Bakillah A.. 1996. Chylomicron assembly and catabolism: role of apolipoproteins and receptors. Biochim. Biophys. Acta. 1300: 151–170. [DOI] [PubMed] [Google Scholar]
- 44.Cluny N. L., Keenan C. M., Lutz B., Piomelli D., and Sharkey K. A.. 2009. The identification of peroxisome proliferator-activated receptor alpha-independent effects of oleoylethanolamide on intestinal transit in mice. Neurogastroenterol. Motil. 21: 420–429. [DOI] [PubMed] [Google Scholar]
- 45.Wang X., Miyares R. L., and Ahern G. P.. 2005. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J. Physiol. 564: 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karwad M. A., Macpherson T., Wang B., Theophilidou E., Sarmad S., Barrett D. A., Larvin M., Wright K. L., Lund J. N., and O’Sullivan S. E.. 2017. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARalpha. FASEB J. 31: 469–481. [DOI] [PubMed] [Google Scholar]
- 47.Godlewski G., Offertaler L., Wagner J. A., and Kunos G.. 2009. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 89: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irving A., Abdulrazzaq G., Chan S. L. F., Penman J., Harvey J., and Alexander S. P. H.. 2017. Cannabinoid receptor-related orphan G protein-coupled receptors. Adv. Pharmacol. 80: 223–247. [DOI] [PubMed] [Google Scholar]
- 49.Sturley S. L., and Hussain M. M.. 2012. Lipid droplet formation on opposing sides of the endoplasmic reticulum. J. Lipid Res. 53: 1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han L., Shen W. J., Bittner S., Kraemer F. B., and Azhar S.. 2017. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-alpha. Future Cardiol. 13: 259–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Améen C., Edvardsson U., Ljungberg A., Asp L., Akerblad P., Tuneld A., Olofsson S. O., Lindén D., and Oscarsson J.. 2005. Activation of peroxisome proliferator-activated receptor alpha increases the expression and activity of microsomal triglyceride transfer protein in the liver. J. Biol. Chem. 280: 1224–1229. [DOI] [PubMed] [Google Scholar]