ABSTRACT
Prion diseases have a wide host range, but prion-infected cases have never been reported in horses. Genetic polymorphisms that can directly impact the structural stability of horse prion protein have not been investigated thus far. In addition, we noticed that previous studies focusing on horse-specific amino acids and secondary structure predictions of prion protein were performed for limited parts of the protein. In this study, we found genetic polymorphisms in the horse prion protein gene (PRNP) in 201 Thoroughbred horses. The identified polymorphism was assessed to determine whether this polymorphism impedes stability of protein using PolyPhen-2, PROVEAN and PANTHER. In addition, we evaluated horse-specific amino acids in horse and mouse prion proteins using same methods. We found only one single nucleotide polymorphism (SNP) in the horse prion protein, and three annotation tools predicted that the SNP is benign. In addition, horse-specific amino acids showed different effects on horse and mouse prion proteins, respectively.
Abbreviations: PRNP: prion protein gene; SNP: single nucleotide polymorphism; CJD: Creutzfeldt-Jakob disease; CWD: chronic wasting disease; TME: transmissible mink encephalopathy; FSE: feline spongiform encephalopathy; MD: molecular dynamics; ER: endoplasmic reticulum; GPI: glycosylphosphatidylinositol; NMR: nuclear magnetic resonance; ORF: open reading frame; GWAS: genome-wide association study; NAPA: non-adaptive prion amplification; HMM: hidden Markov model; NCBI: National Center for Biotechnology Information
KEYWORDS: Horses, prion, PRNP, prion protein, polymorphism, single nucleotide polymorphism, SNP
Introduction
Prion diseases are irreversible neurodegenerative conditions [1] and have a wide range of host variability, encompassing Creutzfeldt-Jakob disease (CJD) in humans, bovine spongiform encephalopathy (BSE) in cattle, scrapie in sheep and goats, chronic wasting disease (CWD) in deer, transmissible mink encephalopathy (TME) in mink, and feline spongiform encephalopathy (FSE) in cats, cheetahs and pumas; however, prion disease in horse has never been reported thus far, even though the life span of the horse is approximately 30 years [2–8].
Previous studies noted that the stability of horse prion protein is related to disease progression. Thus, several studies have focused on identifying the horse-specific structure of prion protein gene (PRNP), which can contribute to the stability of horse prion protein. Two horse-specific structures have been reported, called the β2-α2 loop and salt bridges. Horse-specific amino acids S169, Y224 and F227 are composed of the horse-specific β2-α2 loop and denote remarkable stability among species [9]. In addition, a molecular dynamics (MD) study found 4 salt bridges in horse prion protein and confirmed that the salt bridges bestow upon the most durable structure of horse prion protein that can withstand harsh conditions [10]. Another factor that can contribute to protein stability is genetic polymorphisms. Recent studies have reported disease associated genetic polymorphisms. In humans, more than 20 single nucleotide polymorphisms (SNPs) and mutations on PRNP gene are associated with disease susceptibility [6,11–13]. In addition, two insertion/deletion polymorphisms affect BSE susceptibility in cattle [14–17]. According to the haplotypes of PRNP codons 136, 154 and 171, sheep are subdivided into 5 levels of scrapie risk groups from R1 to R5 [18,19]. Goats have been reported to have SNPs at PRNP codons 142, 143, 146, 154, 171, 211 and 222 that are related to the vulnerability to scrapie [20,21]. In deer and elk, genotype distributions of PRNP codons 95, 96, 132 and 225 showed correlations with the number of CWD-positive animals [22–27]. In recent studies, genetic polymorphisms of prion gene family members have been reported in cattle, goats and chickens [28–31]. However, polymorphisms of the horse PRNP gene have not been investigated thus far. Because the horse is generally believed to be a prion-resistant animal, an investigation of the polymorphisms in the horse PRNP gene is important.
In this study, we investigated genotype and allele distributions of a horse PRNP gene polymorphism by DNA sequencing in 201 Thoroughbred horses. In addition, we also evaluated the impact of the identified SNP and horse-specific amino acids through PolyPhen-2, PROVEAN and PANTHER. Furthermore, we compared amino acid sequences of prion protein among species using ClustalW2 and Wasabi.
Results
Identification and analysis of polymorphisms in the horse PRNP gene
To investigate the genotype and allele frequencies of horse PRNP gene polymorphisms, we performed PCR to amplify the horse PRNP gene. The amplified horse PRNP gene was sequenced in 201 Thoroughbred horses by using an ABI 3730 automatic direct sequencer. The DNA sequences in the open reading frame (ORF) region of the horse PRNP gene sequenced in 201 Thoroughbred horses are identical to the PRNP gene of Equus caballus registered in GenBank (ACG59276.1). Interestingly, we found only one non-synonymous SNP (c.525A> C, N175K) in the ORF of the horse PRNP gene (Figures 1 and 2). Of the 201 Thoroughbred samples, 110 (54.7%) were homozygous for the A allele, 11 (5.5%) were homozygous for the C allele, and 80 (39.8%) were heterozygous at codon 175, with an allele frequency of 0.746:0.254 A:C. Next, to evaluate whether the SNP location was important, we drew a schematic map of horse prion protein and marked N175K on it. Interestingly, N175K did not impede the components of the two horse-specific structure, the β2-α2 loop (residues 167–174) and four salt bridges (E198-R158-H189, R158-D204, E213-H179 and D180-R166) (Figure 3).
Figure 1.
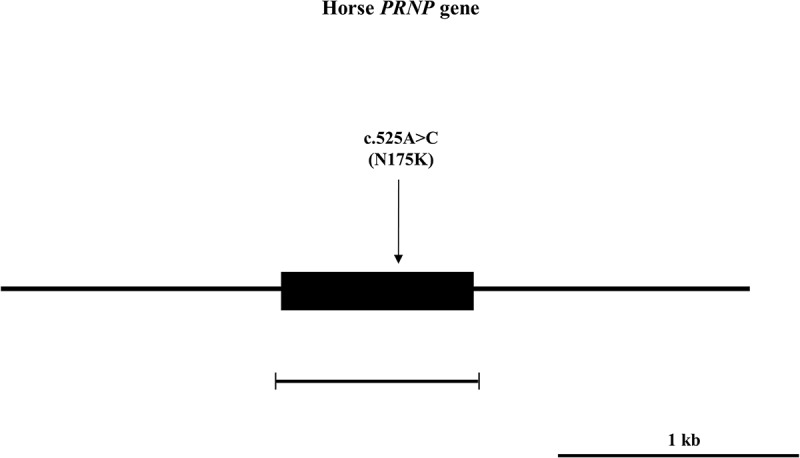
Gene map and polymorphism identified in the horse prion protein gene (PRNP) on chromosome 22. The open reading frame (ORF) is indicated by a shaded block. Horizontal bars with edges indicate the regions sequenced. Arrows indicate the polymorphism found in this study.
Figure 2.
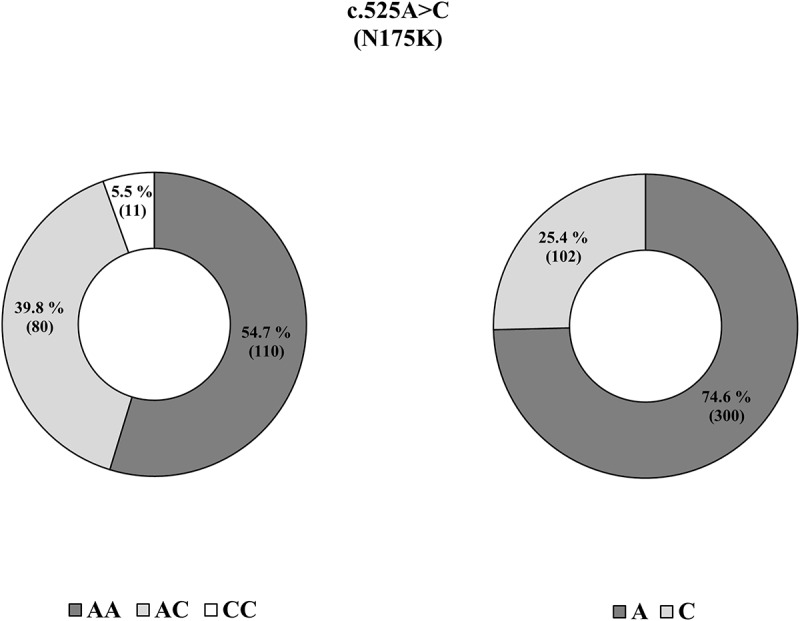
Pie chart for the genotype and allele frequencies of c.525A> C (N175K) in horse PRNP gene. Left pie chart indicates the genotype frequency of c.525A> C (N175K) in the horse PRNP gene. Right pie chart indicates the allele frequency of c.525A> C (N175K) in the horse PRNP gene.
Figure 3.
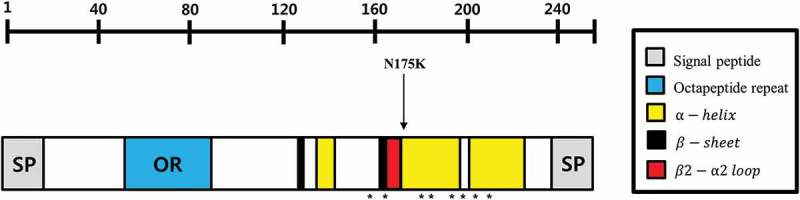
Evaluation of the SNP location on a schematic map of horse prion protein. The protein sequence of horse prion protein was determined by automatic direct sequencing of 201 Thoroughbred horses in this study. Each structure was determined by methods described in the ‘Materials and Methods’ section. Arrows indicate the polymorphism found in this study. The N-terminal endoplasmic reticulum (ER) signal peptide is located on residues 1–19 and the C-terminal glycosylphosphatidylinositol (GPI) signal peptide is located on 231–255. The α-helix is located on residues 145–158, 175–196 and 202–230 and the β-sheet is located on 130–133 and 163–166. The β2-α2 loop is located on residues 167–174. Components of four salt bridges (E198-R158-H189, R158-D204, E213-H179 and D180-R166) are marked by asterisks.
Predicting the impact of the non-synonymous SNP on horse prion protein
To estimate the impact of the non-synonymous SNP (N175K) on horse prion protein, we utilized PolyPhen-2, PROVEAN and PANTHER. PolyPhen-2 predicted that N175K was ‘Benign’ with the score 0.008. PROVEAN estimated N175K to be ‘Neutral’ with the score −0.757. PANTHER evaluated N175K as ‘Probably benign’ with the score 176 (Table 1).
Table 1.
Prediction of protein functional alterations induced by the non-synonymous single nucleotide polymorphism (SNP) in horse prion protein.
| Variation | Method | Score | Prediction |
|---|---|---|---|
| N175K | PolyPhen-2 | 0.008 | Benign |
| PROVEAN | -0.757 | Neutral | |
| PANTHER | 176 | Probably benign |
Evaluation of the effect of horse-specific amino acids
We tried to investigate the effect of horse-specific amino acids. For this, we performed a sequence alignment among species to find horse-specific amino acids. The horse-specific amino acids were determined by ClustW2 and Wasabi based progressive alignment construction and phylogeny-aware methods, respectively. The horse-specific amino acids were as follows: 169S, 224Y, 227F, 228Q and 243V (Figure 4a,4b). First, we substituted horse-specific amino acids with interspecific conserved amino acids sequentially and estimated the substitution effect on horse prion protein using PolyPhen-2, PROVEAN and PANTHER. Notably, the substitution effect did not seem to be significant on horse prion protein (Table 2). Second, we measured the D166S (previous referred as D167S) substitution effect in mouse prion protein, which was performed using the same design as a previous in vivo mutagenesis study [32]. PolyPhen-2 and PANTHER predicted that the substitution effect was ‘Probably damaging’ and ‘Possibly damaging’, respectively (Table 3).
Figure 4.
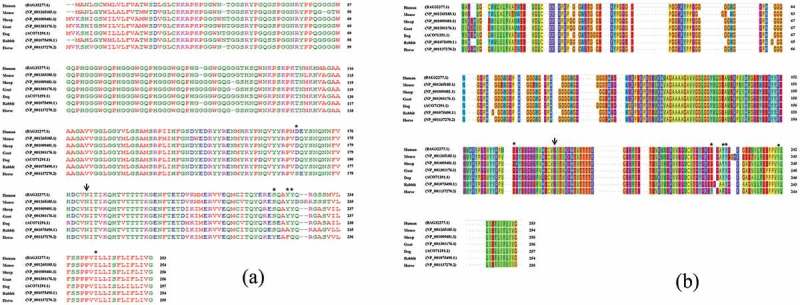
Comparison of amino acid sequences of prion protein in human, mouse, sheep, goat, dog, rabbit and horse. Prion protein sequences were obtained from GenBank at the National Center for Biotechnology Information (NCBI), including those of human (Homo sapiens, BAG32277.1), mouse (Mus musculus, NP_001265185.1), sheep (Ovis aries, NP_001009481.1), goat (Capra hircus, NP_001301176.1), dog (Canis lupus familiaris, ACO71291.1), rabbit (Oryctolagus cuniculus, NP_001075490.1) and horse (Equus caballus, NP_001137270.2). A. Protein sequences were aligned using ClustalW2 based on progressive alignment methods. Colors indicate the chemical properties of amino acids; blue: acidic; red: small and hydrophobic; magenta: basic; green: hydroxyl, sulfhydryl, amine and glycine. The arrow indicates the polymorphism. Asterisks indicate horse-specific residues. B. Protein sequences were aligned using Wasabi based on phylogeny-aware methods. Colors were followed ‘Taylor color’. The arrow indicates the polymorphism. Asterisks indicate horse-specific residues.
Table 2.
Measurement of the effect of substitutions from horse-specific amino acids to interspecific conserved amino acids on horse prion protein.
| Residues | Horse specific | Interspecific conserved | Methods | Score | Prediction |
|---|---|---|---|---|---|
| 169 | S | D | PolyPhen-2 | 0.000 | Benign |
| PROVEAN | 0.515 | Neutral | |||
| PANTHER | 2 | Probably benign | |||
| 224 | Y | S | PolyPhen-2 | 0.005 | Benign |
| PROVEAN | 0.977 | Neutral | |||
| PANTHER | 2 | Probably benign | |||
| 227 | F | Y | PolyPhen-2 | 0.000 | Benign |
| PROVEAN | 0.908 | Neutral | |||
| PANTHER | 2 | Probably benign | |||
| 228 | Q | Y | PolyPhen-2 | 0.000 | Benign |
| PROVEAN | 0.861 | Neutral | |||
| PANTHER | 2 | Probably benign | |||
| 243 | V | I | PolyPhen-2 | NA | Unknown |
| PROVEAN | 0.068 | Neutral | |||
| PANTHER | 2 | Probably benign |
* NA, Not available
Table 3.
Measurement of the effect of substitutions from mouse amino acids to horse-specific amino acid on mouse prion protein.
| Residue | Mouse amino acid | Horse amino acid | Method | Score | Prediction |
|---|---|---|---|---|---|
| 166 | D | S | PolyPhen-2 | 0.991 | Probably damaging |
| PROVEAN | −1.005 | Neutral | |||
| PANTHER | 220 | Possibly damaging |
Discussion
Since prion-infected cases have never been reported in horse, there are many efforts to identify factors that contribute to the resistance of prion disease. Previous studies concentrated on investigating the structural stability of horse prion protein, which participates in prion disease resistance. Thus, we assumed that genetic polymorphism, one of the factors that can be involved in protein stability, could be a factor for the resistance of prion disease in the horse. To identify genetic polymorphisms in the horse PRNP gene, we performed automatic direct sequencing in 201 Thoroughbred horses. Interestingly, we found only one non-synonymous SNP (N175K) in the ORF of the horse PRNP gene. It is a curious result, because polymorphisms in the PRNP gene have a decisive effect on prion diseases susceptibility, unlike other polygenic diseases. This potent propensity was proven by a genome-wide association study (GWAS) that demonstrated that the PRNP gene has an extraordinarily high P-value [33]. In addition, previously reported prion disease-susceptible animals have numerous prion disease-associated SNPs in their genes. Thus, very the low polymorphic feature of the horse PRNP gene is worth noting. Furthermore, since previous studies indicated that Thoroughbred, which considered very inbred status, has several SNPs in the other gene, thus we presumed that very low polymorphic PRNP gene is not caused by inbred status of Thoroughbred [34,35]. However, study just in inbred species may skew data and limit interpretation. Thus, since outbred wild horses did not inhabit in the South Korea, for exact confirmation, further comparison study of horse PRNP gene is needed in other countries that have wild horses.
Because prion disease-infected cases have not been reported in horse, a case-control study could not be performed in this animal. Thus, we devised two ways to evaluate N175K. First, we drew a schematic map that marked horse-specific structures and interpreted the importance of the N175K location. Second, we performed analyzes using PolyPhen-2, PROVEAN and PANTHER to determine the impact of this SNP on horse prion protein. As illustrated in Figure 3, N175K does not impede two horse-specific extraordinary stable structures, the β2-α2 loop and four salt bridges. In addition, the PolyPhen-2, PROVEAN and PANTHER predictions all suggested that N175K is benign (Table 1). Horse-specific amino acid, S169 participates in composing the highly ordered solution structure of the β2-α2 loop. Since this structure contributes to the stability of horse prion protein, the β2-α2 loop with 169S has been considered to be one factor of resistance to prion disease in horse [9]. In addition, four salt bridges, identified by a previous MD study, take part in inhibiting the formation of the β-intermediate state against changes in pH and temperature. Because the β-intermediate state is postulated to be the middle stage of conformational change from an α-helix to β-sheet, the salt bridges have also been considered to be one factor of resistance to prion disease in horse [10]. An important finding was that although N175K polymorphism has approximately 25% of minor allele frequencies, N175K did not impede these two horse-specific structures, and the SNP was predicted to be benign. Because previous study has reported that horse prion protein has more stable structure than other species, horse prion protein which contains N175K may have stable structure. However, since stability of prion protein did not necessarily correlate with prion disease susceptibility, careful interpretation is needed in this study. Indeed, although G114V, D178N, T183A and E200K found in inherited prion disease were predicted as deleterious by in silico analysis, V180I were predicted as ‘benign’ in previous study [36]. In addition, although PolyPhen-2, PROVEAN and PANTHER can evaluate non-synonymous SNPs based on sequence homology, the alteration of protein structure, and the similarity of previous reported pathogenic mutations, those in silico tools cannot estimate all sides of pathogenicity of non-synonymous SNPs. To evaluate impact of N175K, conventional approach in transgenic mouse model expressing horse prion protein with N175K is necessary in the future.
Next, we thought that the partial horse-specific residues and the SNP could not account for all of the prion disease-resistance in horse. Although prion disease-resistance in horse has been reaffirmed through a recent non-adaptive prion amplification (NAPA) study of horse prion protein transgenic mice [37,38], however, a previous study reported that a mouse that contains mouse prion protein with a horse-specific amino acid (166S) develops a spontaneous form of prion disease [32]. Thus, the impact of horse-specific amino acids were elusive. We investigated the effects of horse-specific amino acids by substitution from horse-specific amino acids to interspecies conserved amino acids on horse prion protein. For this, we aligned the prion protein sequences of seven species and found horse-specific amino acids. After substituting horse-specific amino acids with interspecific conserved amino acids sequentially in horse prion protein, we measured the substitution effect using PolyPhen-2, PROVEAN and PANTHER. Interestingly, the substitution effects of horse-specific amino acids are quite low among all horse-specific residues (Table 2). Next, we assessed horse-specific amino acid D166S (previous referred as D167S) effects on mouse prion protein by using the same experimental design that was previous performed in an in vivo transgenic mouse study [25]. Notably, two programs predicted that D166S has a deleterious effect on mouse prion protein. These results suggest that the effect of amino acid substitutions may be different according to species because of the differences in protein sequence, and there is severe deficiency of functional studies that use mutagenesis and ignore interspecific sequence differences. For analysis of species-specific amino acids, it is necessary to analyze the whole structure of proteins. However, prediction results, ‘deleterious’, are not easily interpretable because the programs provide only comprehensive results based internal algorithms. Because in silico analysis provides just preliminary data of protein alteration impact, to interpret this prediction, introduction of structural mimic or electronic mimic of 166S in transgenic mouse model will helpful to understand the mechanism of 166S induced toxicity. In addition, since PRNP knockout mouse showed relatively normal phenotype, additional functional analysis is needed in paralogs of prion protein such as prion-like protein, Doppel or shadow of prion protein, Shadoo. Furthermore, to thoroughly estimate impact of horse-specific amino acids of prion protein, in vivo experiment using simultaneous substitution of whole horse-specific amino acids is more judicious in the future study.
Collectively, we suggest two major points in this study. First, horse has only one benign SNP in the PRNP gene, which does not impede the horse-specific structure and is considered to participate in prion disease resistance. Second, we assessed the impact of horse-specific amino acids and identified a severe deficiency of mutagenesis studies. We hope that these two new results will provide a promising approach to understanding the mechanisms of prion disease.
Materials and methods
Ethics statement
Whole blood of 201 Thoroughbred horses was provided by the Seoul Race Park in the Republic of Korea. All experimental procedures performed in the present study were approved according to the recommendations of the Guiding Principle for Animal Care and Use Committee of Chonbuk National University (IACUC number: CBNU 2016–65).
Genomic DNA extraction
Genomic DNA was extracted from 200 μl of whole blood using the blood genomic DNA Isolation kit (Qiagen, Valencia, California, USA) following the manufacturer’s instructions.
Genetic analysis
The horse PRNP gene was amplified from the genomic DNA using forward and reverse gene-specific primers. The sequences of the primers were as follows: Horse PRNP-F (AGAAGTGCAGAGTGTGACATGC) and Horse PRNP-R (CAAGCGTATTAGCCTACGGGTG). Polymerase chain reaction (PCR) was performed using GoTaq® DNA Polymerase (Promega, Fitchburg, Wisconsin, USA). The PCR mixture contained 20 pmol of each primer, 5 μl of 10 ×Taq DNA polymerase buffer, 1 μl of 10 mM dNTP mixture and 2.5 units of Taq DNA polymerase. The PCR conditions were 94°C for 2 min to denature; 35 cycles of 94°C for 45 sec, 59°C for 45 sec, and 72°C for 1 min 30 sec; and then 1 cycle of 72°C for 10 min to extend the reaction. PCR was performed by an S-1000 Thermal Cycler (Bio-Rad, Hercules, California, USA). The PCR products were obtained by the PCR Purification Kit (Thermo Fisher Scientific, Bridgewater, New Jersey, USA) and directly sequenced with an ABI 3730 automatic sequencer (ABI, Foster City, California, USA). Sequencing results were read by Finch TV software (Geospiza Inc, Seattle, USA) and genotyping was performed.
Schematic map of horse prion protein
Amino acid sequences of horse prion protein were determined by automatic direct sequencing with genomic DNA samples of 201 Thoroughbred horses in this study. The N-terminal endoplasmic reticulum (ER) signal peptide (residues 1–19) was predicted by the SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP/). The C-terminal glycosylphosphatidylinositol (GPI) signal peptide was marked according to a previous study based on the interspecific homology of the GPI signal peptide sequence (residues 231–255). The secondary structure (α-helix: 145–158, 175–196 and 202–230; β-sheet: 130–133 and 163–166) and β2-α2 loop (residues 167–174) of horse prion protein were determined by a previous nuclear magnetic resonance (NMR) study of horse [9]. Four salt bridges (E198-R158-H189, R158-D204, E213-H179 and D180-R166) were confirmed by a previous MD study of horse [10].
Assessment of protein functional alterations
Possible impacts on horse prion protein induced by the substitution of amino acids were predicted by PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/index.shtml), PROVEAN (http://provean.jcvi.org/seq_submit.php) and PANTHER (http://www.pantherdb.org/) [39–41]. PolyPhen-2 provides a position-specific, independent count (PSIC) score (score ranges from 0.0 to 1.0). The prediction results can be presented as three types: ‘benign’, ‘possibly damaging’ and ‘probably damaging’. PROVEAN is a sequence-based predictor that calculates the impact score of protein sequence polymorphisms on protein function. If the final score is below −2.5, protein variants are predicted to be ‘neutral’; otherwise, if the final score is above −2.5, protein variants are predicted to be ‘deleterious’. PANTHER utilizes a hidden Markov model (HMM) based on statistical modeling methods and multiple sequence alignments to perform evolutionary analysis of coding missense SNPs. PANTHER predicts SNPs as two types: ‘deleterious’ (score < −3) or ‘neutral’ (score > −3).
Sequence alignment and secondary structure prediction of horse prion protein
The sequence alignments were carried out using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalo) and Wasabi (http://wasabiapp.org/). ClustalW2 and Wasabi aligned interspecies prion protein sequences based on progressive alignment construction and phylogeny-aware methods, respectively [42,43]. The assays were performed for prion protein sequences from human, mouse, sheep, goat, dog, rabbit and horse. Prion protein sequences were obtained from GenBank at the National Center for Biotechnology Information (NCBI), including those of human (Homo sapiens, BAG32277.1), mouse (Mus musculus, NP_001265185.1), sheep (Ovis aries, NP_001009481.1), goat (Capra hircus, NP_001301176.1), dog (Canis lupus familiaris, ACO71291.1), rabbit (Oryctolagus cuniculus, NP_001075490.1) and horse (Equus caballus, NP_001137270.2).
Funding Statement
This research was supported by the Basic Science Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2015R1D1A1A010599). This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1A6A1A03015876). Y.C. Kim was supported by the BK21 Plus program in the Department of Bioactive Material Sciences.
Acknowledgments
We wish to thank DVM Young-Bak Lim and Min-Hyun Lee for providing blood samples of Thoroughbred horses.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. PMID: 9811807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang L, Li N, Fan B, et al. PRNP polymorphisms in Chinese ovine, caprine and bovine breeds. Anim Genet. 2004;35:457–461. PMID: 15566469. [DOI] [PubMed] [Google Scholar]
- [3].Baron T, Belli P, Madec JY, et al. Spongiform encephalopathy in an imported cheetah in France. Vet Rec. 1997;141:270–271. PMID: 9316242. [DOI] [PubMed] [Google Scholar]
- [4].Moennig V. [Felinfo glossarium. Mad cow disease in the cat]. Tijdschr Diergeneeskd. 1992;117:412–413. PMID: 1641835. [PubMed] [Google Scholar]
- [5].Willoughby K, Kelly DF, Lyon DG, et al. Spongiform encephalopathy in a captive puma (Felis concolor). Vet Rec. 1992;131:431–434. PMID: 1455592. [DOI] [PubMed] [Google Scholar]
- [6].Jeong BH, Lee KH, Kim NH, et al. Association of sporadic Creutzfeldt-Jakob disease with homozygous genotypes at PRNP codons 129 and 219 in the Korean population. Neurogenetics. 2005;6:229–232. PMID: 16217673. [DOI] [PubMed] [Google Scholar]
- [7].Guiroy DC, Williams ES, Yanagihara R, et al. Immunolocalization of scrapie amyloid (PrP27-30) in chronic wasting disease of rocky mountain elk and hybrids of captive mule deer and white-tailed deer. Neurosci Lett. 1991;126:195–198. PMID: 1681473. [DOI] [PubMed] [Google Scholar]
- [8].Marsh RF, Hadlow WJ. Transmissible mink encephalopathy. Rev Sci Tech. 1992;11:539–550. PMID: 1535524. [DOI] [PubMed] [Google Scholar]
- [9].Perez DR, Damberger FF, Wuthrich K. Horse prion protein NMR structure and comparisons with related variants of the mouse prion protein. J Mol Biol. 2010;400:121–128. PMID: 20460128. [DOI] [PubMed] [Google Scholar]
- [10].Zhang J. The structural stability of wild-type horse prion protein. J Biomol Struct Dyn. 2011;29:369–377. PMID: 21875155. [DOI] [PubMed] [Google Scholar]
- [11].Jeong BH, Kim YS. Genetic studies in human prion diseases. J Korean Med Sci. 2014;29:623–632. PMID: 24851016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jeong BH, Lee KH, Lee YJ, et al. PRNP 1368 polymorphism is not associated with sporadic Creutzfeldt-Jakob disease in the Korean population. Eur J Neurol. 2008;15:846–850. PMID: 18549395. [DOI] [PubMed] [Google Scholar]
- [13].Jeong BH, Nam JH, Lee YJ, et al. Polymorphisms of the prion protein gene (PRNP) in a Korean population. J Hum Genet. 2004;49:319–324. PMID: 15148589. [DOI] [PubMed] [Google Scholar]
- [14].Kim YC, Jeong BH. Lack of germline mutation at codon 211 of the prion protein gene (PRNP) in Korean native cattle.. Acta Vet Hung. 2017;65:147–152. PMID: 28244340. [DOI] [PubMed] [Google Scholar]
- [15].Heaton MP, Keele JW, Harhay GP, et al. Prevalence of the prion protein gene E211K variant in U.S. cattle. BMC Vet Res. 2008;4:25 PMID: 18625065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jeong BH, Jin HT, Carp RI, et al. Bovine spongiform encephalopathy (BSE)-associated polymorphisms of the prion protein (PRNP) gene in Korean native cattle. Anim Genet. 2013;44:356–357. PMID: 23134411. [DOI] [PubMed] [Google Scholar]
- [17].Jeong BH, Lee YJ, Kim NH, et al. Genotype distribution of the prion protein gene (PRNP) promoter polymorphisms in Korean cattle. Genome. 2006;49:1539–1544. PMID: 17426768. [DOI] [PubMed] [Google Scholar]
- [18].Hunter N, Foster JD, Goldmann W, et al. Natural scrapie in a closed flock of Cheviot sheep occurs only in specific PrP genotypes. Arch Virol. 1996;141:809–824. PMID: 8678828. [DOI] [PubMed] [Google Scholar]
- [19].Hunter N, Goldmann W, Foster JD, et al. Natural scrapie and PrP genotype: case-control studies in British sheep. Vet Rec. 1997;141:137–140. PMID: 9280041. [DOI] [PubMed] [Google Scholar]
- [20].Laplanche JL, Chatelain J, Westaway D, et al. PrP polymorphisms associated with natural scrapie discovered by denaturing gradient gel electrophoresis. Genomics. 1993;15:30–37. PMID: 8094373. [DOI] [PubMed] [Google Scholar]
- [21].Ortiz-Pelaez A, Georgiadou S, Simmons MM, et al. Allelic variants at codon 146 in the PRNP gene show significant differences in the risk for natural scrapie in Cypriot goats. Epidemiol Infect. 2015;143:1304–1310. PMID: 25140573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brandt AL, Kelly AC, Green ML, et al. Prion protein gene sequence and chronic wasting disease susceptibility in white-tailed deer (Odocoileus virginianus). Prion. 2015;9:449–462. PMID: 26634768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Johnson C, Johnson J, Vanderloo JP, et al. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. J Gen Virol. 2006;87:2109–2114. PMID: 16760415. [DOI] [PubMed] [Google Scholar]
- [24].Johnson CJ, Herbst A, Duque-Velasquez C, et al. Prion protein polymorphisms affect chronic wasting disease progression. PLoS One. 2011;6:e17450 PMID: 21445256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jewell JE, Conner MM, Wolfe LL, et al. Low frequency of PrP genotype 225SF among free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. J Gen Virol. 2005;86:2127–2134. PMID: 16033959. [DOI] [PubMed] [Google Scholar]
- [26].Monello RJ, Galloway NL, Powers JG, et al. Pathogen-mediated selection in free-ranging elk populations infected by chronic wasting disease. Proc Natl Acad Sci U S A. 2017;114:12208–12212. PMID: 29087314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Perucchini M, Griffin K, Miller MW, et al. PrP genotypes of free-ranging wapiti (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol. 2008;89:1324–1328. PMID: 18420812. [DOI] [PubMed] [Google Scholar]
- [28].Kim YC, Jeong BH. Bovine spongiform encephalopathy (BSE) associated polymorphisms of the prion-like protein gene (PRND) in Korean dairy cattle and Hanwoo. J Dairy Res. 2018;85:7–11. PMID: 29468989. [DOI] [PubMed] [Google Scholar]
- [29].Kim YC, Jeong BH. The first report of prion-related protein gene (PRNT) polymorphisms in goat. Acta Vet Hung. 2017;65:291–300. PMID: 28605958. [DOI] [PubMed] [Google Scholar]
- [30].Kim YC, Jeong BH. First report of prion-related protein gene (PRNT) polymorphisms in cattle. Vet Rec. 2018;182:717 PMID: 29666222. [DOI] [PubMed] [Google Scholar]
- [31].Kim YC, Jeong MJ, Jeong BH. The first report of genetic variations in the chicken prion protein gene. Prion. 2018;In Press PMID: 29966485 DOI: 10.1080/19336896.2018.1471922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sigurdson CJ, Joshi-Barr S, Bett C, et al. Spongiform encephalopathy in transgenic mice expressing a point mutation in the beta2-alpha2 loop of the prion protein. J Neurosci. 2011;31:13840–13847. PMID: 21957246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mead S, Uphill J, Beck J, et al. Genome-wide association study in multiple human prion diseases suggests genetic risk factors additional to PRNP. Hum Mol Genet. 2012;21:1897–1906. PMID: 22210626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hirota K, Kakoi H, Gawahara H, et al. Construction and validation of parentage testing for thoroughbred horses by 53 single nucleotide polymorphisms. J Vet Med Sci. 2010;72:719–726. PMID: 20124759. [DOI] [PubMed] [Google Scholar]
- [35].Rooney MF, Porter RK, Katz LM, et al. Skeletal muscle mitochondrial bioenergetics and associations with myostatin genotypes in the Thoroughbred horse. PLoS One. 2017;72:719–726. PMID: 29190290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jahandideh S, Zhi D. Systematic investigation of predicted effect of nonsynonymous SNPs in human prion protein gene: a molecular modeling and molecular dynamics study. J Biomol Struct Dyn. 2014;32:289–300. PMID: 23527686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Christen B, Hornemann S, Damberger FF, et al. Prion protein NMR structure from tammar wallaby (Macropus eugenii) shows that the beta2-alpha2 loop is modulated by long-range sequence effects. J Mol Biol. 2009;389:833–845. PMID: 19393664. [DOI] [PubMed] [Google Scholar]
- [38].Bian J, Khaychuk V, Angers RC, et al. Prion replication without host adaptation during interspecies transmissions. Proc Natl Acad Sci U S A. 2017;114:1141–1146. PMID: 28096357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. PMID: 20354512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Choi Y, Chan AP. PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31:2745–2747. PMID: 25851949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Thomas PD, Campbell MJ, Kejariwal A, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res . 2003;13:2129–2141. PMID: 12952881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 2016;11:4673–4680. PMID: 7984417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kauff F, Cox CJ, Lutzoni F. WASABI: an automated sequence processing system for multigene phylogenies. Syst Biol. 2007;56:523–531. PMID: 17562476. [DOI] [PubMed] [Google Scholar]


