ABSTRACT
Sporadic Creutzfeldt-Jakob disease (sCJD) is a fatal progressive neurodegenerative disease. Multimodal approaches, including electroencephalogram, diffusion-weighted imaging (DWI) of brain MRI, and cerebrospinal fluid biomarkers, have been applied to increase the diagnostic accuracy of sCJD. Although previous studies suggested DWI could be the most useful modality for sCJD diagnosis, whether metabolism changes underlying in sCJD are still poorly understood. To the best of our knowledge, there are only one case using the technique of arterial spin labeling (ASL) to detection and follow-up of perfusion changes in CJD. Herein, we described a 71-year-old woman presented with progressive cognitive decline, behavioral and psychological symptoms for two months. The patient died one month later after her admission. As far as we know, this is the first report using the combination of fluorodeoxyglucose positron emission tomography and ASL to explore the metabolism changes in sCJD. Our case exemplifies the difficulty clinicians may face in the diagnosis of sCJD.
KEYWORDS: Creutzfeldt-Jakob disease, prions, rapidly progressive dementia, positron emission tomography, arterial spin labeling
Introduction
Prion disease is a rare and fatal neurodegenerative disorder that affects mammals and humans, with a mortality rate of 100%. Sporadic Creutzfeldt-Jakob disease (sCJD) is the most common form of prion disease [1]. It is generally characterized by rapidly progressing dementia (RPD), behavioral and psychiatric disorders, cerebellar ataxia, vision disturbances, akinetic mutism state and myoclonus [2]. To date, the multimodal imaging studies such as diffusion-weighted imaging (DWI) hyperintensity, electroencephalography (EEG), and cerebrospinal fluid (CSF) biomarkers are utilized in conjunction with the clinical picture to try to make the diagnosis of CJD without brain biopsy.
Although previous studies suggested DWI could be the most useful modality for sCJD diagnosis, whether some metabolism changes underlying in sCJD are still poorly understood. Only quite a few studies using the 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) to investigate the metabolism changes in sCJD [3]. To the best of our knowledge, there are only one case using the technique of arterial spin labeling (ASL) to detection and follow-up of perfusion changes in CJD [4]. We herein described a 71-year-old woman presenting with typical clinical features, the findings of MRI, triphasic morphology of EEG and positive 14–3-3 proteins in CSF. As far as we know, this is the first report using the combination of FDG-PET and ASL to explore the metabolism changes in sCJD.
Case
A 71-year-old woman presented with progressive cognitive decline, behavioral and psychological symptoms such as visual hallucinations for two months. Initial neurological examination revealed rapidly progressive dementia, ataxia, tremor and involuntary movements, myoclonus, visual neglect, mutism, startle myoclonus and dystonia, urinary incontinence. The biomarkers of autoimmune encephalitis were unremarkable. The 14–3-3 proteins in CSF were positive. Cranial MRI indicated diffusion restriction revolved in entire cortex more on the right, presenting with hyperintensive signal in the cortex (Figure 1A). The EEG showed periodic discharges over bilateral hemisphere with triphasic morphology (Figure 1B). The findings of FDG-PET (Figure 2) and ASL (Figure 3) both showed extensive hypoperfusion in bilateral cortex, thalamus and cerebellum, especially lateralized to the left hemisphere. The patient died three months after the initial onset of symptoms. The patient had no risk factors for familial, new variant, or iatrogenic CJD. sCJD was the final diagnosis.
Figure 1.
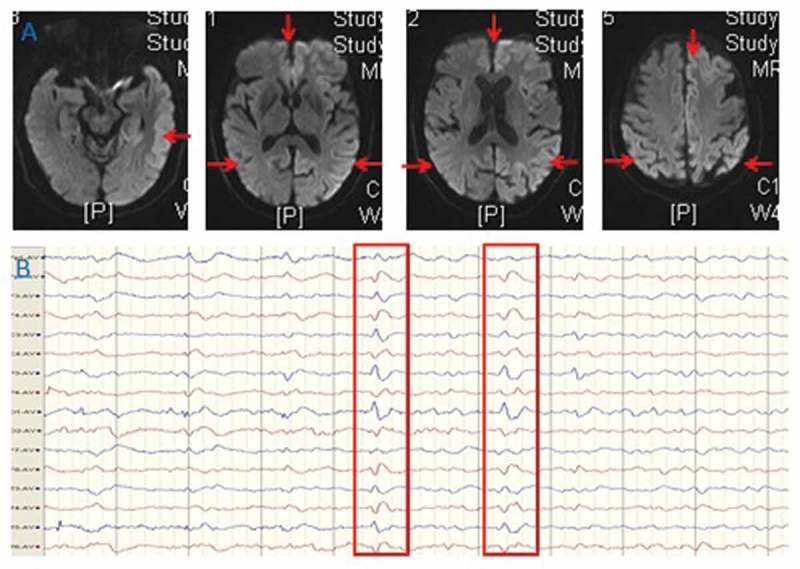
MRI (A) findings of bilateral cortical diffusion restriction presenting with hyperintensive signal in the cortex on diffusion weighted imaging. EEG (B) showed slowing and periodic discharges over bilateral hemisphere with triphasic morphology.
Figure 2.
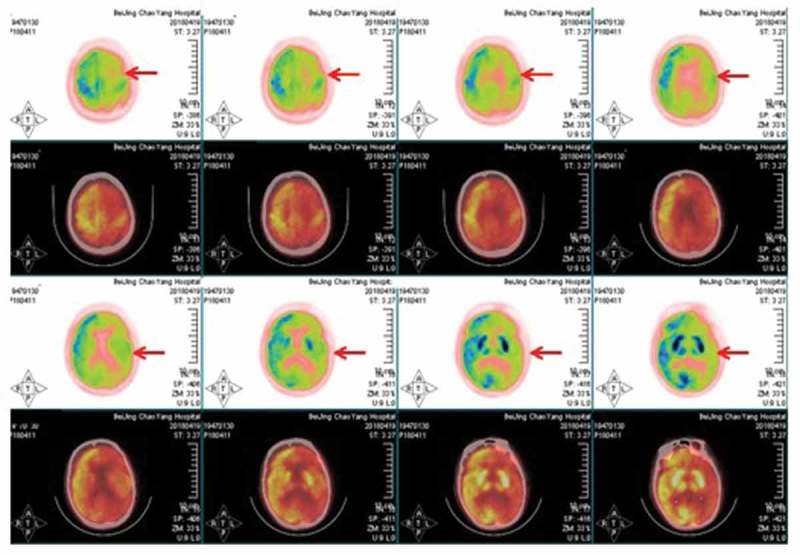
FDG-PET showed hypoperfusion in bilateral cortex, thalamus and cerebellum, especially lateralized to the left hemisphere.
Figure 3.
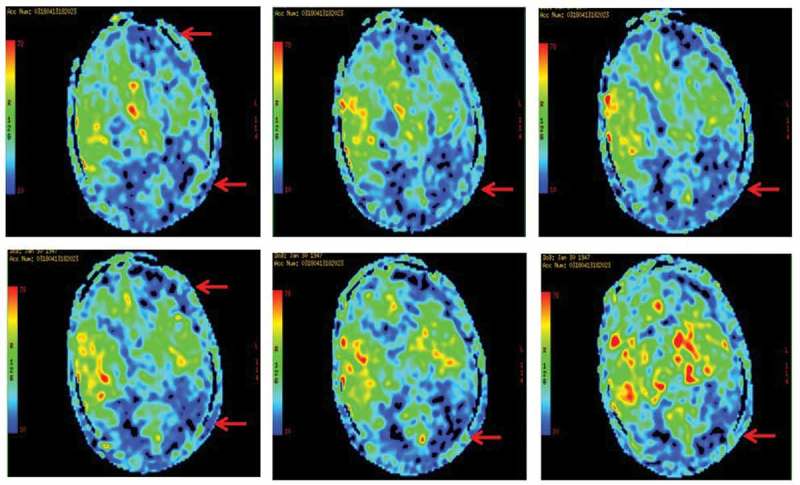
ASL indicated severe extensive hypoperfusion in the cerebral cortex, basal ganglia, especially lateralized to the left hemisphere.
Discussion
sCJD is a prion disease consequent to accumulation of misfolded pathogenic prion protein in the brain. As an untreatable prion encephalopathy, early and accurate diagnosis of sCJD is essential to avoid iatrogenic transmission and to distinguish CJD from other potentially treatable dementia [5]. Common presenting symptoms are RPD, behavioral abnormalities, mood disturbance, sleep disorders, startle myoclonus, extra-pyramidal signs and cerebellar abnormalities. Current clinical diagnostic criteria mostly depend on clinical features and periodic sharp-wave complexes on EEG, DWI hyperintensity, and the presence of 14–3-3 in CSF. Although DWI could be the most useful modality for the diagnosis of sCJD [6,7], however, the gold standard for definitive diagnosis is still considered to be histopathological confirmation. Therefore, the diagnosis of sCJD is often challenging in clinical practice.
Supportive investigations such as EEG, MRI and CSF biomarkers have a relatively low diagnostic sensitivity and specificity in sCJD. Considering to brain metabolism, however, a recent study indicated the [18F]-AV-1451 PET was not suitable for patients with RPD due to sCJD, due to different pathological process [8]. As for the technique of FDG-PET, it has an established role in the diagnostic workup of patients with sCJD [9,10]. We have summarized a table showing the changes in FDG-PET in sCJD patients reported from literature (Table 1). To date, there are only one case using ASL to detection and follow-up of perfusion changes in CJD [4]. In this study, ASL showed severe hypoperfusion in the cerebral cortex, basal ganglia and thalami but this was least marked in the thalami [4]. However, the combination of FDG-PET and ASL is quite rare. To the best of our knowledge, our study is the first case using combined FDG-PET and ASL to explore the metabolism changes in patients with sCJD.
Table 1.
The changes in FDG-PET in sCJD patients reported from literature.
| Literature | Population | Number/Sex | Age range | Clinical features | FDG-PET findings |
|---|---|---|---|---|---|
| Henkel K,2002 [11] | Germany | 8 (5F 3M) | 35–78 years | dementia, cerebellar, myoclonus, pyramidal/extrapyramida, visual, speech | spread hypometabolism in CJD, with a reduction of cerebral glucose metabolism in at least one temporal or parietal region |
| Engler H,2003[12] | Sweden | 9(5F 4M) | 56 ± 8 years | dementia, aphasia, apraxia, ataxia, myoclonus, rigidity, chorea, akinetic mutism, pyramidal symptoms | pronounced regional decrease in glucose brain metabolism, which were most pronounced in the cerebellum and the frontal, occipital and parietal cortices |
| Hamaguchi T,2005[13] | Japan | 8(4F 4M) | 30–75 years | dementia, ataxia, and pyramidal and extrapyramidal signs | thalamic hypoperfusion or hypometabolism on cerebral blood flow |
| Okamura N,2010 [14] | Japan | 2(1F 1M) | 58–63 years | subacutely progressive dementia, gait disturbance, psychotic symptoms, dysarthria and myoclonus | no obvious retention of BF-227 with positron emission tomography |
| Zhang WJ,2011 [15] | USA | 1F | 72 year | falls, ataxia, bizarre behavior and rapid cognitive deterioration | basal ganglia and patchy cortical hypometabolism |
| Zhang Y,2012 [16] | USA. | 1F | 58 year | left-sided decreased sensation, ataxia, and left “alien” hand and leg. | hypoperfusion and hypometabolism in the right frontoparietal cortices, including the primary sensorimotor cortex, and temporal cortex |
| Xing XW,2012 [17] | China | 14 (6F 8M) | 36–68 years | NA | hypometabolism was observed in the basal ganglia in 12 patients (85.71%), and 8 patients (57.14%) had decreased FDG uptake in the thalamic nuclei on PET scans |
| Kim EJ, 2012 [18] | Korea | 11 (6F 5M) | 36–75 years | gait disturbance, visual disturbance, obtundation, dysarthria, memory impairment, tremor, dementia | decreased glucose metabolism in bilateral parietal, frontal, and occipital cortices. |
| Engler H, 2012 [19] | Uruguay | 1M | 64 year | dizziness, memory problems, disorientated, visual hallucinations, speech difficulties, ataxia, myoclonic jerks | extreme low glucose uptake in the left brain hemisphere |
| Zhao W,2013 [20] | China | 57(24F33M) | 36–75 years | sub-acute onset with progressive dementia | abnormalities of hypo-metabolism in the cerebral cortex were clearly detected by PET. |
| Renard D,2013 [3] | Belgium | 9 (4 F 5M) | 52–86 years | rigidity and dystonia, shuffling gait, postural tremor, Shuffling gait, monotone speech, chorea, bradykinesia | hypometabolism in the medial parietal cortex bilaterally, and in the left-sided lateral and medial frontal and the lateral parietal cortex |
| Furukawa F,2014 [21] | Japan | 1 M | 52 year | gait instability, gait unsteadiness, cognitive impairment, dysarthria and hallucinations | low glucose metabolism in the thalami and cerebellar cortices |
| Euskirchen P,2014 [22] | Germany | 1F | 65 year | gait disturbance and vertigo, difficulties to speak and write | reduced FDG metabolism in the basal ganglia with spatial correlation to MR lesions |
| Ortega-Cubero S, 2015 [23] | Spain | 9 (2M7F) | 39–77 years | psychological disorder, cognitive impairment, cerebellar syndrome | characterised by a cortical-subcortical pattern |
| Prieto E,2015 [24] | Spain | 12 (8F 4M) | 39–77 years | psychiatric symptoms, ataxia, cognitive decline, vestibular symptoms | hypometabolism that affected both subcortical (bilateral caudate, thalamus) and cortical (frontal cortex) structures |
| Matias-Guiu JA,2017 [10] | Spain | 1 M | 80 year | gait disturbance, unsteadiness and weakness of right limbs, disturbance in smooth pursuit and saccadic ocular movements, mild hemiparesis and bilateral ataxia, spontaneous and action myoclonus | large regions of hypometabolism including left frontoparietal lobes as well as bilateral thalamus |
| Miyazawa N,2017 [25] | Japan | 1M | 78 year | memory impairment, severe aphasia and apraxia. | hypometabolism in the posterior cingulate and precuneus cortex, and putamen and caudate nucleus |
| Miyazawa N,2017 [25] | Japan | 1F | 68 year | myoclonus and disturbance of consciousness | hypometabolism in the same locations as the foci on MRI and in the cerebellum. |
| Renard D,2017 [26] | France | 15 (10F 5M) | 52–86 years | cerebellar ataxia, visual signs, pyramidal signs, myoclonus, limb apraxia, limb dystonia, sensory loss, parkinsonism, corticobasal syndrome | entire CJD group showed lateralized frontal and parietal hypometabolism |
| Mente KP, 2017 [27] | USA | 8 (4F 4M) | 2–32 years | dementia, insomnia, myoclonus, pyramidal ataxia, visual, behavior, extrapyramidal | (1) hypometabolism was found in a few regions, most frequently in the posterior cingulate gyrus and precuneus, both of which are in the parietal lobe. (2) hypermetabolism was identified in the limbic and mesolimbic structures, including insula, medial temporal lobe, nucleus accumbens, olfactory cortex, and parahippocampal gyrus. |
| Duignan J,2018 [28] | Ireland | 1F | 71 year | cognitive decline, progressive dysphasia and visual hallucinations. | hypometabolism in the temporal and parietal lobes with involvement of the posterior cingulate gyrus and precuneus bilaterall |
sCJD, sporadic Creutzfeldt-Jakob disease. FDG-PET, fluorodeoxyglucose positron emission tomography. NA, not available.
The strengths of our case were listed as follows. Firstly, our case was typical for sCJD due to very rapid onset of cognitive deficits, myoclonus, ataxia, visual neglect, and tremor. Brain imaging, CSF biomarkers, and neurophysiological findings also revealed typical findings for sCJD as well. Although sCJD and dementia with Lewy bodies (DLB) have overlapping clinical symptoms, however, the given this patient’s clinical presentation was more consistent with CJD than DLB. As a result, our case exemplifies the difficulty clinicians may face in the diagnosis of sCJD. Secondly, in spite that the features of DWI have been pivotal in the diagnostic workup of sCJD and to be the best predictor for sCJD [7,11], however, due to the complementary advantages, the combined techniques of PET and ASL may give us some more important information to detect the metabolism changes underlying in sCJD. Thus, our findings of perfusion changes such as PET and ASL have been proved to be more promising values in clinical practice [12]. As far as we know, this is the first report using the combination of FDG-PET and ASL to explore the metabolism changes in sCJD. Thirdly, our study provides an update on the neuroimaging patterns of sCJD, emphasizing the relevance of MRI and PET/ASL, and highlighting clinical, genetic, and imaging correlations in sCJD. Although CJD is typically associated with diffuse cerebral pathology, our case also conform it could also present with unilateral neurologic symptoms and concordant MRI and EEG findings [13].
Conclusion
Herein, we reported a sCJD with the typical clinical presentations, neuroimaging features, triphasic morphology of EEG, positive 14–3-3 proteins in CSF, also with the evidences of hypometabolism using PET and ASL. One of the limitation was the histopathology was not available in our case. Novel ultrasensitive seeding assays such as the real-time quaking-induced conversion of CSF could provide promising diagnostic information than tests for surrogate markers of sCJD in CSF in future study.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81301016) and the Beijing Municipal Administration of Hospitals’ Youth Programme (QML20150303).
Disclosure statement
No potential conflicts of interest are disclosed
References
- [1].Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus. 2015;39(5):E2. [DOI] [PubMed] [Google Scholar]
- [2].Iwasaki Y. Creutzfeldt-Jakob disease. Neuropathology. 2017;37(2):174–188. [DOI] [PubMed] [Google Scholar]
- [3].Renard D, Vandenberghe R, Collombier L, et al. Glucose metabolism in nine patients with probable sporadic Creutzfeldt-Jakob disease: FDG-PET study using SPM and individual patient analysis. J Neurol. 2013;260(12):3055–3064. [DOI] [PubMed] [Google Scholar]
- [4].Chen S, Guan M, Shang JK, et al. Reduced cerebral blood flow in genetic prion disease with PRNP D178N-129M mutation: an arterial spin labeling MRI study. J Clin Neurosci. 2015;22(1):204–206. [DOI] [PubMed] [Google Scholar]
- [5].Bizzi A, Peoc’h K. Amended diagnostic protocol increases the early diagnosis of sporadic Creutzfeldt-Jakob disease. Neurology. 2018;91:155–156. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [6].Suzuki K, Kawasaki A, Nagashima T, et al. Diffusion-weighted MRI abnormalities antedate the onset of sporadic Creutzfeldt-Jakob disease. Neurology. 2016;87(8):843–845. [DOI] [PubMed] [Google Scholar]
- [7].Forner SA, Takada LT, Bettcher BM, et al. Comparing CSF biomarkers and brain MRI in the diagnosis of sporadic Creutzfeldt-Jakob disease. Neurol Clin Pract. 2015;5(2):116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Day GS, Gordon BA, Perrin RJ, et al. In vivo [(18)F]-AV-1451 tau-PET imaging in sporadic Creutzfeldt-Jakob disease. Neurology. 2018;90(10):e896–e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Duignan J, Healy GM, Hughes NM, et al. FDG-PET diagnoses of sporadic Creutzfeldt-Jakob disease: radiology- Pathology correlation. Qjm. 2018. [Epub ahead of print] DOI: 10.1093/qjmed/hcy071 [DOI] [PubMed] [Google Scholar]
- [10].Matias-Guiu JA, Guerrero-Marquez C, Cabrera-Martin MN, et al. Amyloid- and FDG-PET in sporadic Creutzfeldt-Jakob disease: correlation with pathological prion protein in neuropathology. Prion. 2017;11(3):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Henkel K, Zerr I, Hertel A, et al. Positron emission tomography with [(18)F]FDG in the diagnosis of Creutzfeldt-Jakob disease (CJD). J Neurol. 2002;249(6):699–705. [DOI] [PubMed] [Google Scholar]
- [12].Engler H, Lundberg PO, Ekbom K, et al. Multitracer study with positron emission tomography in Creutzfeldt-Jakob disease. Eur J Nucl Med Mol Imag. 2003;30(1):85–95. [DOI] [PubMed] [Google Scholar]
- [13].Hamaguchi T, Kitamoto T, Sato T, et al. Clinical diagnosis of MM2-type sporadic Creutzfeldt-Jakob disease. Neurology. 2005;64(4):643–648. [DOI] [PubMed] [Google Scholar]
- [14].Okamura N, Shiga Y, Furumoto S, et al. In vivo detection of prion amyloid plaques using [(11)C]BF-227 PET. Eur J Nucl Med Mol Imag. 2010;37(5):934–941. [DOI] [PubMed] [Google Scholar]
- [15].Zhang WJ, Westover MB, Keary CJ. Premortem diagnosis of sporadic Creutzfeldt-Jakob disease aided by positron-emission tomography imaging. AJNR Am J Neuroradiol. 2011;32(1):E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang Y, Minoshima S, Vesselle H, et al. A case of Creutzfeldt-Jakob disease mimicking corticobasal degeneration: FDG PET, SPECT, and MRI findings. Clin Nucl Med. 2012;37(7):e173–175. [DOI] [PubMed] [Google Scholar]
- [17].Xing XW, Zhang JT, Zhu F, et al. Comparison of diffusion-weighted MRI with 18F-fluorodeoxyglucose-positron emission tomography/CT and electroencephalography in sporadic Creutzfeldt-Jakob disease. J Clin Neurosci. 2012;19(10):1354–1357. [DOI] [PubMed] [Google Scholar]
- [18].Kim EJ, Cho SS, Jeong BH, et al. Glucose metabolism in sporadic Creutzfeldt-Jakob disease: a statistical parametric mapping analysis of (18) F-FDG PET. Eur J Neurol. 2012;19(3):488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nennesmo I, Kumlien E, et al. Imaging astrocytosis with PET in Creutzfeldt-Jakob disease: case report with histopathological findings. Int J Clin Exp Med. 2012;5(2):201–207. [PMC free article] [PubMed] [Google Scholar]
- [20].Zhao W, Zhang JT, Xing XW, et al. Chinese specific characteristics of sporadic Creutzfeldt-Jakob disease: a retrospective analysis of 57 cases. PLoS One. 2013;8(3):e58442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Furukawa F, Ishibashi S, Sanjo N, et al. Serial magnetic resonance imaging changes in sporadic Creutzfeldt-Jakob disease with valine homozygosity at codon 129 of the prion protein gene. JAMA Neurol. 2014;71(9):1186–1187. [DOI] [PubMed] [Google Scholar]
- [22].Euskirchen P, Buchert R, Koch A, et al. Sporadic Creutzfeldt-Jakob disease with mesiotemporal hypermetabolism. J Neurol Sci. 2014;345(1–2):278–280. [DOI] [PubMed] [Google Scholar]
- [23].Ortega-Cubero S, Pagola I, Luquin MR, et al. Clinical and neuroimaging characteristics of 14 patients with prionopathy: a descriptive study. Neurologia. 2015;30(3):144–152. [DOI] [PubMed] [Google Scholar]
- [24].Prieto E, Dominguez-Prado I, Riverol M, et al. Metabolic patterns in prion diseases: an FDG PET voxel-based analysis. Eur J Nucl Med Mol Imag. 2015;42(10):1522–1529. [DOI] [PubMed] [Google Scholar]
- [25].Miyazawa N. Creutzfeldt-Jakob disease mimicking alzheimer disease and dementia with lewy bodies-findings of FDG PET with 3-dimensional stereotactic surface projection. Clin Nucl Med. 2017;42(5):e247–e248. [DOI] [PubMed] [Google Scholar]
- [26].Renard D, Castelnovo G, Collombier L, et al. FDG-PET in Creutzfeldt-Jakob disease: analysis of clinical-PET correlation. Prion. 2017;11(6):440–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mente KP, O’Donnell JK, Jones SE, et al. Fluorodeoxyglucose positron emission tomography (FDG-PET) correlation of histopathology and MRI in prion disease. Alzheimer Dis Assoc Disord. 2017;31(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fragoso DC, Goncalves Filho AL, Pacheco FT, et al. Imaging of Creutzfeldt-Jakob disease: imaging patterns and their differential diagnosis. Radiographics. 2017;37(1):234–257. [DOI] [PubMed] [Google Scholar]
- [29].Yung B, Moeller JJ, Bitar M, et al. Teaching neuroimages: hemispheric-onset Creutzfeldt-Jakob disease with concordant MRI and EEG findings. Neurology. 2010;75(16):e66. [DOI] [PubMed] [Google Scholar]


