Abstract
Purpose/Objective:
To assess the safety and efficacy of upfront treatment using bortezomib in combination with standard radiation therapy (RT) and temozolomide, followed by adjuvant bortezomib and temozolomide for up to 24 cycles in patients with newly-diagnosed glioblastoma multiforme (GBM).
Patients and Methods:
Twenty-four newly-diagnosed GBM patients were enrolled. Patients received standard external beam regional RT with concurrent temozolomide commencing 3–6 weeks after surgery, followed by adjuvant temozolomide and bortezomib for up to 24 cycles or until tumor progression. During RT, bortezomib was given at 1.3 mg/m2 on days 1, 4, 8, 11, 29, 32, 36, and 39. Post RT, bortezomib was given at 1.3 mg/m2 on days 1, 4, 8, and 11 every 4 weeks.
Results:
No unexpected adverse events occurred from the addition of bortezomib. Efficacy analysis showed median progression free survival (PFS) of 6.2 months (95% CI, 3.7–8.8) with promising PFS rates at 18 months and beyond compared to historical norms (25.0% at 18 and 24 months, 16.7% at 30 months). In terms of overall survival (OS), the median OS was 19.1 months (95% CI, 6.7–31.4) with improved OS rates at 12 months and beyond (87.5% at 12, 50.0% at 24, 34.1% at 36 throughout 60 months) compared to historical norms. Median PFS was 24.7 months (95% CI 8.5–41.0) in 10 MGMT methylated and 5.1 months (95% CI 3.9–6.2) in 13 unmethylated patients. The estimated median OS was 61 months in the methylated (the upper bound of 95% CI could not be reached) and 16.4 months (95% CI 11.8–21.0) in the unmethylated patients.
Conclusion:
Addition of bortezomib to current standard radio-chemotherapy in newly-diagnosed GBM patients was tolerable. The PFS and OS rates appeared promising with more benefit to MGMT methylated patients. Further clinical investigation is warranted in a larger cohort of patients.
Keywords: bortezomib, glioblastoma, radiotherapy, temozolomide, MGMT methylation
Introduction
Glioblastoma multiforme (GBM) is the most common and aggressive primary malignant brain tumor in adults. Despite current standard treatment consisting of maximal tumor resection followed by radiation and temozolomide, the prognosis remains dismal for GBM patients receiving standard treatment with median overall survival of 14.6–16.1 months and 2-year survival rate is 26.5–30.1% (1–3).
Bortezomib is an FDA approved proteasome inhibitor indicated for multiple myeloma and mantle cell lymphoma, and has been investigated in various solid tumors as single agent or in combination (4). The ubiquitin proteasome system controls universal cellular function for protein degradation and is essential in regulation of development, differentiation, cell proliferation, signal transduction and apoptosis (4, 5). The mechanism of action of bortezomib was suggested through suppression of NFκB signaling pathway (4, 5). Bredel et al. reported NFκBIA deletion as a negative prognostic marker in GBM patients without EGFR amplification, proposing that NFκB inhibition by bortezomib may be beneficial for GBM patients (6). Preclinical studies have found that bortezomib caused growth arrest of human GBM cell lines and GBM explants via NFκB inhibition (7, 8). In a phase I clinical trial in patients with various central nervous system malignancies, Kubicek et al. have demonstrated that the combination of bortezomib and concurrent temozolomide and radiotherapy was well tolerated (9).
To investigate whether adding bortezomib to radiation and temozolomide can be tolerated and can improve efficacy in newly-diagnosed GBM, we combined bortezomib with standard treatment of radiation with concurrent temozolomide followed by adjuvant temozolomide. Here we report safety and efficacy data of this phase II trial.
Patients and Methods
Patient eligibility
The institutional review boards of the University of California – Los Angeles and Columbia University approved the protocol. All patients signed the approved informed consent form.
Eligible patients must be 18 years or older with the following criteria: Karnofsky performance status (KPS) of 60 or higher, newly-diagnosed GBM without prior therapy other than surgery, adequate bone marrow function (WBC > 3,000/µl, ANC > 1,500/mm3, platelet count of >100,000/mm3, and hemoglobin > 10 gm/dl), liver function (SGOT < 2.5 times and bilirubin < 1.5 times of their upper limit of normal values), and renal function (creatinine < 1.5 mg/dL), without other concurrent serious medical or psychiatric illnesses. Treatment began between two to six weeks from surgery. Patients were required to have 400 mg or more frozen tumor tissue at the time of the surgery. Therefore, patients who had biopsy were excluded unless they underwent another surgery and obtained enough frozen tissue. Patients were excluded if they had a history of New York Heart Association class III or IV heart failure, uncontrolled angina, clinically significant abnormality on electrocardiogram, myocardial infarction or stroke within 6 months of enrollment, or if they were pregnant, received Gliadel wafer during the surgery, had a clinically significant peripheral neuropathy or positive hepatitis B surface antigen or active hepatitis C infection. Patients who were diagnosed or received treatment for another malignancy within 3 years of enrollment were also excluded, except for patients with history of complete resection of basal cell carcinoma or squamous cell carcinoma of the skin, an in situ malignancy, or low-risk prostate cancer after curative therapy.
Treatment plan (Figure 1.)
Figure 1. Treatment and Evaluation Plan.
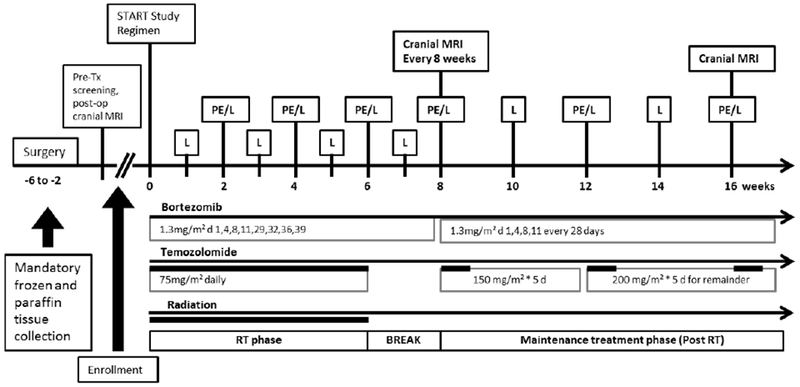
Study Regimen: Bortezomib is given intravenously/subcutaneously at 1.3 mg/ m2 on days 1, 4, 8, 11, 29, 32, 36, and 39 during radiation as early as 14 days after surgery. Temozolomide is given daily (75mg/m2) during radiation, followed by 5 days out of 28 with a dosage of 150–200 mg/m2, for up to 24 cycles. Bortezomib at 1.3 mg/m2 is given on days 1, 4, 8, and 11 of each subsequent 28 day cycle. Both bortezomib and temozolomide will continue until progression or up to 24 cycles.
Evaluation (L: Laboratory testing, PE: physical examination)
PE/L: physical examination on day 28 of every cycle and at study discontinuation. Laboratory blood testing weekly during radiation and every cycle thereafter.
MRI: baseline screening within 21 days of day 1; then at day 56; then every 2 cycles (56 days +/− 3 days)
Radiation therapy phase (RT phase):
During the RT phase, patients were treated with radiation for 6 weeks which started within 3 to 6 weeks from surgery. Radiotherapy was given by external beam in daily fractions of 2.0 Gy to a planned total dose of 60.0 Gy to the tumor (10).
The initial target volume was the contrast-enhancing lesion and surrounding edema seen on MRI/CT with a 2 cm margin. If there was no edema present, then a 2.5 cm margin was used. After 46 Gy in 23 fractions, a cone-down tumor volume, which consisted of the contrast enhancing lesion (without edema) on the pre-operative scan with a 2.5 cm margin, was treated with an addition of 14 Gy in 7 fractions.
The gross tumor volume (GTV) for the initial target volume (GTV1) was the contrast-enhancing lesion plus surrounding edema if it exists. The initial CTV (CTV1) was a 2 cm margin if edema was present and 2.5 cm margin if there was no edema. The initial PTV (PTV1) was 3–5 mm. The GTV for the boost (GTV2) was the contrast enhancing lesion with a 2.5 cm CTV2 and a 3–5 mm PTV2.
The inhomogeneity across the target volume was kept to a minimum. The minimum dose to the target volume was kept within 5% of the dose at the center of the volume. The maximum dose was no higher than 5% of the dose at the center of the target volume. Of note, several patients received 1.8 Gy fractions to a total dose of 59.4 Gy.
During RT phase, patients received oral temozolomide at 75 mg/m2 daily for 42 days and bortezomib injection at 1.3 mg/m2 on days 1, 4, 8, 11, 29, 32, 36 and 39. Bortezomib was initially given via intravenous (IV) infusion, but the protocol was later modified to subcutaneous (SC) injection at ~10 months after the first patient had begun treatment (e.g., August 2012), due to the reported similar efficacy with improved safety profile (11, 12).
Maintenance treatment phase (post-RT phase):
Three to six weeks after completion of RT, patients began the maintenance phase of temozolomide with bortezomib, given in a 28-day cycle for up to 24 cycles if tolerated and progression-free. Oral temozolomide was given at 150 mg/m2/day on days 1–5 of 28-day cycle for the first cycle. If the patient tolerated this dosing, subsequent cycles were increased to 200 mg/m2/day for five days every 28 days.
Bortezomib was given at 1.3 mg/m2 IV/SC on days 1, 4, 8, and 11 of 28-day cycles. Patients 2 and 3 received bortezomib via IV infusion only, patients 7 to 24 received bortezomib via SC injection only, and patients 1, 4, 5 and 6 started with IV bortezomib infusions and switched to SC injection.
Evaluation (Figure. 1)
A pre-treatment evaluation which included a complete history, physical, vital signs, and neurological examinations, as well as brain magnetic resonance imaging (MRI) with and without gadolinium contrast was performed within 21 days prior to treatment initiation. Complete blood count, serum chemistries, coagulation tests, and urinalysis were obtained within 14 days prior to enrollment. Serum pregnancy tests were performed for women of childbearing potential. Biomarker profile including MGMT methylation and IDH1/IDH2 mutation status were examined.
During the RT phase, complete blood count was acquired weekly. Serum chemistries, general physical examinations, and neurological exams were performed biweekly. During maintenance treatment phase with temozolomide and bortezomib, complete blood count and serum chemistries were obtained on days 21 and 28 of each 28-day cycle. Physical and neurological exams were performed every 4 weeks for one year, and every 8 weeks thereafter. Brain MRI with and without gadolinium contrast was performed two weeks after radiation completion and then every 8 weeks thereafter.
Assessment of response and toxicity
RANO (Response Assessment in Neuro-Oncology) criteria were applied to evaluate imaging response using brain MRI with and without contrast (13). Adverse events were graded based on National Cancer Institute Common Toxicity Criteria (Version 3.0).
All patients were followed for progression free survival and overall survival data. One patient was lost to follow up after 20 months on trial.
Statistical analysis
The primary endpoint was overall survival (OS). The secondary endpoints include safety and progression free survival (PFS). The initial sample size calculation for this one-arm trial was based on an endpoint of an 18-month survival rate with a 36-month accrual period (1–2 patients/month). The following assumptions were made: a) survival time followed an exponential distribution; b) the 18-month survival rate was 55% for this new treatment and 40% for the standard treatment (1); c) the 18-month survival rate was estimated using the Kaplan-Meier method; and d) one side Z-test was used with Type I error of 0.05 and power of 0.70. These resulted in a sample size of 50 patients. The study was closed by sponsor in April 2015 due to a slow rate of accrual with a final enrollment of 24 patients. Descriptive statistics including medians and ranges for the variables of population characteristics were presented. Survival analyses were performed using STATA (Data Analysis and Statistical Software). Comparisons on survival data between our data and historical norm were performed in a descriptive manner in this initial cohort of cases. Log rank tests were employed as a post hoc analysis to compare progression free and overall survival times between MGMT methylated and unmethylated patients. P<0.05 was defined as significant. The study was registered with clinicaltrial.gov (NCT00998010).
Results
Patient Profiles
A total of 25 patients with pathologically confirmed newly-diagnosed GBM were screened, and 24 were enrolled between October 2011 and April 2015. One failed screening due to pulmonary embolism onset one day before enrollment. Twenty-one patients were enrolled at University of California – Los Angeles and 3 were enrolled at Columbia University.
Basic characteristics of patients and molecular markers are shown in Table 1. Our cohort included 13 male and 11 female patients with a median age of 57 years old (range 27–75), and a median KPS score at the time of enrollment of 90 (range 60–100). Thirteen patients had gross total resection and 11 patients had subtotal resection. Molecular biomarkers were tested in 24 patients for IDH1 and 21 for IDH2 mutation status: 3 (13%) were found positive for IDH1 mutation and 0 (0%) were positive for IDH2 mutation (Table 1). MGMT methylation was tested in 23 patients: 10 (43%) were methylated and 13 (57%) were unmethylated (Table 1).
Table 1.
Baseline characteristics of study patients at enrollment (n=24)
| Characteristic | Value (%) |
|---|---|
|
| |
| Age in years: median (range) | 57 (27-75) |
| Gender - no. (%) | |
| Male | 13 (54 ) |
| Female | 11 (46) |
| Karnofsky performance score - no. (%) | |
| 60-80 | 8 (33) |
| 90-100 | 16 (67) |
| Pre-surgery lesion location- no. (%) | |
| Temporal lobe | 9 (38) |
| Frontal lobe | 9 (38) |
| Parietal lobe | 4 (17) |
| Occipital lobe | 2 (8) |
| Extent of resection - no. (%) | |
| Sub-total resection | 11 (46) |
| Gross total resection | 13 (54) |
| Use of glucocorticoids at baseline - no. (%) | |
| Yes | 10 (42) |
| No | 14 (58) |
| MGMT methylation status - no. (%) | |
| Total # of patients tested | 23 |
| Methylated | 10 (43) |
| Unmethylated | 13 (57) |
| IDH mutation status - no. (%) | |
| Total # of patients tested (IDH1/IDH2) | 24/21 |
| Mutant type (IDH1/IDH2) | 3/0 (13/0) |
| Wild type (IDH1/IDH2) | 21/21 (88/100) |
The median interval from histopathological diagnosis/initial surgery to treatment initiation was 5 weeks (range 3–6) and from diagnosis to completion or discontinuation from the trial was 26 weeks (range 7–116). Median follow up period was 21.8 months (range 5.5–61.4). As of April 2016, all 24 patients were either taken off study or completed 24 cycles of adjuvant therapy. The reasons for discontinuation from the trial include: 4 (17%) patients completed 24 cycles of adjuvant temozolomide and bortezomib therapy without progression, 14 (58%) had tumor progression, 2 (8%) were non-compliant, 2 (8%) withdrew from the trial voluntarily due to difficulty to maintain clinic visit, and 2 (8%) had adverse effects including allergic reaction in 1 patient and refractory neutropenia in the other patient as described below.
Safety
The most common grade 2 toxicities observed were hematologic events including lymphopenia (63%), neutropenia (21%) and thrombocytopenia (17%) and non-hematologic events including fatigue (38%), headache (29%), skin rash/injection site reaction (17%), seizure (17%) and cognition disturbances (17%) (see Table E1, online supplementary material). Table 2 shows grade 3/4 toxicities observed during both RT and post-RT phases for each patient. During the RT phase, grade 3 and grade 4 toxicities were observed in 6 (25%) patients and 1 (4%) patient respectively. Grade 3 toxicities included rash, asymptomatic hyponatremia, neutropenia, constipation, and headache. Hydrocephalus and pulmonary embolism were considered unrelated to treatment given the onset was after three day of the treatment and the symptoms of hydrocephalus had developed before the treatment. Grade 4 toxicity contained gait abnormality, which was considered related to hydrocephalus and unrelated to the trial medications. During post-RT phase, 7 (29%) patients experienced grade 3 hematologic toxicities and none (0%) experienced any grade 4 toxicities. The adverse events were reversible. With the follow-up periods ranging from 5.5 to 61.4 months, we did not see unexpected adverse events, delayed neuro-toxicity or correlative abnormal brain MRI findings.
Table 2.
Observed grade 3 (G3) and grade 4 (G4) toxicities
| Events | During radiation therapy phase | During maintenance treatment phase | ||
|---|---|---|---|---|
| No. of patients (%) | No. of patients (%) | |||
| G 3 | G 4 | G 3 | G 4 | |
| Rash/drug reaction | 1 (4) | 0 | 0 | 0 |
| Hyponatremia (most likely related to disease) | 1 (4) | 0 | 0 | 0 |
| Leukopenia | 0 | 0 | 3 (13) | 0 |
| Lymphopenia | 0 | 0 | 7 (29) | 0 |
| Neutropenia | 1 (4) | 0 | 1 (4) | 0 |
| Headache | 1 (4) | 0 | 0 | 0 |
| Constipation | 1 (4) | |||
| Hydrocephalusa)b) (most likely related to disease) | 2 (8) | 0 | 0 | 0 |
| Pulmonary embolism (PE) a) (most likely related to disease) | 1 (4) | 0 | 0 | 0 |
| Mood alteration b) (most likely related to hydrocephalus) | 1 (4) | 0 | 0 | 0 |
| Gait abnormality b) (most likely related to hydrocephalus) | 0 | 1 (4) | 0 | 0 |
| Any events | 9 (38) | 1 (4) | 11 (46) | 0 |
| Patients with adverse events c) | 6 (25) | 1 (4) | 7 (29) | 0 |
Note:
One patient developed G3 hydrocephalus and G3 PE on the 4th day after starting the trial. The patient only received one dose of bortezumab, 3 days of temozolomide and 3 days of radiation therapy. The symptoms resolved after shunt placement and anticoagulation. The patient resumed treatment. The hydrocephalus and PE were most likely related to the GBM itself.
The other patient had G2 equivalent gait abnormality before clinical trial and progressed into G4 gait abnormality in the middle of RT phase. G3 hydrocephalus was found. G3 mood alteration started at the same time when hydrocephalus was diagnosed. The symptoms resolved after shunt placement. Thus, the gait abnormality and mood alteration were most likely caused by hydrocephalus. Hydrocephalus is most likely due to GBM disease or worsening by radiation effect.
Overall patients with adverse events were 13/24 (54%).
Among all 24 patients, 2 patients were taken off from the study due to adverse effects from the trial. One patient had a grade 3 skin allergy while being on temozolomide, bortezomib and Bactrim during RT phase. Upon stopping all 3 medications, the patient’s rash improved. Assuming the allergy was caused by Bactrim, we switched Bactrim to Mepron and resumed the patient on RT, temozolomide and bortezomib. However, the rash worsened and the treatment was again stopped. The patient was taken off from trial during week 6 of RT phase and resumed treatment after the allergic reaction resolved completely. Another patient developed grade 2 neutropenia while on both temozolomide and bortezomib during maintenance treatment phase. Then the patient developed grade 3 neutropenia after holding bortezomib but continuing dose reduced temozolomide. The patient was taken off the study and discontinued temozolomide at week 37 because of refractory neutropenia.
Efficacy
Among 24 patients, 1 (4%) was lost to follow up after last visit on July 24 of 2015, 15 (63%) were deceased and 8 (33%) were alive as of 4/14/2017, when survival data were last confirmed. Median progression free survival (PFS) was 6.2 months (95% CI, 3.7–8.8) from the time of diagnosis (Table 3). PFS rates were 54.2% (95% CI 32.7–71.4) at 6 months, 29.2% (95% CI 13.0–47.6) at 12 months, 25.0% (95% CI 10.2–43.1) at 18 months and 24 months, 16.7% (95% CI 5.2–33.7) at 30 months, and 12.5% (95% CI 3.1–28.7) at 36 months. Median overall survival (OS) was 19.1 months (95% CI, 6.7–31.4) from the time of diagnosis (Table 3). The OS rates were 87.5% (95% CI 66.1–95.8) at 12 months, 50.0% (95% CI 29.1–67.8) at 24 months, and 34.1% (95% CI 15.4–53.9) at 36 to 60 months. The PFS rate and OS rate appeared to be promising at 18 months and beyond (Table E2, online supplementary materials) compared to historical norms.
Table 3.
Progression free survival (PFS) and overall survival (OS)
| PFS | OS | |
|---|---|---|
| Median survival (95% CI) in month | 6.2 (3.7-8.8) | 19.1 (6.7-31.4) |
| Median survival Rate (%) (95% CI) | ||
| At 6 months | 54.2 (32.7-71.4) | 95.8 (73.9-99.4) |
| At 12 months | 29.2 (13.0-47.6) | 87.5 (66.1-95.8) |
| At 18 months | 25.0 (10.2-43.1) | 54.2 (32.7-71.4) |
| At 24 months | 25.0 (10.2-43.1) | 50.0 (29.1-67.8) |
| At 30 months | 16.7 (5.2-33.7) | 45.5 (25.2-63.7) |
| At 36 months | 12.5 (3.1-28.7) | 34.1 (15.4-53.9) |
| At 48 months | NA | 34.1 (15.4-53.9) |
| At 60 months | NA | 34.1 (15.4-53.9) |
NA: Data not available.
CI: confidence interval
Twenty three of 24 patients (96%) were tested for MGMT methylation status, and 10 (43%) were methylated. As expected, we observed improved median PFS in MGMT methylated patients comparing to unmethylated patients (Figure 2a and Figure E1 with 95% confidence regions in online supplementary materials, p=0.00004, Log rank test): median PFS was 24.7 months (95% CI 8.5–41.0) in methylated patients and 5.1 months (95% CI 3.9–6.2) in unmethylated patients (Table 4). Similarly, the OS in methylated patients was significantly greater than unmethylated patients (Figure 2b and Figure E2 with 95% confidence regions in online supplementary materials, p=0.0002, Log rank tests). The estimated value of median OS was 61 months for the 10 MGMT methylated patients; however, the upper bound of 95% confidence interval could not be calculated because 7 of 10 (70%) methylated patients are still alive at the time when data was analyzed. Instead, we calculated the mean OS for methylated patients, which showed 49.4 months (95% CI 38.3–60.6 months) (Table 4). In unmethylated patients, median OS was 16.4 months (95% CI 11.8–21.0) and mean OS was 15.6 months (95% CI 12.3–18.9) (Table 4). The OS of MGMT methylated patients in the trial appeared to be greater than historical MGMT methylated cohorts (Table E3, online supplementary materials).
Figure 2a.
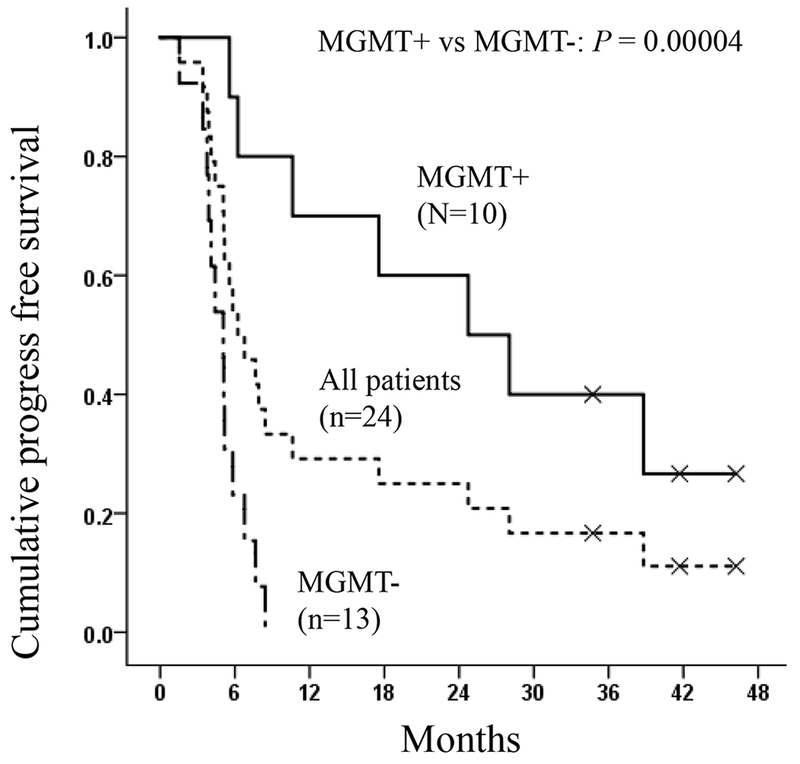
Median progression-free survival (PFS) curves for all patients and patients with methylated and unmethylated MGMT status. MGMT+: methylated MGMT; MGMT−: unmethylated MGMT
Table 4.
Progression free survival (PFS) and overall survival (OS) in accordance to MGMT methylation status
| PFS in month | OS in month | |
|---|---|---|
| Patients with MGMT+ (n=10) |
24.7 (8.5-41.0) a) | 61 (NC) a) 49.4 (38.3-60.6) b) |
| Patients with MGMT− (n=13) |
5.1 (3.9-6.2) a) | 16.4 (11.8-21.0) a) 15.6 (12.3-18.9) b) |
| Log rank test | P=0.00004 | P=0.0002 |
MGMT+: MGMT methylated; MGMT−: MGMT unmethylated.
Median value (95% Confidence Interval bounds). NC: the upper bound of 95% confidence interval could not be calculated because 7 out of 10 patients were still alive at the time of the data analysis.
Mean value (95% Confidence Interval bounds), which was used for statistical comparison because of the above reason in a).
Figure 2b.
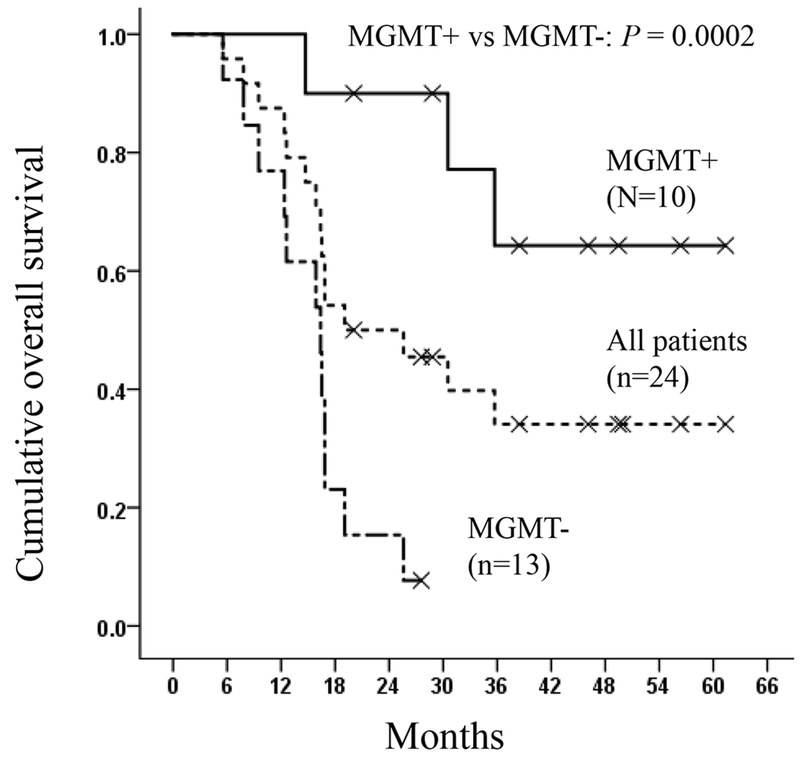
Median overall survival (OS) curves for all patients and patients with methylated and unmethylated MGMT status. MGMT+: methylated MGMT; MGMT−: unmethylated MGMT
Post-trial treatments for recurrence
Among all 24 patients, 3 had no progression at the time of the last follow-up and 1 lost follow-up. The remaining 20 patients had documented recurrence. The treatments for recurrence were at attending physician’s discretion. For first recurrence, 4 patients were treated with temozolomide re-challenge, 9 with bevacizumab single agent, 4 with bevacizumab plus lomustine (CCNU), 1 with bevacizumab plus onartuzumab, 1 with bevacizumab plus pembrolizumab, and 1 with nivolumab. For further tumor progression, treatment therapies consisted of traditional chemotherapy agents (CCNU, carboplatin or etoposide) with and without bevacizumab. Four patients received stereotactic radiation therapy during further recurrences.
Discussion
Despite current standard treatment combining maximal tumor resection followed by radio-chemotherapy, prognosis of GBM patients remains poor. Consequently, finding new agents to integrate into standard treatment for newly-diagnosed GBM is required to improve patient survival. Bortezomib is an FDA approved proteasome inhibitor for hematologic malignances that has shown efficacy towards GBM cell lines in vitro and in vivo (7–8, 14–15). Therefore, we sought to gain a greater insight into the use of bortezomib as a treatment for newly-diagnosed GBM patients, by combining it into current standard of care and examined its efficacy as well as safety. We found that bortezomib treatment in combination with standard RT and temozolomide, followed by adjuvant bortezomib and temozolomide was tolerable without unanticipated adverse events, and observed promising survival benefit particularly in the MGMT methylated population.
Kubicek et al. (9) conducted a phase I trial of bortezomib with concurrent temozolomide (TMZ) and radiotherapy with dose escalation in 27 patients with CNS malignancies, including 19 GBM patients. Bortezomib was given during concurrent RT/TMZ phase only. The patient population was heterogeneous including various primary glioma patients in newly-diagnosed or recurrent setting and two metastatic CNS tumor patients. The study tested bortezomib with dose escalation rated at 0.7, 1.0 and 1.3mg/m2 on days 1, 4, 8 and 11 of a 21-day cycle during RT/TMZ phase only. Their results indicated that bortezomib at 1.3 mg/m2 is well tolerated and safe in combination with temozolomide and radiotherapy. Accordingly, our protocol used consistent dose (1.3 mg/m2) of bortezomib and recruited only newly-diagnosed GBM patients. In addition, bortezomib treatment was continued during maintenance treatment phase up to 24 cycles.
We found that the combination therapy with radiation, temozolomide, and bortezomib were well tolerated. One patient was taken off the study due to grade 3 skin allergy while the patient was on bortezomib, temozolomide, Bactrim and then Mepron. The medications that caused skin allergy might be Bactrium or Mepron and/or bortezomib as switching Bactrim to Mepron did not relieve the rash. Another patient had refractory neutropenia while the patient was on both bortezomib and temozolomide and was taken off study. Based on our experience and past clinical trials with temozolomide at our institution, the refractory neutropenia was most likely attributable to temozolomide because the patient developed worsening neutropenia after holding bortezomib while continuing dose reduced temozolomide. With the follow-up periods ranging from 5.5 to 61.4 months, we did not see unexpected adverse events, or delayed neuro-toxicity on clinical examination or brain MRIs. Many of the common toxicities such as hematologic adverse events and fatigue were attributable to RT/TMZ toxicities, similar to the common adverse events reported in temozolomide studies (1–3, 16) and from our daily treatment experience with temozolomide. The overall grade 3/4 toxicities were observed in 25% and 29% of patients during RT phase and post-RT phase respectively (Table 2). There was only 1 (4%) grade 4 toxicity: gait abnormality during RT phase (Table 2). This patient had grade 2 gait abnormality before the study which progressed into grade 4 gait abnormality in the middle of RT phase. This progression is most likely caused by hydrocephalus that was related to GBM or radiation effect, because the symptoms resolved after shunt placement even with continued treatment. Overall, when compared with a recent study by Gilbert et al., the grade 4 adverse events in our patients (4% during RT phase and 0% during post RT phase) were less while the grade 3 adverse events were more (2). When compared with a recent study by Chinot et al., the total adverse events of ≥ grade 3 are comparable between theirs (51.3%) and ours (54%) (3). The more frequent grade 2 lymphopenia during RT phase could be due to the addition of bortezomib to temozolomide; however, the significant increased grade 2 and grade 3 lymphopenia adverse events in our trial during post RT phase are likely because of 24 cycles of treatment duration in comparison to 6–12 cycles of treatment duration in the other trial (2, 16). Other possible reasons include small sample of this study and generous adverse effect documentation- unrelated adverse events but occurred during the clinical trial period (Table 2). Similar pattern for reporting adverse effects was seen in other clinical trial (16).
We changed the administration of bortezomib from intravenous route to subcutaneous route at 10 months after the enrollment of the first patient, as Moreau et al. showed subcutaneous bortezomib significantly reduced development of peripheral neuropathy when compared with intravenous administration (11, 12). In our patient population, 3 developed symptoms suggestive of peripheral neuropathy; 2 of whom received bortezomib both via intravenous and subcutaneous routes and 1 of whom via subcutaneous injection only.
Our single-arm data shows that adding bortezomib to RT/TMZ may potentially improve overall survival. The most promising results observed are the higher OS rates in our patients at 12 months and beyond (Table E2, online supplementary materials). While the median PFS in our patients does not show much difference from historical data, we observed increased PFS rates at 18 months and beyond (Table E2, online supplementary materials). Due to small sample size, statistical comparison was not performed between our survival data and historical data. Nevertheless, our data were encouraging for newly diagnosed GBM when bortezomib was combined with radiation therapy and temozolomide. In preclinical models, bortezomib has been shown to be a radiosensitizer (17). That could be one of the reasons for lacking of efficacy in the treatment of recurrent GBM with bortezomib and vorinostat (a histone deacetylase inhibitor) when radiotherapy was not used (18).
Similar to other studies (3, 19), our results clearly showed MGMT methylated patients had significant longer PFS and OS than unmethylated patients (Table 4, Figure 2). Moreover, MGMT methylated patients appear to derive enhanced benefit to the addition of bortezomib to RT/TMZ therapy (Table E3, online supplementary materials). Interestingly, this clinical outcome is different from a study showing through GBM cell lines that adding bortezomib to temozolomide overcomes MGMT-mediated GBM resistance to temozolomide (20).
There are some limitations of our study. First, we were only able to enroll 24 patients. Although we have not reached the planned statistical power, our data from these 24 patients have shown that the median OS rate (54%) at 18-month has approximated our assumed OS of 55%, in comparison to 39%, 36% and 43% in historical norms (1–3) (Table E2, online supplementary material), suggesting a potential of better efficacy of our treatment. Second, our patients all underwent surgical resections, which was not the case in most of the historical studies. While in Gilbert’s study, no patients with unresected tumors were assessed for efficacy, whereas in Stupp’s and Chinot’s studies, 17% and 13%, respectively, of their patients had only biopsy without surgical resections (1–3). These differences could have resulted in longer survival in our patients. However, compared to the median OS (16.1 months and 15.8 months) of pure surgical resection patients in Gilbert’s trial (2) and Stupp’s online supplemental data (1), the overall survival of 19.1 months in our patients are still favorable.
Conclusion
In this phase II study investigating the safety and efficacy of adding bortezomib to radiotherapy with temozolomide in the treatment of newly-diagnosed GBM, we found that this regimen did not cause unexpected adverse events. The overall toxicity is tolerable. The overall survival rates appear promising. For the patients with MGMT methylation, there appear to be enhanced benefit of bortezomib in terms of PFS and OS compared to historical outcomes for MGMT methylated patients. Further clinical investigation is warranted with a larger sample size to validate our findings.
Supplementary Material
Acknowledgement
I confirm that I have mentioned all organizations that funded my research in the Acknowledgements section of my submission, including grant numbers where appropriate.
Footnotes
Conflict of interest:
The authors whose names are listed immediately below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Xiao-Tang Kong MD PhD
Nhung T. Nguyen
Yoon J. Choi MD
Guicheng Zhang, MD PhD
HuyTram N. Nguyen
Emese Filka MD
Stacey Green MSN
P. Leia Nghiemphu MD
William H. Yong MD
Linda M. Liau MD PhD
Tania Kaprealian MD
Whitney B. Pope MD PhD
Andrew Lassman, MD
Tim Cloughesy MD
The authors whose names are listed immediately below report the following details of affiliation or involvement in an organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this manuscript. Please specify the nature of the conflict on a separate sheet of paper if the space below is inadequate.
Albert Lai, MD, PhD – Grant from Millennium/Takeda (This is an investigator initiated industry sponsored study)
**Author disclosure forms from all authors were uploaded.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014;370(8):699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinot OL, Wick W, and Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014;370:709–722 [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Frezza M, Schmitt S et al. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr Cancer Drug Targets 2011;11(3):239–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dou QP, and Zonder JA. Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system. Curr Cancer Drug Targets 2014;14(6):517–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredel M, Scholtens DM, Yadav AK, et al. NFκBIA deletion in glioblastomas. N Engl J Med 2011;364:627–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin D, Zhou H, Kumagai T, et al. Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme. Oncogene 2005;24:344–54 [DOI] [PubMed] [Google Scholar]
- 8.Styczynski et al. Activity of Bortezomib in glioblastoma. Anticancer research 2006;26:4499–504 [PubMed] [Google Scholar]
- 9.Kubicek GJ, Werner-Wasik M, Machtay M, et al. Phase I trial using proteasome inhibitor bortezomib and concurrent temozolomide and radiotherapy for central nervous system malignancies. Int J Radiation Oncology Biol Phys 2009;74:433–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai A, Filka E, McGibbon B, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma mutiforme: Interim analysis of safety and tolerability. Int J Rad Oncol Biol Phys 2008;71(5):1372–1380 [DOI] [PubMed] [Google Scholar]
- 11.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomized, phase 3, non-inferiority study. Lancet Oncol 2011;12:431–40 [DOI] [PubMed] [Google Scholar]
- 12.Moreau P, Karamanesht II, Domnikova N, et al. Pharmacokinetic, pharmacodynamics and covariate analysis of subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma. Clin Pharmacokinet 2012;51:823–29 [DOI] [PubMed] [Google Scholar]
- 13.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for High-grade gliomas: Response assessment in Neuro-Oncology working group. J Clin Oncol 2010;28(11):1963–1972 [DOI] [PubMed] [Google Scholar]
- 14.Bota DA, Alexandru D, Keir ST, et al. Proteasome inhibition with bortezomib induces cell death in GBM stem-like cells and temozolomide-resistant glioma cell lines, but stimulates GBM stem-like cells’ VEGF production and angiogenesis. J Neurosurg 2013;119:1415–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Unterkircher T, Cristofanon S, Vellanki SH, et al. Bortezomib primes glioblastoma, including glioblastoma stem cells, for TRAIL by increasing tBid stability and mitochondrial apoptosis. Clin Cancer Res 2011;17(12):4019–4031 [DOI] [PubMed] [Google Scholar]
- 16.Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543 [DOI] [PubMed] [Google Scholar]
- 17.Davies AM, Lara PN, Mack PC, et al. Incorporating bortezomib into the treatment of lung cancer. Clin Cancer Res 2007;13(15):4647s–4651s [DOI] [PubMed] [Google Scholar]
- 18.Friday BB, Anderson SK, Buckner J, Yu C, Giannini C, Geoffroy F, Schwerkoske J, Mazurczak M, Gross H, Pajon E, Jaeckle K, Galanis E. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro Oncol. 2012. February;14(2):215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352(10):997–1003 [DOI] [PubMed] [Google Scholar]
- 20.Vlachostergios PJ, Hatzidaki E, Befani C, et al. Bortezomib overcomes MGMT-related resistance of glioblastoma cell lines to temozolomide in a schedule-dependent manner. Invest New Drugs 2013;31:1169–1181 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


