Abstract
Background:
The prevalence of type 2 diabetes (T2D) has nearly doubled since 1980. Elevated body mass index (BMI) is the leading risk factor for T2D, mediated by inflammation and oxidative stress. Arsenic shares similar pathogenic processes, and may contribute to hyperglycemia and β-cell dysfunction.
Objectives:
We assessed a unique situation of individuals living in Northern Chile with data on lifetime arsenic exposure to evaluate the relationship between arsenic and T2D, and investigate possible interactions with BMI.
Methods:
We analyzed data collected from October 2007-December 2010 from an arsenic-cancer case-control study. Information on self-reported weight, height, smoking, diet, and other factors were obtained. Diabetes was defined by self-reported physician-diagnoses or use of hypoglycemic medication. A total of 1053 individuals, 234 diabetics and 819 without known diabetes were included.
Results:
The T2D odds ratio (OR) for cumulative arsenic exposures of 610–5279 and ≥5280 µg/L-years occurring 40 years or more before interview were 0.97 (95% CI: 0.66–1.43) and 1.53 (95% CI: 1.05–2.23), respectively. Arsenic-associated T2D ORs were greater in subjects with increased BMIs. For example, the ORs for past cumulative exposures ≥5280 µg/L-years was 1.45 (95% CI: 0.74–2.84) in participants with BMIs <25 kg/m2 but 2.64 (95% CI: 1.14–6.11) in those with BMIs ≥30 kg/m2 (synergy index = 2.49, 95% CI: 0.87–7.09). Results were similar when people with cancer were excluded.
Conclusions:
These findings identify increased odds of T2D with arsenic exposure, which are significantly increased in individuals with excess BMI.
Keywords: Arsenic, Type 2 diabetes (T2D), Obesity, Body Mass Index (BMI), Synergy
1. Introduction
More than 200 million individuals worldwide are exposed to arsenic-contaminated drinking water above the World Health Organization (WHO)’s permissible limit of 10 µg/L (Naujokas et al. 2013). Ingested arsenic is an established carcinogen and prevalent at high concentrations in drinking water sources in Taiwan, Bangladesh, India, Chile, Argentina, the US, and elsewhere (ATSDR 2007; IARC 2004, 2012). In the US, an estimated 12% of all public water systems have arsenic concentrations near 10 µg/L (U.S. EPA 2000), the current US regulatory standard. Millions more people are likely exposed to even higher arsenic water concentrations from private wells, which are not regulated (Steinmaus 2005). Arsenic also occurs in apple juice, chicken, wine, and beer (Marshall 2012; Nachman et al. 2013; Schute 2013; Wilson et al. 2012), and the US Food and Drug Administration (FDA) found arsenic in almost all 193 brands of rice, rice baby foods, and rice cereals tested (U.S. FDA 2012). Arsenic is also common at industrial waste sites and is currently ranked first on the Superfund hazardous waste site priority list in terms of toxicity and prevalence of exposure (ATSDR 2013).
Type 2 diabetes (T2D) is a chronic condition of increasing prevalence, affecting an estimated 415 million individuals worldwide, projected to increase to 642 million by the year 2040 (Zimmet et al. 2016). In Chile, the prevalence of diabetes mellitus has increased from 4.2% in 2003 to 12.3% in 2016 (Ministerio de Salud, 2017). Although obesity is the primary risk factor for T2D, other factors may also play a role in either directly causing T2D or in enhancing the role of excess BMI on T2D risks (Thayer et al. 2012). Arsenic has been linked to T2D in areas with high exposures (Huang et al. 2011; Maull et al. 2012), but studies of lower exposures (e.g., <100 µg/L) have produced very mixed and unclear results (Smith, 2013). Although the primary mechanism of arsenic toxicity is unknown, it has been shown to affect several mechanistic pathways that are linked to both obesity and T2D. For example, both arsenic and obesity have been associated with mitochondrial dysfunction and with increases in reactive oxygen species (ROS), two processes that are thought to play an important role in T2D development (Pan et al. 2016; Tseng et al. 2004; Bournat and Brown 2010). Given these and other shared pathologic processes, we hypothesized that arsenic and obesity might interact to increase T2D risk.
Many of the water sources in Northern Chile are contaminated with naturally-occurring arsenic, with concentrations ranging from <10 to >800 µg/L. This geographical area contains the Atacama Desert, which is the driest inhabited place on earth. Because it is so dry, almost everyone in the area lives in one of the cities or towns in the area, and each city and town has its own single water supply. Extensive historical records of arsenic concentrations in all this area’s major water sources are available, and because of this, comprehensive estimates of people’s lifetime arsenic exposure, from birth through adulthood, can be made simply by knowing the cities or towns in which they have lived (Ferreccio et al. 2000). These types of lifetime exposure data are not available anywhere else in the world. The largest city in the area, Antofagasta, experienced a period of very high arsenic exposure (average of 860 µg/L) starting in 1958, when two rivers with high arsenic concentrations were diverted to the city for drinking. The high exposures ended in the 1970s, when arsenic treatment plants were installed. Except for the installation of arsenic treatment plants in Antofagasta and several other cities, arsenic concentrations in drinking water have been very stable over time (Ferreccio et al. 2000).
For this paper, we used this unique exposure scenario to assess the effects of arsenic exposure on T2D development, and to evaluate whether arsenic and obesity may act synergistically to increase T2D risk. To our knowledge, the present study is the first to examine the possible interaction between arsenic and obesity on the development of T2D.
2. Materials and Methods
2.1. Study area and subject ascertainment
Study design details are published elsewhere (Steinmaus et al. 2013). Briefly, all subjects were participants in an arsenic-cancer case-control study which involved two contiguous regions (Regions I and II) in Northern Chile. Institutional review board approval was obtained in both the US and Chile. Participation was voluntary, and written informed consent was obtained from all subjects or next of kin. Cancer cases in the underlying study included all people who: (i) had newly diagnosed primary lung, bladder, or kidney cancer between October 2007 and December 2010; (ii) lived in Regions I or II at the time of diagnosis; (iii) were >25 years old when diagnosed; and (iv) were able to provide interview data or had a close relative who could. Cases were ascertained using a rapid case ascertainment system established for the study which involved all pathologists, hospitals, and radiologists in the study area, and most cases were interviewed within three months of diagnosis. Hospital cancer committees and death records were used to identify missing cases. Cancer-free controls, frequency matched to cancer cases by sex and five-year age groups, were randomly selected from the Chile Electoral Registry. This Registry contains >95% of people over age 50 years based on population numbers for Regions I and II recorded in the Chilean Census.
2.2. Participant interviews
Participants were interviewed in person using a standardized study questionnaire. For deceased participants, we interviewed the next of kin (proxy). Participants were asked to provide all lifetime residences, water sources (e.g., public water, bottled) and water filter use at each residence, and all jobs held for at least six months. Participants were also asked about specific occupational exposures including asbestos, arsenic, silica, and solvents. Questions regarding tobacco smoke included age at which smoking began, periods of no smoking, years smoked, number of cigarettes smoked per day, and childhood or adult secondhand smoke exposure. Participants were also asked about typical water intake, both currently and 20 years ago. Typical dietary intakes in the year preceding interview and 20 years earlier were assessed using a modified version of the National Cancer Institute’s Diet History Questionnaire. A 14-point socioeconomic status (SES) scoring system was developed by asking subjects about household items (e.g. computer), cars, and use of domestic help. Information on all medical conditions (e.g. hypertension) and medications were collected. For the analyses presented here, diabetics were defined as people who self-reported physician-diagnosed diabetes or who used a hypoglycemic medication.
2.3. Body mass index (BMI)
All subjects and proxy respondents were asked to provide the subjects’ adult height and typical weight at ages 20 and 40. People without cancer were also asked to provide their typical weight in the 10 years preceding the interview, and people with cancer were also asked to provide their typical weight in the 10 years preceding their cancer diagnosis. BMI at each period in time was calculated as weight (kg)/height (m2). Category cut-off points were based on the WHO definition for overweight and obesity in adults of 25 mg/kg2 and 30 mg/kg2, respectively, for both men and women.
2.4. Exposure indices
For each subject, each residence in Chile was linked to a water arsenic measurement for that location and the years the subject lived there. Using this process, we could assign an arsenic concentration to each year of each subject’s life. Arsenic water records for all cities and towns in Regions I and II, and for all large cities in Chile outside these regions, were collected from governmental agencies, research studies, and water suppliers (Ferreccio et al. 2000). Overall, arsenic water concentrations could be linked to >95% of all subject residences. Use of bottled water or sources with reverse osmosis filtering were assigned arsenic concentrations of zero. The yearly arsenic exposure estimates for each subject were then used to calculate several metrics of arsenic exposure. These included lifetime cumulative exposure (the sum of each subjects’ yearly water concentrations), average exposure (the mean of all of each subject’s yearly water concentrations), and lifetime highest (the single highest arsenic water concentration at any year in a subject’s life). Results are given for each of these metrics since it is unknown which might be most strongly associated with arsenic-related diabetes. Forty-year lag periods, which ignored all arsenic exposure in the 40 years preceding cancer diagnosis (for people with incident cancer) or interviews (for people without incident cancer), were applied in some analyses. This was done because exposures in the area were highest >40 years ago (before arsenic treatment plants were installed in several cities). Category cut-off points are tertiles unless otherwise stated.
2.5. Statistical analysis
Odds ratios (OR) for T2D were calculated using unconditional logistic regression for various categories and metrics of arsenic exposure. No heterogeneity in results was observed by sex in analyses of arsenic and T2D, thus males and females were combined. Our inclusion of lung, bladder and kidney cancer cases from the underlying cancer case-control study could potentially introduce bias if these types of cancers were associated with T2D. In unadjusted analyses we found that T2D was more common among the bladder cancer cases than among cancer-free controls (OR=1.34, 95% CI: 0.95–1.88). Although not statistically significant, the fact that this OR was moderately above 1.0 suggests including these bladder cancer cases could introduce some bias.
For this reason, these subjects were excluded here. Neither lung nor kidney cancers were associated with T2D in our study (see Results section and Table 1). As such, all kidney and lung cancer cases were included in most of the analyses. The advantage of including these cases is that it leads to a greater sample size and thus more precise estimates of odds ratios. Possible bias from including these cases was assessed by performing separate analyses with these subjects excluded.
Table 1.
Sociodemographic characteristics of study participants with and without diabetes
| Diabetes |
|||
|---|---|---|---|
| Yes n |
No n |
OR (95% CI) | |
| Total | 234 | 819 | |
| Sex | |||
| Male | 144 | 556 | 1.00 (Ref) |
| Female | 90 | 263 | 1.32 (0.98–1.79) |
| Age (years old) | |||
| <60 | 46 | 258 | 1.00 (Ref) |
| >60–70 | 88 | 285 | 1.73 (1.17–2.57) |
| >70 | 100 | 276 | 2.03 (1.38–3.00) |
| Cancer case-control status | |||
| Control | 145 | 489 | 1.00 (Ref) |
| Cancer cases: all | 89 | 330 | 0.91 (0.67–1.23) |
| Lung | 67 | 234 | 0.97 (0.70–1.34) |
| Kidney | 22 | 96 | 0.77 (0.47–1.27) |
| Proxy | |||
| No | 187 | 657 | 1.00 (Ref) |
| Yes | 47 | 162 | 1.02 (0.71–1.47) |
| Mining work | |||
| No | 186 | 637 | 1.00 (Ref) |
| Yes | 48 | 182 | 0.90 (0.63–1.29) |
| Cigarette smoking | |||
| Never | 84 | 252 | 1.00 (Ref) |
| Former | 103 | 322 | 0.96 (0.69–1.34) |
| Current | 47 | 245 | 0.58 (0.39–0.86) |
| Average cigarettes (per day)* | |||
| >0–5 | 15 | 82 | 1.00 (Ref) |
| 6–15 | 19 | 61 | 1.70 (0.80–3.62) |
| >15 | 13 | 102 | 0.70 (0.31–1.55) |
| Cumulative pack years* | |||
| Low | 18 | 87 | 1.00 (Ref) |
| Middle | 27 | 146 | 0.89 (0.47–1.72) |
| High | 2 | 12 | 0.81 (0.17–3.91) |
| Adult secondhand smoke** | |||
| No | 58 | 188 | 1.00 (Ref) |
| Yes | 26 | 64 | 1.32 (0.77–2.27) |
| Childhood secondhand smoke** | |||
| No | 59 | 181 | 1.00 (Ref) |
| Yes | 25 | 71 | 1.08 (0.63–1.86) |
| BMI recent (kg/m2)# | |||
| <25 | 66 | 346 | 1.00 (Ref) |
| 25–30 | 105 | 344 | 1.60 (1.14–2.25) |
| ≥30 | 63 | 129 | 2.56 (1.72–3.82) |
| BMI age 40 (kg/m2) | |||
| <25 | 93 | 472 | 1.00 (Ref) |
| 25–30 | 84 | 255 | 1.78 (1.28–2.46) |
| ≥30 | 49 | 84 | 2.96 (1.95–4.49) |
| BMI age 20 (kg/m2) | |||
| <25 | 159 | 638 | 1.00 (Ref) |
| 25–30 | 55 | 148 | 1.49 (1.05–2.13) |
| ≥30 | 20 | 33 | 2.43 (1.36–4.35) |
| Hypertension | |||
| No | 149 | 653 | 1.00 (Ref) |
| Yes | 85 | 166 | 2.24 (1.64–3.08) |
| Fruit and vegetable intake## | |||
| ≤1/day | 14 | 74 | 1.00 (Ref) |
| 1–2/day | 50 | 208 | 1.27 (0.66–2.43) |
| >2/day | 114 | 362 | 1.66 (0.91–3.06) |
| Race | |||
| Hispanic/European descent | 171 | 600 | 0.99 (0.71–1.37) |
| Other | 63 | 219 | 1.00 (Ref) |
| SES scores | |||
| Low | 73 | 208 | 1.00 (Ref) |
| Middle | 80 | 364 | 0.63 (0.44–0.90) |
| High | 81 | 247 | 0.93 (0.65–1.35) |
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio; SES, socioeconomic
Among current smokers only
Among never smokers only
Typical BMI in the ten years preceding cancer diagnosis (cancer cases) or interview (cancer-free controls)
Dietary data only collected in non-proxy subjects
Potential biological interactions between arsenic and increased BMI were assessed by calculating T2D odds ratios for various levels of arsenic exposure in analyses stratified by healthy (<25 kg/m2), overweight (≥25 to <30 kg/m2), and obese (≥30 kg/m2) BMI. BMI based on subjects typical height and weight in the ten years preceding cancer diagnosis (cancer cases) or interview (cancer-free controls) was used in our main analyses, although BMIs at other periods (ages 20 or 40 years) were also assessed. Greater than additive interaction was assessed using the Rothman synergy index (Rothman 1976). Here, T2D ORs were calculated separately for people with elevated BMI (≥30 kg/m2) and low arsenic, people with elevated arsenic and low BMI (<30 kg/m2), and people with both elevated arsenic and elevated BMI, using people with low BMI and low arsenic as the reference group. For these analyses, high and low arsenic was defined as exposures above or below the upper tertile cut-off point for cumulative exposure lagged 40 years. The impact of BMI on arsenic-related T2D risk was displayed visually by calculating T2D ORs for a 10,000 µg/L-year increase in cumulative arsenic exposure (lagged 40 years) in analyses of subjects below each integral of BMI, beginning at 20 and ending at 34 kg/m2 (Figure 1). Here, 10,000 µg/L-year was selected because this was the approximate difference in the means of the upper and lower tertiles. Potential confounding variables entered into the final logistic regression models included sex, age (age <60, 60–70, >70 years), BMI (in analyses not stratified by BMI; <25, 25–<30, ≥30 kg/m2), and smoking (average cigarettes per day of 0, >0–<10, ≥10 when smoking). These were entered a priori since each is a known risk factor for T2D, as shown in the directed acyclic causal graph in Supplementary Figure 1. Age can potentially confound analyses of cumulative exposure so additional analyses were done entering age in 5-year categories, as a continuous variable (each 1 year), and by restricting subjects to narrower age groups (e.g. ages 60–70). Sixteen subjects with no BMI information were excluded. The impact on results of adjustments for other factors potentially related to arsenic exposure or diabetes risk was also assessed. These factors included mining work (ever vs. never), occupational arsenic exposure (ever vs. never), typical fruit and vegetable consumption (above or below one serving per day), water intake (typical intake in L/day), education (high school diploma), adult and child second hand smoke, SES scores (above or below the lower tertile), hypertension, and race (European vs. other descent). The impact of including proxy respondents was evaluated by performing analyses after excluding these subjects. Analyses were conducted using STATA version 13.1 (StataCorp, College Station, TX) and SAS version 9.2 (SAS Institute Inc., Cary NC).
Figure 1.
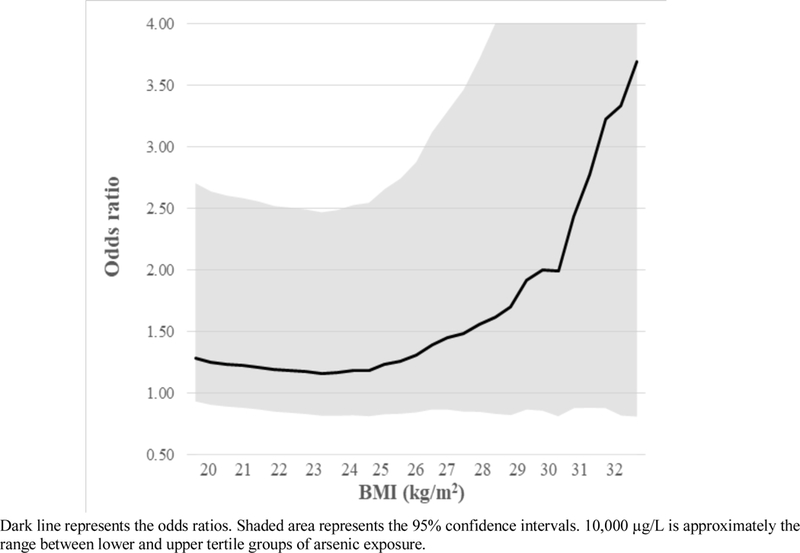
Odds ratios for diabetes for high arsenic exposure (each 10,000 µg/L-year increase in cumulative exposure lagged 40 years) by increasing levels of BMI
3. Results
A total of 1053 subjects were included in the main analyses, 634 cancer-free participants and 419 cancer cases, and 234 and 819 people with and without known diabetes. The mean age, BMI, and unlagged cumulative arsenic exposure in these participants were 65.7 years (standard deviation (SD)=11.2), 26.4 kg/m2 (SD=4.6), and 6527.3 µg/L-years (SD=5646.8), respectively. Clear evidence of an association between T2D and either kidney or lung cancer was not seen, with unadjusted T2D odds ratios for both cancers below 1.0 (Table 1). Adjustment for age, sex, and smoking gave similar results. Subjects with T2D were more likely to be females, above the age of 70, not currently smoking, hypertensive, and have elevated BMIs (≥30 mg/kg2) (Table 1).
Compared with subjects in the lowest tertile of cumulative arsenic exposure lagged 40 years (0–609 µg/L-years), adjusted T2D ORs were 0.97 (95% CI: 0.66–1.43) and 1.53 (95% CI: 1.05– 2.23) for those in the middle (610–5279 µg/L-years) and highest (≥5280 µg/L-years) exposure categories, respectively (Table 2). Similar ORs were seen for unlagged cumulative and average lifetime exposure metrics (Table 2), and in analyses using other arsenic category cut-offs (Table S1). Additional adjustments for daily fruit and vegetable consumption, typical water intake, SES scores, occupation, hypertension, and race had little impact on results. For example, the T2D ORs for cumulative exposures lagged 40 years ≥5280 µg/L were 1.53 (95% CI: 1.05–2.23) and 1.61 (95% CI: 1.04–2.50), respectively, before and after adjustment for these additional factors. Entering age in 5-year categories, as a continuous variable, and restricting subjects to narrower age groups (e.g. ages 60–75) gave similar results, as did removing cancer cases first diagnosed with diabetes within two years of their cancer diagnosis. In addition, arsenic-T2D ORs were similar after excluding cancer cases (Table S2). For example, the T2D OR for lagged cumulative arsenic exposures ≥5280 vs. <610 µg/L-years was 1.53 (95% CI:1.05–2.23) in the analyses that included these cases and 1.56 (95% CI: 0.97–2.51, p-trend=0.03) in analyses where they were excluded. Similar findings were also observed when bladder cancer cases were included; the T2D OR for lagged cumulative arsenic exposures ≥5280 vs. <610 µg/L-years was 1.59 (95% CI: 1.13–2.23). In analyses excluding proxy subjects, arsenic-related T2D ORs were mostly higher. For example, the T2D OR for the highest vs. lowest tertile of cumulative arsenic exposure (lagged 40 years) was 1.67 (95% CI: 1.10–2.54) after excluding proxy subjects.
Table 2.
Diabetes odds ratios for tertiles of various metrics of arsenic water concentrations
| Arsenic metric | Arsenic level |
Diabetes |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
|
|---|---|---|---|---|---|
| Yes | No | ||||
| Cumulative exposure: 0 year lag (µg/L-years) |
0–2416 | 70 | 281 | 1.00 (Ref) | 1.00 (Ref) |
| 2417–8664 | 74 | 276 | 1.08 (0.75–1.55) | 1.14 (0.78–1.66) | |
| ≥8665 | 90 | 262 | 1.38 (0.97–1.97) | 1.50 (1.03–2.19) | |
| p-trend | 0.06 | 0.03 | |||
| Cumulative exposure: 40 year lag (µg/L-years) |
0–609 | 70 | 276 | 1.00 (Ref) | 1.00 (Ref) |
| 610–5279 | 68 | 278 | 0.96 (0.66–1.40) | 0.97 (0.66–1.43) | |
| ≥5280 | 93 | 254 | 1.44 (1.01–2.06) | 1.53 (1.05–2.23) | |
| p-trend | 0.016 | 0.009 | |||
| Highest year exposure: 0 year lag (µg/L) |
0–60 | 85 | 326 | 1.00 (Ref) | 1.00 (Ref) |
| 61–635 | 68 | 222 | 1.17 (0.82–1.69) | 1.28 (0.88–1.85) | |
| ≥636 | 81 | 271 | 1.15 (0.81–1.62) | 1.28 (0.89–1.84) | |
| p-trend | 0.63 | 0.25 | |||
| Highest year exposure: 40 year lag (µg/L) |
0–60 | 102 | 364 | 1.00 (Ref) | 1.00 (Ref) |
| 61–286 | 49 | 176 | 0.99 (0.68–1.46) | 1.04 (0.70–1.55) | |
| ≥287 | 80 | 268 | 1.07 (0.76–1.49) | 1.16 (0.82–1.64) | |
| p-trend | 0.69 | 0.39 | |||
| Average lifetime exposure: 0 year lag (µg/L) |
0–38 | 74 | 276 | 1.00 (Ref) | 1.00 (Ref) |
| 39–129 | 72 | 279 | 0.96 (0.67–1.39) | 1.02 (0.70–1.48) | |
| ≥130 | 88 | 264 | 1.24 (0.87–1.77) | 1.43 (0.98–2.07) | |
| p-trend | 0.16 | 0.04 | |||
| Average exposure: 40 year lag (µg/L) |
0–22 | 73 | 273 | 1.00 (Ref) | 1.00 (Ref) |
| 23–222 | 74 | 272 | 1.02 (0.71–1.46) | 0.99 (0.68–1.44) | |
| ≥223 | 84 | 263 | 1.19 (0.84–1.71) | 1.36 (0.93–1.97) | |
| p-trend | 0.28 | 0.07 | |||
Abbreviations: CI, confidence interval; OR, odds ratio; Ref, reference group
Adjusted for age (<60, 60–70, >70 years old), sex, BMI (<25, 25–30, ≥30 kg/m2), cancer status, smoking (never-smokers, >0 to <10, ≥10 cigarettes/day)
When analyses were stratified by BMI, ORs for T2D associated with arsenic were markedly higher in subjects who were obese (BMI ≥30 mg/kg2) (Table 3). For example, adjusted T2D ORs for cumulative arsenic exposures lagged 40 years of ≥5280 vs. <610 µg/L-years were 1.45 (95% CI: 0.74–2.84) in those with healthy BMIs, but 2.64 (95% CI: 1.14–6.11) in obese subjects. When cancer cases were excluded, the corresponding ORs were 1.41 (95% CI: 0.58–3.38) and 2.23 (95% CI: 0.74–6.70), respectively (Table S3). A similar pattern was seen for other arsenic exposure metrics although with somewhat lower ORs (data not shown). Figure 1 shows the T2D ORs for each 10,000 µg/L increase in cumulative arsenic exposure (lagged 40 years) by increasing levels of BMI. As shown, arsenic-related T2D ORs increase as BMI increases beginning at BMIs of about 24–25 kg/m2. Similar patterns were seen for unlagged cumulative exposure and average lifetime arsenic exposure although with slightly lower ORs.
Table 3.
Diabetes odds ratios for categories of cumulative arsenic water concentrations lagged 40 years stratified by healthy (<25 kg/m2), overweight (≥25 to <30 kg/m2), and obese (≥30 kg/m2) BMI
| BMI level (kg/m2) |
Arsenic level (µg/L) |
Diabetes |
Unadjusted OR (95% CI) |
Adjusted* OR (95% CI) |
|
|---|---|---|---|---|---|
| Yes | No | ||||
| Healthy (<25) |
0–609 | 18 | 102 | 1.00 (Ref) | 1.00 (Ref) |
| 610–5279 | 16 | 116 | 0.78 (0.38–1.61) | 0.75 (0.36–1.56) | |
| ≥5280 | 32 | 123 | 1.47 (0.78–2.78) | 1.45 (0.74–2.84) | |
| p-trend | 0.08 | 0.09 | |||
| Overweight (≥25 to <30) |
0–609 | 27 | 113 | 1.00 (Ref) | 1.00 (Ref) |
| 610–5279 | 34 | 117 | 1.22 (0.69–2.15) | 1.12 (0.62–2.00) | |
| ≥5280 | 43 | 111 | 1.62 (0.94–2.80) | 1.34 (0.76–2.35) | |
| p-trend | 0.08 | 0.31 | |||
| Obese (≥30) |
0–609 | 25 | 61 | 1.00 (Ref) | 1.00 (Ref) |
| 610–5279 | 18 | 45 | 0.98 (0.48–2.00) | 1.04 (0.49–2.20) | |
| ≥5280 | 18 | 20 | 2.20 (1.00–4.83) | 2.64 (1.14–6.11) | |
| p-trend | 0.04 | 0.02 | |||
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio; Ref, reference group
Adjusted for age (<60, 60–70, >70 years old), sex, cancer status, smoking (never-smokers, >0 to <10, ≥10 cigarettes/day)
Similar findings among cancer cases vs controls
The ORs used in the calculations of the Rothman synergy index are shown in Table 4. The T2D adjusted ORs for people with elevated BMI and low arsenic, people with elevated arsenic and low BMI, and people with both elevated arsenic and BMI were 1.99 (95% CI: 1.30–3.05), 1.41 (95% CI: 0.99–2.00), and 4.48 (95% CI: 2.25–8.92), respectively, with a Rothman synergy index of 2.49 (95% CI: 0.87–7.09) (Table 4). When cancer cases were excluded, the corresponding ORs were 2.29 (95% CI: 1.36–3.87), 1.43 (95% CI: 0.90–2.28), and 6.39 (95% CI: 2.63–15.55), with a Rothman synergy index of 3.12 (95% CI: 0.91–10.70) (Table S4). Clear evidence of synergy was not seen for BMI at ages 20 or 40 (data not shown).
Table 4.
Diabetes odds ratios and Rothman synergy indices by categories of low and high BMI and arsenic
| BMI* | Arsenic* | Diabetes |
Unadjusted OR (95% CI) |
Adjusted# OR (95% CI) |
|
|---|---|---|---|---|---|
| Yes | No | ||||
| Low | Low | 95 | 448 | 1.00 (Ref) | 1.00 (Ref) |
| High | Low | 43 | 106 | 1.91 (1.26–2.91) | 1.99 (1.30–3.05) |
| Low | High | 75 | 234 | 1.51 (1.07–2.13) | 1.41 (0.99–2.00) |
| High | High | 18 | 20 | 4.24 (2.16–8.33) | 4.48 (2.25–8.92) |
| Rothman synergy index | 2.28 (0.82–6.36) | 2.49 (0.87–7.09) | |||
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio; Ref, reference group
Low and high BMI defined as < and ≥30 kg/m2. Low and high arsenic defined as <5280 and ≥5280 cumulative arsenic water concentration lagged 40 years
Adjusted for age (<60, 60–70, >70 years old), sex, cancer status, smoking (never-smokers, >0 to <10, ≥10 cigarettes/day)
4. Discussion
Overall, we found evidence of an association between high exposures to arsenic in drinking water and increased risks of T2D. These results are consistent with several other studies which have found associations at high exposure levels (e.g. arsenic water concentrations >200 µg/L) (Huang et al. 2011; Maull et al. 2012). We did not find evidence of strong associations at more moderate exposure levels (e.g. <200 µg/L). However, our study did not have sufficient statistical power to detect ORs much below 1.5 in each tertile, which may have limited our ability to detect associations at these more moderate exposure levels. A unique aspect of our study is that we identified higher arsenic-associated T2D ORs in subjects with higher BMIs, results that provide evidence that arsenic and excess BMI interact in a greater than additive manner to increase the risk of T2D. The consistency of findings across several arsenic exposure metrics and cut-off points further support these conclusions (Table S1).
Findings from other investigations support the biological plausibility of our results. For example, a number of studies have shown that arsenic can play a role in glucose dysfunction and insulin resistance (Tseng 2004). Arsenic increases the production of ROS and free radicals, which have been implicated in chronic inflammation and apoptosis of pancreatic β-cells (Martin et al. 2017). Arsenic also upregulates the expression of inflammatory genes such as NFkB, tumor necrosis factor- α(TNF- α), and interleukin (IL)-6 (Tseng 2004). These inflammatory factors can injure the pancreas and interfere with glucose-mediated insulin secretion (Martin et al. 2017; Walton et al. 2004). Several environmental pollutants have been shown to impair thermogenic adipose tissue involved in glucose and lipid metabolism (Zhang et al. 2016), and arsenic’s effect on adiposity, specifically on brown and beige adipocytes, may also be a mechanism by which it affects diabetes risk (Maull et al. 2012).
The results from previous studies also support our findings that arsenic and obesity may act synergistically. In Taiwan, although T2D was not specifically assessed, chronic arsenic exposure and obesity were reported to synergistically contribute to insulin resistance in children (Lin et al. 2014). In Bangladesh, some evidence of synergy for T2D was seen between arsenic in water and BMIs >25 kg/m2, although <10% of subjects had diabetes and <1% were obese (Pan et al., 2013). In mice, chronic arsenic exposure was shown to act synergistically with high fat diet-induced obesity to produce glucose intolerance (Paul et al. 2007; 2011).
Although the mechanism by which arsenic and excess BMI may act synergistically is unknown, several possibilities exist. Obesity is associated with chronic inflammation and pro-inflammatory markers like C-reactive protein (CRP), TNF- α, interleukins (IL)-6, −8, −12, plasminogen activator inhibitor (PAI)-1, vascular endothelial growth factor (VEGF), and monocyte chemoattractant protein (MCP)-1 (Dutta et al. 2015; Ramos et al. 2003; van Kruijsdijk et al. 2009; Weisberg et al. 2003). These same markers are also increased by arsenic. For example, in two studies from arsenic-exposed regions in West Bengal, increased IL-6, IL-8, IL-12 and MCP-1, CRP, TNF- α were observed in subjects chronically exposed to arsenic via drinking water (Das et al. 2012; Sinha et al. 2014). Another mechanism may involve mitochondrial dysfunction (Divya et al. 2015; Pan et al. 2016; Bournat and Brown 2010; Jelenik and Roden 2013). Obesity is associated with a decreased number and function of mitochondria. This decline in density and function impairs fatty acid oxidation, contributing to lipid accumulation, increases in ROS and stress kinases, and decreased glucose uptake (Koves et al. 2008; Bournat and Brown 2010). A number of studies have also linked arsenic to increases in ROS (Tseng et al. 2004; Maull et al. 2012), and mitochondrial dysfunction has been shown to be a significant contributor to arsenic-induced ROS (Divya et al. 2015; Pan et al. 2016). For example, arsenic has been shown to downregulate the expression of key proteins involved in mitochondrial respiration and biogenesis in both adipose tissue and skeletal muscle (Divya et al. 2015). Chronic arsenic exposure increases oxidative stress, which may induce changes in the mitochondrial membrane potential that decrease the expression of important ROS scavengers, affecting glucose transporters and insulin-stimulated glucose uptake (Divya et al. 2015; Pan et al. 2016). Overall, arsenic and obesity have been shown to impact a variety of the same processes thought to be involved in T2D development. Importantly though, the actual mechanism by which arsenic impacts T2D and the mechanism by which it may interact with obesity are unknown, and further research is needed to explore these issues.
Misclassification of BMI could have resulted from our use of self-reported height and weight. The literature suggests that women and men tend to under- and over-estimate their weights, respectively (Perry et al. 1995). Importantly though, despite these individual-level errors, self-reports seem to be fairly accurate for classifying subjects relative to one another. For example, in a study of 6,101 subjects in the US National Health and Nutrition Examination Survey (NHANES), measured weights from 10–20 years prior were recalled incorrectly by an average of 3.9 lbs. (Kovalchik 2009). However, the correlation coefficient between measured and recalled weights was 0.96. This high correlation suggests that despite widespread under- or over-estimation, recalled weight can be used to fairly accurately place subjects into low and high categories, like those used in our study. In addition, despite the presence of inherent biases in the self-reporting of anthropometric data, research suggests self-reported weight, height, and BMI to be adequate estimates in population studies (Fonseca et al. 2004). Data from the NHANES show that shifting social attitudes since the 1990s regarding obesity have increased the accuracy of self-reported weight and height metrics, leading to more accurate BMI categorizations from questionnaires (Stommel and Osier 2013).
Another potential issue is confounding. Obesity and increasing age are by far the greatest risk factors for T2D but our results were either adjusted for or stratified on both of these factors. Other factors including diet, smoking, race and SES can be related to both obesity and T2D but these were either not associated with or were only weakly associated with arsenic exposure in our study, or adjusting for them had little impact on results. Other factors like rare genetic or medical conditions are likely not prevalent enough to cause major confounding in this study (Axelson, 1978). Overall, while residual confounding by the factors we evaluated or confounding by some unknown variable is possible, this seems an unlikely cause for the associations observed in this study.
It has been estimated that 22.5% of diabetics in Chile are not diagnosed (Ministerio de Salud, 2009). Chile has a public health care system with wide access. As such, the bias from under-diagnosis of diabetes would most likely be non-differential and towards the null, not towards the positive results we identified. Correcting the OR of 1.53 we identified for cumulative arsenic exposure >5280 µg/L-year for a non-differential under-diagnosis rate of 22.5% would give an OR of 1.57, a relatively small increase. Misclassification of arsenic exposure may have occurred from missing exposure data, inexact residential history, or arsenic exposure from non-water sources (Steinmaus et al. 2015). Since arsenic exposure was assessed similarly in diabetics and non-diabetics, any bias would likely be non-differential, and most likely bias ORs towards the null. Furthermore, arsenic exposure assessment was primarily based on the cities and towns where the subjects lived, and errors in the subjects’ recall of their residential history is most likely minimal. Non-water sources of arsenic exposure are probably negligible compared to historical exposures from water, as most of the food in this region is imported due to the arid climate and limited land use for agriculture. Air and food samples tested for arsenic revealed relatively low arsenic concentrations, with similar levels in the parts of our study area with and without high arsenic water concentrations, and generally accounted for inorganic arsenic intakes of roughly <1–13 µg/day (Ferreccio and Sancha 2006). In contrast, intakes from water would be about 1720 µg/day in those drinking 2 L/day of water with arsenic concentrations of 860 µg/L, the level in the large city of Antofagasta during the high exposure period.
We evaluated whether the cancer case-control study design we used here could have introduced major bias, and found fairly strong evidence that it did not. Cancer cases were ascertained identically throughout the study region, regardless of arsenic exposure levels (Steinmaus et al. 2014). Most importantly, bias might have occurred if the cancer cases in our study had higher rates of T2D than the cancer-free controls. Importantly, we did not find any evidence of an association between T2D and lung and kidney cancer, the two cancer types we included here. We did find evidence that bladder cancer might be related to T2D in our study population, but these subjects were excluded from our main analyses. In addition, we repeated all analyses after excluding all cancer cases, and found that these exclusions had little impact on the odds ratio estimates, although precision was reduced by having smaller numbers.
The non-cancer participants used as controls in our underlying cancer case-control study were randomly selected from the Chilean voter registration list, matched to cancer cases by age and sex. At the time of selection, the cities of residence of these participating controls were similar to the population distribution of the 2002 Chilean census, providing evidence that they were a good representation of the study area given the matching criteria (Steinmaus et al. 2014). Matching of controls to cancer cases was done in our original study to help limit the impact of these variables on our arsenic-cancer analyses. Importantly though, this matching was done independently of either arsenic exposure or of T2D status. As such, it is unlikely to have introduced selection bias or to have caused the elevated ORs for T2D we identified. Overall, we found little evidence that the underlying case-control study design used here caused major bias.
5. Conclusions
Overall, our study is the first human epidemiologic investigation of T2D to identify a synergistic relationship between arsenic and obesity. Clearly, obesity is the primary risk factor for T2D and most efforts aimed at preventing and treating T2D should focus on preventing or reducing obesity. However, the synergistic relationship we identified between arsenic and BMI suggests that arsenic might worsen or increase the risk of obesity-related T2D. Given the current obesity epidemic and the widespread occurrence of arsenic exposure worldwide, this synergistic relationship could have significant public health implications. Because our findings are novel, future research in arsenic exposed areas like Northern Chile, which have a wide range of arsenic exposure, good data on lifetime exposure, and adequate information on potential confounding variables, should seek to replicate these findings. The mechanism of the synergistic relationship we identified is unknown although several possibilities exist. New information on this mechanism could help further support the biologic plausibility for our findings and might provide insights into new ways to diagnose, prevent, or treat T2D.
Supplementary Material
Acknowledgments:
This project was funded by grants R01 ES014032 and P42 ES04705 from the National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
The authors declare they have no actual or potential competing financial interests.
References
- ATSDR. 2013. Priority List of Hazardous Substances Agency for Toxic Substances and Disease Registry, Atlanta GA: Available: http://www.atsdr.cdc.gov/spl/ [accessed 05/18/2015]. [Google Scholar]
- ATSDR. 2007. Toxicological Profile for Arsenic U.S., Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta, GA: Available: https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf [accessed 05/18/2015]. [PubMed] [Google Scholar]
- Axelson O 1978. Aspects on confounding in occupational health epidemiology. Scand J Work Environ Health 4: 85–89. [DOI] [PubMed] [Google Scholar]
- Bournat JC, Brown CW. 2010. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes 17(5):446–52. PMID: 20585248 PMCID: PMC5001554 DOI: 10.1097/MED.0b013e32833c3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das N, Paul S, Chatterjee D, Banerjee N, Majumder NS, Sarma N, et al. 2012. Arsenic exposure through drinking water increases the risk of liver and cardiovascular diseases in the population of West Bengal, India. BMC Public Health 10(12):639 PMID: 22883023 PMCID: PMC3441389 DOI: 10.1186/1471-2458-12-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya PS, Pratheeshkumar P, Son YO, Vinod RR, Hitron AJ, Kim D, Dai J, et al. 2015. Arsenic induces insulin resistance in mouse adipocytes and myotubes via oxidative stress-regulated mitochondrial sirt3-FOXO3a signaling pathway. Toxicol Sci 146(2):290–300. PMID: 25979314 DOI: 10.1093/toxsci/kfv089. [DOI] [PubMed] [Google Scholar]
- Dutta K, Prasad P, Sinha D. 2015. Chronic low level arsenic exposure evokes inflammatory responses and DNA damage. Int J Hyg Environ Health 218(6):564–74. PMID: 26118750 DOI: 10.1016/j.ijheh.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Ferreccio C, González C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. 2000. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology 11:673–79. PMID: 11055628. [DOI] [PubMed] [Google Scholar]
- Ferreccio C, Sancha AM. 2006. Arsenic exposure and its impact on health in Chile. J Health Popul Nutr 24:164–75. PMID: 17195557. [PubMed] [Google Scholar]
- Fonseca MeJ, Faerstein E, Chor D, Lopes CS. 2004. Validity of self-reported weight and height and the body mass index within the “Pró-saúde” Study. Rev Saude Publica 38:392–398. PMID: 15243669 DOI: /S0034–89102004000300009. [DOI] [PubMed] [Google Scholar]
- Huang CF, Chen YW, Yang CY, Tsai KS, Yang RS, Liu SH. 2011. Arsenic and diabetes:Current perspectives. Kaohsiung Journal of Medical Sciences 27(9):402–10. DOI:10.1016/j.kjms.2011.05.008. [DOI] [PubMed] [Google Scholar]
- IARC. 2004. Arsenic and arsenic compounds International Agency for Research on Cancer Monographs; 100C Available: http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-6.pdf [accessed 05/15/2017]. [Google Scholar]
- IARC. 2012. A review of human carcinogens: Arsenic, metals, fibres, and dusts International Agency for Research on Cancer; 100C Available: http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C.pdf [accessed 05/15/2017]. [Google Scholar]
- Jelenik T and Roden M. 2013. Mitochondrial Plasticity in Obesity and Diabetes Mellitus. Antioxid Redox Signal 19(3):258–68. DOI: 10.1089/ars.2012.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchik S 2009. Validity of adult lifetime self-reported body weight. Public Health Nutr 12(8):1072–7. PMID: 18789171 DOI: 10.1017/S1368980008003728 [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7(1):45–56. PMID: 18177724 DOI: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lin HC, Huang YK, Shiue HS, Chen LS, Choy CS, Huang SR, Han BC, et al. 2014. Arsenic methylation capacity and obesity are associated with insulin resistance in obese children and adolescents. Food Chem Toxicol 74:60–7. DOI: 10.1016/j.fct.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Marshall T 2012. Arsenic in apple juice: How much is too much? Tampa Bay Times Available: http://www.tampabay.com/news/health/arsenic-in-apple-juice-how-much-is-too-much/1079395 [accessed 09/08/2017].
- Martin EM, Styblo M, Fry RC. 2017. Genetic and epigenetic mechanisms underlying arsenic-associated diabetes mellitus: a perspetive of the current evidence. Epigenomics 9(5):701–10. PMID: 28470093 PMCID: PMC5480787 [Available on 2018-05-01] DOI: 10.2217/epi-2016-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Sc. 2012. Evaluation of the association between arsenic and diabetes: A National Toxicology Program workshop review. Environ Health Perspect 120:1658–1670. PMID: 22889723 PMCID: PMC3548281 DOI: 10.1289/ehp.1104579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio de Salud. Gobierno de Chile. Departmento de Epidemiologia (2017). “Encuesta Nacional de Salud 2016–2017: Primaeros resultatdos.” Retrieved 4/5/2018, from www.minsal.cl/wp…/ENS-2016-17_PRIMEROS-RESULTADOS.pdf.
- Ministerio de Salud. 2009. Implementación del enfoque de riesgo en el Programa de Salud Cardiovascular Retrieved 4/5/2018, from http://buenaspracticasaps.cl/wp-content/uploads/2014/07/MINSAL-2009-enfoque-riesgo-CV.pdf2009.
- Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. 2013. Roxarsone, inorganic arsenic, and other arsenic species in chicken: A U.S.-based market basket sample. Environ Health Perspect 121:818–24. PMID: 23694900 PMCID: PMC3701911 DOI:10.1289/ehp.1206245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, et al. 2013. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environmental Health Perspect 121:295–302. PMID: 23458756 PMCID: PMC3621177 DOI: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WC, Seow WJ, Kile ML, Hoffman EB, Quamruzzaman Q, Rahman M, Mahiuddin G, Mostofa G, Lu Q, Christiani DC. 2013. Association of low to moderate levels of arsenic exposure with risk of type 2 diabetes in Bangladesh. Am J Epidemiol 15;178:1563–70. DOI: 10.1093/aje/kwt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Jiang L, Zhong L, Geng C, Jia L, Liu S, et al. 2016. Arsenic induces apoptosis by the lyosomal-mitochondrial pathway in INS-1 cells. Environ Toxicol 31(2):133–41. PMID: 25077447 DOI: 10.1002/tox.22027. [DOI] [PubMed] [Google Scholar]
- Paul DS, Hernández-Zavala A, Walton FS, Adair BM, Dedina J, Matousek et al. 2007. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol 222(3):305–14. PMID: 17336358 PMCID: PMC2680915 DOI: 10.1016/j.taap.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DS, Walton FS, Saunders RJ, Styblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. 2011. Environ Health Perspect 119(8):1104–9. PMID: 21592922 PMCID: PMC3237360 DOI:10.1289/ehp.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry GS et al. 1995. The validity of self-reports of past body weights by U.S. adults. Vol.Epidemiology 6(1): 61–66. PMID: 7888448. [DOI] [PubMed] [Google Scholar]
- Ramos EJ, Xu Y, Romanova I, Middleton F, Chen C, Quinn R, et al. 2003. Is obesity an inflammatory disease? Surgery 134(2):329–35. PMID: 12947337 DOI: 10.1067/msy.2003.267. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. 1976. The estimation of synergy or antagonism. Am J Epidemiol 103(5):506–511. PMID: 1274952. [DOI] [PubMed] [Google Scholar]
- Schute N 2013. Arsenic in beer may come from widely used filtering process National Public Radio; Available: http://www.npr.org/sections/thesalt/2013/04/08/176587506/arsenic-in-beer-may-come-from-widely-used-filtering-process [accessed 07/15/2017]. [Google Scholar]
- Sinha D, Mukherjee B, Bindhani B, Dutta K, Saha H, Prasad P, et al. 2014. Chronic low level arsenic exposure inflicts pulmonary and systemic inflammation. J Cancer Sci 6:62–9. DOI:10.4172/1948-5956.1000250. [Google Scholar]
- Smith AH. 2013. Arsenic and diabetes. Environ Health Perspect 121(3):A70–1. DOI: 10.1289/ehp.1206100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C 2005. Arsenic in drinking water: Health effects and current issues Available:https://www.cdc.gov/NCEH/tracking/webinars/sep05/steinmaus.pdf [accessed 07/11/2017].
- Steinmaus C, Ferreccio C, Acevedo J, Yuan Y, Liaw J, Durán V, et al. 2014. Increased lung and bladder cancer incidence in adults after in utero and early-life arsenic exposure. Cancer Epidemiol Biomarkers Prev 23:1529–1538. PMID: 24859871 PMCID: PMC4344186 DOI:10.1158/1055-9965.EPI-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Castriota F, Ferreccio C, Smith AH, Yuan Y, Liaw J, et al. 2015. Obesity and excess weight in early adulthood and high risks of arsenic-related cancer in later life. Environ Res 142:594–601. PMID: 26301739 PMCID: PMC4664040 DOI: 10.1016/j.envres.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus CM, Ferreccio C, Romo JA, Yuan Y, Cortes S, Marshall G, et al. 2013. Drinking water arsenic in Northern Chile: High cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev 22:623–630. PMID: 23355602 PMCID: PMC3848421 DOI:10.1158/1055-9965.EPI-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel M, Osier N. 2013. Temporal changes in bias of body mass index scores based on self-reported height and weight. Int J Obes 37:461–67. PMID: 22546777 PMCID: PMC3595465 DOI: 10.1038/ijo.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA. 2012. Role of Environmental Chemicals in Diabetes and Obesity: A National Toxicology Program Workshop Review. Environ Health Perspect 120(6):779–89. PMID: 22296744 PMCID: PMC3385443 DOI: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CH. 2004. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol 197:67–83. PMID: 15163543 DOI: 10.1016/j.taap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. 2000. Arsenic occurrence in public drinking water supplies EPA-815-R-000-23 Washington, DC: U.S. Environmental Protection Agency; EPA-815-R-00-23 Available:https://www.epa.gov/sites/production/files/2015-09/documents/2005_11_10_arsenic_occurrence-appendices.pdf [accessed 06/15/2017]. [Google Scholar]
- U.S. FDA. 2012. Arsenic in rice: Full analytical results from rice/rice product sampling - September 2012 U.S. Food and drug administration; Available: http://www.fda.gov/Food/FoodborneIllnessContaminants/Metals/ucm319916.htm [accessed 11/29/12]. [Google Scholar]
- van Kruijsdijk RC, van der Wall E, Visseren FL. 2009. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev 18(10):2569–78. PMID: 19755644 DOI: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- Walton FS, Harmon AW, Paul DS, Drobná Z, Patel YM, Styblo M. 2004. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol Appl Pharmacol 198(3):424–33. PMID: 15276423 DOI: 10.1016/j.taap.2003.10.026 [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–808. PMID: 14679176 PMCID: PMC296995 DOI: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D, Hooper C, Shi X. 2012. Arsenic and lead in juice: apple, citrus, and apple-base. J Environ Health 75:14–20. PMID: 23270108. [PubMed] [Google Scholar]
- Zhang G, Sun Q, Liu C. 2016. Influencing factors of thermogenic adipose tissue activity. Front Physiol 7(29):1–11. PMID: 26903879 PMCID: PMC4742553 DOI: 10.3389/fphys.2016.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Magliano DJ, Bennett PH. 2016. Diabetes mellitus statistics on prevalence and mortality: facts and fallacies. Nat Rev Endocrinol 12:616–22. PMID: 27388988 DOI: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


