Non-communicable diseases kill 40 million people each year, accounting for 70% of all deaths globally. In this context, 17.7 million people die annually as a consequence of cardiovascular diseases, whereas cancer accounts for 8.8 million deaths per year (1). Evidence linking cardiovascular diseases with a higher incidence of cancer has been suggested previously in community-based observational trials (2). In 2013, Hasin et al. found an interconnection between heart failure and consequent cancer diagnosis (3). These findings were reproduced in a prospective cohort study of patients with myocardial infarction-induced heart failure (4). Banke et al. observed similar results in a large Danish heart failure cohort (5). Importantly though, these epidemiologic studies do not differentiate between heart failure with preserved or reduced ejection fraction, which may be relevant as these conditions appear distinct.
Shared risk factors and biological mechanisms possibly explain this relationship. Indeed, cardiovascular risk factors such as unhealthy diet, tobacco smoking, obesity, diabetes, and hypertension have been found to be associated with an increased cancer risk (6). Moreover, it has been speculated that cardiovascular drugs (i.e. angiotensin converting enzyme inhibitors and β-blockers), radiation during diagnostic assessment, epigenetic mechanisms, and regenerative signaling are all potential links connecting both illnesses (2,6). With regards to common molecular pathways, chronic inflammation and oxidative stress are likely candidates, as they play central roles in the pathophysiology of both cardiovascular diseases and cancer (6).
Nonetheless, despite the evidence described in the aforementioned population studies and shared risk factors/biological mechanisms, the possibility that other comorbidities may explain the association between cardiovascular disease and cancer cannot be ruled out (7). Moreover, inasmuch as clinical assessment of heart failure patients occurs regularly, an apparent higher risk of cancer may reflect merely earlier diagnosis rather than a higher incidence (7).
The study by Meijers et al. (8) in this issue of Circulation presents new evidence suggesting heart failure triggers subsequent colon cancer. The authors report compelling data using the APCmin mouse strain, which harbors a nonsense mutation in APC leading to persistence of beta-cateinin and a susceptibility to spontaneous intestinal adenoma formation (9–11). Six weeks after the induction of myocardial infarction with subsequent heart failure (left ventricular ejection fraction~32% one week post myocardial infarction), these mice developed an increased number and size of intestinal polyps compared to the sham-operated control. Moreover, they found an association between tumor growth and left ventricular ejection fraction and cardiac fibrosis, both of which correlate with the magnitude of heart failure-induced myocardial remodeling.
To exclude alterations in hemodynamics as the cause of their findings, Meijers et al. transplanted hearts from APCmin mice subjected one week earlier to myocardial infarction or sham operation heterotopically into the cervical region of other APCmin mice (HTx). In this procedure, the external jugular vein and common carotid artery of the recipient mouse were anastomosed with the pulmonary trunk and aorta respectively of the transplanted heart. The performance of the recipient native heart was unaltered. Interestingly, six weeks after the procedure, APCmin mice that were transplanted with infarcted hearts manifested elevated numbers and size of polyps in comparison to mice receiving sham-operated hearts (Figure A).
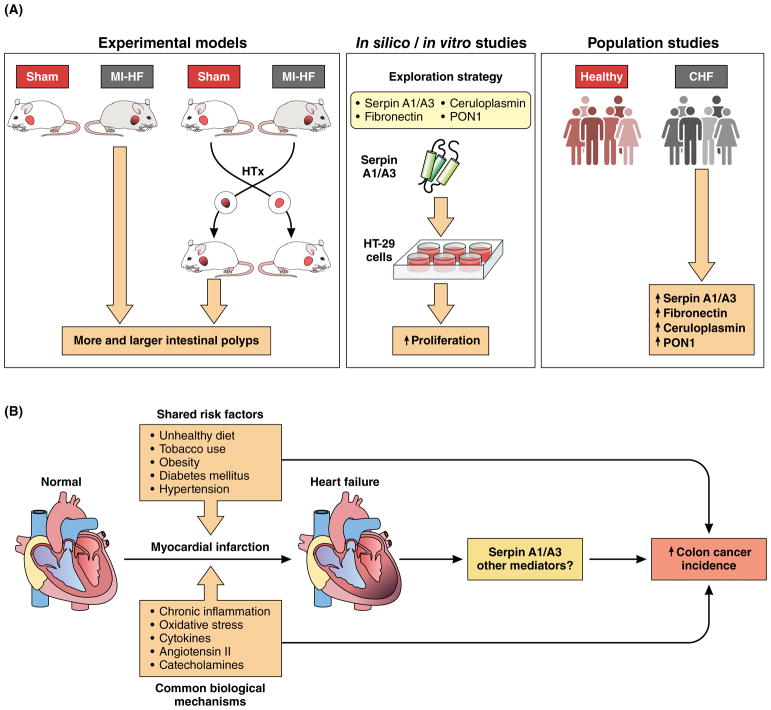
Figure. Failing heart stimulates colon tumor growth.
A, Experimental models (left panel) showed that myocardial infarction-induced heart failure (MI-HF) stimulates pre-cancerous colon tumor growth in APCmin mice. Left ventricular ejection fraction was markedly decreased after myocardial infaction in MI-HF mice compared to sham-operated mice (Sham). MI-HF mice also developed more and larger intestinal polyps than Sham mice. Failing hearts heterotopically (HTx) transplanted to APCmin recipient mice trigger the same tumorigenic effect. In silico studies (middle panel). Exploration strategy identified alpha-1-antitrypsin (SerpinA1), alpha-1-antichymotrypsin (SerpinA3), fibronectin (FN), cerulopasmin (CP), and paraoxonase 1 (PON1) as potential circulating factors responsible for promoting tumorigenesis. In vitro studies (middle panel) showed that Serpin A1/A3 elicited proliferation of the HT-29 cancer cell line. Population studies (right panel) showed that plasma levels of SerpinA1, SerpinA3, FN, CP and PON1 were increased in chronic heart failure (CHF) patients compared to healthy patients. B, Proposed model, whereby post-MI induced HF promotes colon tumor growth. Shared risk factors, such as unhealthy diet, tobacco smoking, obesity, diabetes mellitus and hypertension are potential links between both diseases. Mechanistically, chronic inflammation, oxidative stress, cytokines, angiotensin II and catecholamines are all plausible mediators contributing to this connection as they play a role in both cancer and cardiovascular diseases.
Important questions arise from these novel findings. Can these observations be extrapolated to other types of cancer? Would heart failure of non-ischemic origin elicit the same tumorigenic response? Since heart failure triggered tumor growth, could it also promote metastasis?
Pursuing mechanism, Meijers et al. reviewed the literature concerning proteins released after myocardial infarction and identified five of potential relevance: alpha-1-antitrypsin (SerpinA1), alpha-1-antichymotrypsin (SerpinA3), fibronectin (FN), cerulopasmin (CP), and paraoxonase 1 (PON1). The mRNA levels of all these proteins were elevated in the left ventricles of failing APCmin mice, but this result was replicated only for SerpinA3, FN and Pon1 in the HTx model (Figure A). The authors went on to show that the addition of SerpinA1 and SerpinA3 to the cell culture media promoted proliferation of the colorectal cell line HT-29 (Figure A). These findings would have been strengthened by the demonstration of increases in the protein abundance of these candidate mediators in the heart and/or blood. In addition, such measurements would have allowed the investigators to probe whether levels of these mediators correlate with tumor load. This limitation notwithstanding, these data are quite provocative.
Finally, this study provides evidence for the translational value of the reported results by showing increased plasma levels of their 5 candidate proteins in the plasma of 101 patients with chronic heart failure compared to 180 healthy patients enrolled in the Prevention of Renal and Vascular ENd-stage Disease (PREVEND) study (12,13) (Figure A). Also, they observed that augmented heart failure biomarkers, as well as inflammation-related proteins, were predictive of incident cancer independent of cancer risk factors.
While the authors’ findings shed light on possible molecules mediating the observed effects, a more comprehensive mechanistic assessment will be required to definitively delineate links between heart disease and cancer (Figure B). Moreover, an unbiased proteomics approach – rather than a candidate approach – would likely be better suited to reveal the most important mediators connecting the failing heart with tumor growth. Ultimately, however, multifaceted experimental approaches to reduce the abundance of these mediators will be required to prove cause-and-effect.
Although further research is needed to confirm and deepen these findings, these are potentially groundbreaking results that will stimulate further delineation of the connections between heart disease and cancer. This study highlights how heart disease impacts cancer just as other work has demonstrated important effects of cancers (14) and cancer treatments (15) on cardiac structure and function. We may be at the gates of a new scientific research field.
Acknowledgments
Dr Kitsis thanks the Wilf Family for their generous support. We also thank to Dr. R. Bravo for his help with figures.
FUNDING SOURCES
Dr Kitsis was supported by grants from the National Institutes of Health (R01HL128071, R01HL130861, R01HL138475, R01CA170911), DOD (PR151134P1), AHA (15CSA26240000), Fondation Leducq (RA15CVD04), and the Dr. Gerald and Myra Dorros Chair in Cardiovascular Disease. Dr. Lavandero was supported by Comision Nacional de Investigacion Cientifica y Tecnologica (CONICYT), Chile, Fondo de Financiamiento Centros de Excelencia en Investigación en Áreas Prioritarias (FONDAP) grant 51300011 and Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) grant 1161156. J.A.R was supported by post-doctoral FONDECYT grant 3160298.
Footnotes
DISCLOSURES
None.
References
- 1.World Health Organization (WHO) NLM classification: WT 500. Geneva, Switzerland: 2014. Global status report on noncommunicable diseases. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1. [Google Scholar]
- 2.Hasin T, Iakobishvili Z, Weisz G. Associated risk of malignancy in patients with cardiovascular disease: evidence and possible mechanism. Am J Med. 2017;130:780–785. doi: 10.1016/j.amjmed.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 3.Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, Cerhan JR, Roger VL. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62:881–886. doi: 10.1016/j.jacc.2013.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasin T, Gerber Y, Weston SA, Jiang R, Killian JM, Manemann SM, Cerhan JR, Roger VL. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol. 2016;68:265–271. doi: 10.1016/j.jacc.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banke A, Schou M, Videbaek L, Møller JE, Torp-Pedersen C, Gustafsson F, Dahl JS, Køber L, Hildebrandt PR, Gislason GH. Incidence of cancer in patients with chronic heart failure: a longterm follow-up study. Eur J Heart Fail. 2016;3:260–266. doi: 10.1002/ejhf.472. [DOI] [PubMed] [Google Scholar]
- 6.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deswal A, Basra SS. Incident cancer in patients with heart failure: causation or mere association? J Am Coll Cardiol. 2013;62:887–888. doi: 10.1016/j.jacc.2013.04.087. [DOI] [PubMed] [Google Scholar]
- 8.Meijers WC, Maglione M, Bakker SJL, Oberhuber R, Kieneker LM, de Jong S, Nagengast WB, Lyon AR, van der Vegt B, van Veldhuisen DJ, Westenbrink BD, van der Meer P, Silljé HHW, de Boer RA. The failing heart stimulates tumor growth by circulating factors. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.117.030816. in press. [DOI] [PubMed] [Google Scholar]
- 9.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 10.Moser AR, Luongo C, Gould KA, McNeley MK, Shoemaker AR, Dove WF. ApcMin: a mouse model for intestinal and mammary tumorigenesis. Eur J Cancer. 1995;31A:1061–1064. doi: 10.1016/0959-8049(95)00181-h. [DOI] [PubMed] [Google Scholar]
- 11.Hankey W, Frankel WL, Groden J. Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: implications for therapeutic targeting. Cancer Metastasis Rev. 2018;37:159–172. doi: 10.1007/s10555-017-9725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroten NF, Ruifrok WPT, Kleijn L, Dokter MM, Silljé HH, Heerspink JL, Bakker SJL, Kema IP, van Gilst WH, van Veldhuisen DJ, Hillege HL, de Boer RA. Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: An open-label, blinded end point, randomized prospective trial (VitD-CHF trial) Am Heart J. 2013;166:357–364 e2. doi: 10.1016/j.ahj.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Hillege HL, Janssen WMT, Bak AAA, Diercks GFH, Grobbee DE, Crijns HJGM, Van Gilst WH, De Zeeuw D, De Jong PE. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–526. doi: 10.1046/j.1365-2796.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 14.Cosper PF, Leinwand LA. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res. 2011;71:1710–1720. doi: 10.1158/0008-5472.CAN-10-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. 2016;375:1457–1467. doi: 10.1056/NEJMra1100265. [DOI] [PubMed] [Google Scholar]