Introduction
In choroiditis, fundoscopic examination is very limited. Only the choroidal foci of sufficient importance causing yellow-white discoloration can be visible through the retinal pigment epithelium (RPE). This is the reason why several inflammatory choroidal entities, with different pathophysiologic mechanisms, were grouped under the general term “white dot syndromes”.1 With the advent of indocyanine green angiography (ICGA), we gained access to the choroidal compartment which allowed the differentiation between the two main mechanisms at the origin of choroidal inflammatory pathology: choriocapillaris diseases (inflammatory choriocapillaropathies/choriocapillaritis) and stromal diseases (stromal choroiditis). Primary inflammatory choriocapillaropathies include multiple evanescent white dot syndrome (MEWDS), acute posterior multifocal placoid pigment epitheliopathy (APMPPE), idiopathic multifocal choroiditis (MFC), serpiginous choroiditis as well as acute macular neuroretinopathy such as acute zonal occult outer retinopathy (AZOOR).2, 3 In these conditions, ICGA shows patchy or geographic hypofluorescent areas of variable sizes more clearly visible on the late frames. These areas correspond to areas of hypo or non-perfusion of the choriocapillaris. Recently, optical coherence tomography angiography (OCT-A), a new imaging technique which allows visualization of the retinal and choroidal vasculature, was developed. It has the advantage of being fast and easy to acquire, non-invasive, and depth-selective.4 OCT-A of active lesions of APMPPE and serpiginous choroiditis revealed areas of non-perfused choriocapillaris which corresponded topographically to hypofluorescent areas in ICGA,5, 6 supporting the theory of choriocapillaris hypo and/or non-perfusion as the origin of these diseases. However, a recent study by Pichi et al.7 has created doubt about choriocapillaritis being the origin of the morphological and functional alterations in MEWDS,7 as OCT-A seems not to show any alterations in choriocapillaris circulation. We present in detail the reasons why choriocapillaritis should not be discarded as the origin of the pathological lesions in MEWDS.
Arguments in favor of MEWDS being a primary choriocapillaritis
Choriocapillaritis entities belong to the same nosological group
Numerous reports indicate that primary choriocapillaritis entities (i.e., MEWDS, APMPPE, MFC, serpiginous choroiditis, and intermediary forms) belong to the same nosological group.8, 9, 10, 11 These are young patients who present with uniform symptoms described as blurred vision, photopsias, and visual field disturbances12, 13, 14 probably caused by ischemic damage to photoreceptor outer segments due to inflammatory non-perfusion of the choriocapillaris. In more than 50% of primary choriocapillaritis patients, a viral flu-like episode precedes the onset of the disease.8, 11 MEWDS patients conform perfectly to all points of this nosological ensemble. For more than two decades, this group has also been united by ICG angiographic findings showing diverse patterns of hypofluorescence depending on the level and importance of choriocapillaris involvement. Most reports for nearly three decades have interpreted these ICGA signs as choriocapillaris non-perfusion.
Therefore, it is very unlikely that these ICGA findings are suddenly attributed to a new, questionable mechanism solely for MEWDS and not other choriocapillaritis entities.
Different choriocapillaritis entities can occur in the same patient
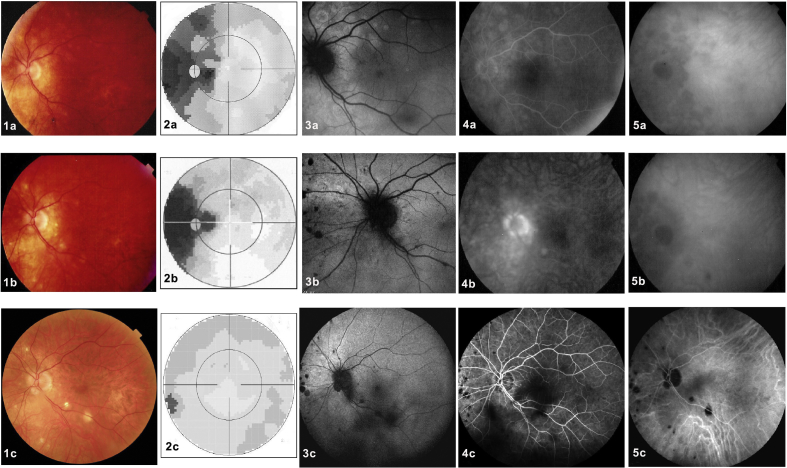
In addition to similar nosological characteristics, indicating a similar physiopathological process and the involvement of a similar structure, namely the choriocapillaris, numerous reports have shown that more than one type of choriocapillaritis can occur in the same patient,15, 16, 17, 18, 19, 20 underlining the unitarian character of this group of disorders. MEWDS patients that have evolved to MFC have been described, supporting the hypothesis of a common mechanism. Fig. 1 shows an overlapping case of MEWDS with MFC.
Fig. 1.
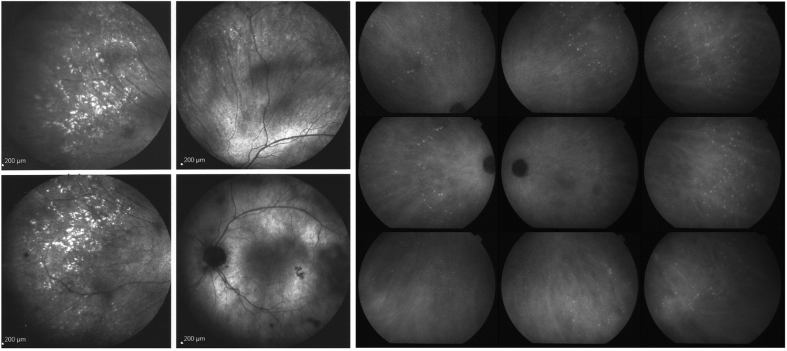
An example of two different choriocapillaritis entities occurring in the same patient. A 40-year-old woman presented with decreased visual acuity in her left eye associated with photopsias. Best corrected visual acuity (BCVA) was 20/20 in the right eye and 20/32 in the left eye. Anterior segment biomicroscopy and intraocular pressure were normal in both eyes. (1a) Color fundus photography of the left eye revealed subtle deep retinal white spots in the posterior pole around the optic disk and in the midperipheral nasal retina. (2a) The visual field revealed enlargement of the blind spot and a temporal scotoma. (3a) Hyperautofluorescent areas on fundus autofluorescence (FAF), which were (4a) early hyperfluorescent in fluorescein angiography (FA), and (5a) late hypofluorescent on indocyanine green angiography (ICGA) co-localized with the fundus lesions. These are all disease-defining characteristics of multiple evanescent white dot syndrome (MEWDS). Recovery was complete at 10 weeks without any treatment. One year later, the patient presented again with scotomas accompanied by photopsias in her left eye. (1b) The fundus exhibited chorioretinal scars nasally to the optic disc, characteristic of idiopathic multifocal choroiditis (MFC). (2b) Visual field testing revealed a new temporal scotoma. (3b) Both FAF and (4b) FA revealed only faint lesions, (5b) whereas on late ICGA, new hypofluorescent lesions were evident, a disease pattern corresponding to MFC. Lesions disappeared a month after a sub-Tenon injection of triamcinolone acetonide (40 mg). (2c) At the last follow-up, the visual field was normal, (1c) but chorioretinal scars were visible in the color fundus photograph, (2c) the visual field recovered, and (3c) the FAF, (4c) FA, and (5c) ICGA normalized except for the presence of scars.
Absence of OCT-A signs does not mean absence of perfusion pathology in the choriocapillaris
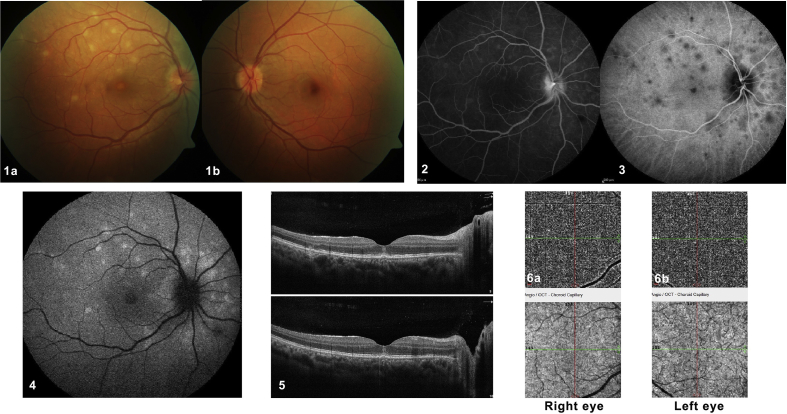
Pichi et al.7 based their hypothesis of absence of involvement of the choriocapillaris due to the lack of abnormalities on OCT-A and suggested that MEWDS is a primary disease of the photoreceptor/RPE complex. In our opinion, a normal OCT-A is not enough to discard the long-lasting theory that MEWDS is a choriocapillaritis. OCT-A generates images of the retinal and choroidal vasculature by detecting the movement of red blood cells inside the vessels between two consecutive scans of the same area.21 As this method detects vascular blood flow within a determined time frame, there is a slowest and fastest detectable blood flow.22 If the vascular flow is outside this range, the OCT-A will not capture it. At the posterior pole, the choriocapillaris is a dense and complex network of capillaries22, 23, 24 in which the blood flows slowly.25 With current commercially available technology, evaluation of the choriocapillaris is still beyond the capacity of OCT-A, which is not able to identify small low-flow vessels.22, 23, 26, 27 As MEWDS is an end-capillary disease with very slow flow in these tiny capillaries, we deduce that the reason for the lack of abnormalities in OCT-A is simply because this device is unable to detect the much reduced vascular flow at this level. Fig. 2 illustrates a typical MEWDS case with normal OCT-A.
Fig. 2.
A classical case of multiple evanescent white dot syndrome (MEWDS) with absent signs on optical coherence tomography angiography (OCT-A). A 31-year-old woman presented with acute blurred vision in her right eye. Upon ocular examination, her best corrected visual acuity (BCVA) was 20/50 in the right eye and 20/20 in the left eye. Biomicroscopy of the anterior segment and intraocular pressure were normal in both eyes. (1a) Color fundus photography of the right eye revealed deep retinal white spots in the posterior pole and around the optic disc. (2) These lesions corresponded topographically to early hyperfluorescent lesions in fluorescein angiography (FA), (3) late hypofluorescent lesions on indocyanine green angiography (ICGA), and (4) hyperautofluorescent lesions on fundus autofluorescence (FAF). (5a and 5b) Cross-sectional optical coherence tomography (OCT) scans demonstrated disruption of the ellipsoid zone corresponding to the deep retinal white spots seen by color photography and FAF. These are all disease-defining characteristics of multiple evanescent white dot syndrome (MEWDS). (6a and 6b) When analyzing the optical coherence tomography angiography (OCT-A), we found no visible pathological alterations or differences between the two eyes. Recovery was complete after 10 weeks without any treatment.
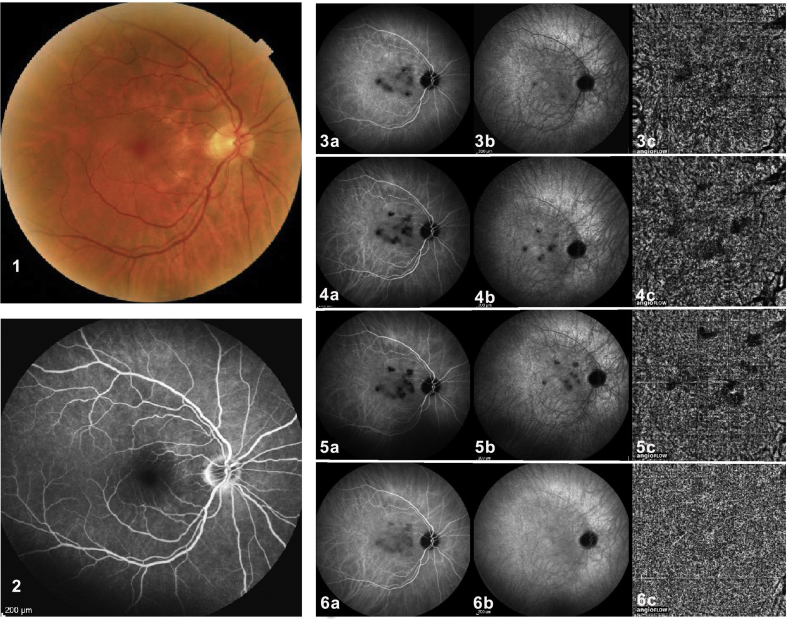
Primary inflammatory choriocapillaropathies are a disease spectrum ranging from benign diseases, such as MEWDS, to chorioretinal-destroying diseases, such as APMPPE, MFC, and serpiginous choroiditis.8 In our opinion, the clinical and angiographic phenotype is determined by the extension, severity, and level of vaso-occlusive process. MEWDS is probably characterized by a reversible involvement of small vessels, whereas larger vessels are involved in APMPPE, MFC, and serpiginous choroiditis. The lack of sensitivity of OCT-A in detecting fine choriocapillaris non-perfusion areas can be further illustrated by a case report of serpiginous choroiditis (Fig. 3).5
Fig. 3.
Comparison of indocyanine green angiography (ICGA) and optical coherence tomography angiography (OCT-A) findings in a case of serpiginous choroiditis. A 65-year-old man presented with blurred vision in his left eye for 2 months. Ocular examination revealed a best corrected visual acuity (BCVA) of 1.25 in both eyes. Biomicroscopy of the anterior segment and intraocular pressure were normal in both eyes. Color fundus photography revealed typical serpiginoid lesions in the left fundus (not shown) but a (1) quasi-normal right eye showing a few dyspigmented areas. (2) Fluorescein angiography (FA) was normal in the right eye. (3a: intermediate & 3b: late) Indocyanine green angiography (ICGA) revealed hypofluorescent areas in the right and left eyes (not shown), indicating areas of non-perfusion in the posterior pole choriocapillaris. Based on the bilateral involvement and the clinical presentation, FA, ICGA, and the negative work-up for systemic or infectious disease, the patient was diagnosed with serpiginous choroiditis. (4a and 4b) After sub-Tenon triamcinolone acetonide therapy and (5a and 5b) subsequent mycophenolic acid therapy, ICGA signs did not change substantially. (6a and 6b) After treatment with oral prednisone, cyclosporine, and mycophenolic acid, hypoperfused areas decreased substantially on ICGA and were only faintly visible on intermediate phase ICGA frames. (3c, 4c, and 5c) OCT-A revealed non-perfused areas in parallel with ICGA lesions before aggressive triple immunosuppressive treatment was introduced. (6a) After aggressive therapy, faint hypofluorescent lesions were only seen on intermediate ICGA frames and (6b) not on late frames. (6c) No anomaly was seen any more on OCT-A, indicating that ICGA was more sensitive than OCT-A for occult subclinical hypoperfusion, and that a normal image on OCT-A does not exclude hypoperfusion.
Hypofluorescence on ICGA can not be explained by absence of fixation of ICG as diseased areas tend to fix ICG
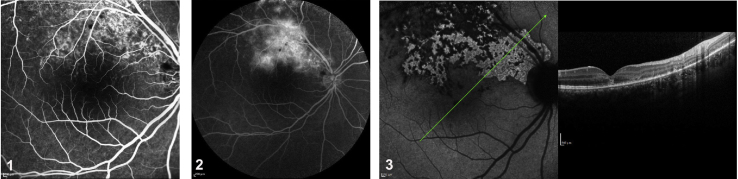
Pichi et al.7 found that hypofluorescent lesions scattered throughout the entire fundus in the late phase of ICGA in 100% of the eyes studied and that these lesions seemed to exceed the lesions documented by fundus photography and fluorescein angiography (FA). The authors defend choriocapillaris hypoperfusion is not present in MEWDS, and the justification for the hypofluorescent lesions on ICGA is the presence of diminished ICG uptake by the disrupted RPE cells. We do not agree with this hypothesis. Chang et al.28 showed in vitro uptake of ICGA by RPE cells, and ICG fluorescence was greater, not diminished, in disrupted RPE cells than in intact RPE cells due to increased permeability through the damaged cell membranes. Clinically, diseased RPE is not characterized by an absence of fixation of the ICG molecules but by increased fixation. We illustrate this increased uptake with three clinical examples of RPE damage with late hyperfluorescence, one as a consequence of mechanical trauma with Berlin's edema (Fig. 4) and the other two caused by inflammatory damage (Fig. 5).
Fig. 4.
Increased uptake of indocyanine green (ICG) in damaged areas. This is a case of Berlin's edema with photoreceptor and retinal pigment epithelium (RPE) damage after a blunt trauma. (1) Hyperfluorescence in the early and (2) late phases of fluorescein angiography (FA). (3) In the area corresponding to the absence of photoreceptors and irregularities of the RPE on optical coherence tomography (OCT) (right), there is hyperfluorescence in this damaged area, indicating fixation of the ICG molecule (left).
Fig. 5.
Case of ocular sarcoidosis with choroiditis (left) and case of ocular tuberculosis with choroiditis (right). Note the hyperfluorescent lesions on indocyanine green angiography (ICGA).
Primary outer segment/RPE disease presents differently
It has been proposed that MEWDS is a pure “photoreceptoritis”,7 with the loss of photoreceptors’ inner and outer segments being the origin of the classical clinical picture of MEWDS. When considering cases of pure photoreceptoritis as described by Aleman et al.,29 the clinical picture is characterized by a quite different phenotype. We present an illustrative case of primary photoreceptoritis in Fig. 6, presenting without fundus lesions nor FA nor ICGA lesions.
Fig. 6.
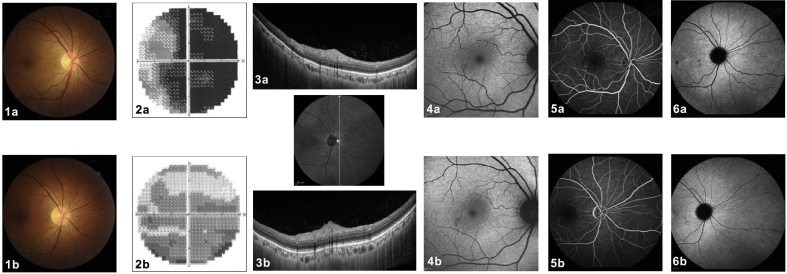
Photoreceptoritis. A 46-year-old man presented with sudden and profound visual loss in his right eye. Upon ocular examination, the best corrected visual acuity (BCVA) was hand motion in the right eye and 20/20 in the left eye. Anterior segment biomicroscopy and intraocular pressure were normal in both eyes. (1a) Color fundus photographs of the right eye revealed no abnormalities. (2a) The visual field of the right eye revealed global and severe sensitivity depression (mean defect −25.75 dB). (3a) Cross-sectional optical coherence tomography (OCT) through the nasal periphery demonstrated an absence of the hyperreflective ellipsoid zone. (4a) Fundus autofluorescence (FAF) showed no significant alterations. (5a) The retinal vasculature was normal on fluorescein angiography (FA) and (6a) no perfusion anomaly (no hypofluorescent areas) was seen on indocyanine green angiography (ICGA). Five months later, BCVA improved to 1/20 in the right eye. (1b) Fundus color photography was unchanged. (2b) The visual fields improved (mean defect −11.96 dB) and (3b) cross-sectional follow-up OCT, scanned in the same location, demonstrated the reappearance of a still disrupted hyperreflective ellipsoid zone, explaining the better visual field performance. (4b) FAF, (5b) FA, and (6b) ICGA did not present relevant abnormalities. This case represents a primary photoreceptoritis with no other clinical signs and no sign of a choriocapillaris perfusion anomaly.
Final comment
Beyond the remarkable iconographic contribution of the study by Pichi et al.,7 the physiopathogenic explanation the authors give for these lesions is difficult to endorse for two main reasons. First, OCT-A is not sensitive enough to detect end-choriocapillaris perfusion disturbances. Second, ICGA hypofluorescent areas can not be explained by a masking effect or a speculative lack of indocyanine fixation.
OCT-A detects intravascular flow, but to detect the presence or absence of flow, vessels have to be of a certain size and have sufficient flow. The device is excellent for analyzing retinal perfusion, including superficial and deep retinal circulation, as well as choroidal neovessels, where flow is substantial. Thus, the device is very much suited for analyzing retinal perfusion in diabetic retinopathy, where it can show rarefication of peri-foveal vessels, as well as following high-flow neovessels in wet age-related macular degeneration.30, 31 In contrast, the choriocapillaris is characterized by a low pressure/low flow and labile circulation, especially in its terminal network and, therefore, is probably not detected by OCT-A. As such, anomalies can not be judged, making this device unsuitable for detecting choriocapillaris perfusion disturbances.32 We showed that, even in choriocapillaritis entities, such as serpiginous choroiditis, in which choriocapillaris perfusion is severely impaired in larger vessels, OCT-A performs less suitably than ICGA in identifying choriocapillaris non-perfusion areas.5 MEWDS is the least severe of the choriocapillaritis entities, as involved areas are rarely confluent; in contrast to APMPPE, more adequately named acute multifocal ischemic choriocapillaritis (AMIC) by Deutman,33 in which choriocapillaris non-perfusion usually involves larger confluent areas. This constellation of a lesion process situated at the end-circulatory level of the choriocapillaris vascular network, where pressure and flow are low, explains why OCT-A appears normal in MEWDS, as it is not suited for the analysis of these structures.
The second problem is the authors’ unconvincing interpretation of the ICGA hypofluorescent dark areas in 100% of their MEWDS patients. The masking effect is well-known to play a minor role in ICGA, as the infrared wavelengths can “see” through the RPE, regardless of whether it is damaged or edematous. Only heavily pigmented or thick lesions can produce masking on ICGA, but certainly not tissue edema or alterations in one or several tissue layers. In our choriocapillaritis case, masking effect could be an explanation if there was only slight attenuation of the hypofluorescence. However, the heavy hypofluorescence regularly seen in MEWDS cannot be caused by a masking effect. The lack of alleged impregnation of RPE by indocyanine green is even more dubious in explaining hypofluorescent dark areas. Consequently, hypofluorescent areas seen on ICGA frames in MEWDS can only result from impaired circulation not allowing the indocyanine dye to properly reach these areas. In MEWDS, perfusion disturbances are completely reversible after a few weeks, which is not the case in more severe choriocapillaritis cases, such as APMPPE or serpiginous choroiditis. Hypofluorescent areas co-localize with areas of increased fundus autofluorescence (FAF), with areas of disruption of outer segments on optical coherence tomography (OCT), and with visual field defects, characterizing MEWDS as in other choriocapillaritis.
In summary, we demonstrated the crucial role of choriocapillaris hypoperfusion or non-perfusion as the origin of choriocapillaritis entities, including MEWDS, resulting in ischemic damage to the outer retina and possibly the RPE.
Footnotes
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Ben Ezra D., Forrester J.V. Fundal white dots: the spectrum of a similar pathological process. Br J Ophthalmol. 1995;79(9):856–860. doi: 10.1136/bjo.79.9.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dell'Omo R., Pavesio C.E. Multiple evanescent white dot syndrome (MEWDS) Int Ophthalmol Clin. 2012;52(4):221–228. doi: 10.1097/IIO.0b013e31826647ed. [DOI] [PubMed] [Google Scholar]
- 3.Gross N.E., Yannuzzi L.A., Freund K.B., Spaide R.F., Amato G.P., Sigal R. Multiple evanescent white dot syndrome. Arch Ophthalmol. 2006;124(4):493–500. doi: 10.1001/archopht.124.4.493. [DOI] [PubMed] [Google Scholar]
- 4.Pichi F., Sarraf D., Morara M., Mazumdar S., Neri P., Gupta V. Pearls and pitfalls of optical coherence tomography angiography in the multimodal evaluation of uveitis. J Ophthalmic Inflamm Infect. 2017;7(1):20. doi: 10.1186/s12348-017-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Ameen A., Herbort C.P., Jr. Serpiginous choroiditis imaged by optical coherence tomography angiography. Retin Cases Brief Rep. 2018;12(4):279–285. doi: 10.1097/ICB.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 6.Klufas M.A. Optical coherence tomography angiography reveals choriocapillaris flow reduction in placoid chorioretinitis. Ophthalmol Retina. 2017;1(1):77–91. doi: 10.1016/j.oret.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Pichi F., Srvivastava S.K., Chexal S. En face optical coherence tomography and optical coherence tomography angiography of multiple evanescent white dot syndrome: new insights into pathogenesis. Retina. 2016;36(suppl 1):S178–S188. doi: 10.1097/IAE.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 8.Luca Cimino A.M., Carl P., Herbort . Primary inflammatory choriocapillaropathies. In: Pleyer B.M.U., editor. Uveitis and Imunological Disorders. Springer; 2004. [Google Scholar]
- 9.Abu-Yaghi N.E., Hartono S.P., Hodge D.O., Pulido J.S., Bakri S.J. White dot syndromes: a 20-year study of incidence, clinical features and outcomes. Ocul Immunol Inflamm. 2011;19(6):426–430. doi: 10.3109/09273948.2011.624287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jampol L.M., Becker K.G. White spot syndromes of the retina: a hypothesis based on the common genetic hypothesis of autoimmune/inflammatory disease. Am J Ophthalmol. 2003;135(3):376–379. doi: 10.1016/s0002-9394(02)02088-3. [DOI] [PubMed] [Google Scholar]
- 11.Crawford C.M., Igboeli O. A review of the inflammatory chorioretinopathies: the white dot syndromes. ISRN Inflamm. 2013;2013:783190. doi: 10.1155/2013/783190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hangai M., Fujimoto M., Yoshimura N. Features and function of multiple evanescent white dot syndrome. Arch Ophthalmol. 2009;127(10):1307–1313. doi: 10.1001/archophthalmol.2009.250. [DOI] [PubMed] [Google Scholar]
- 13.Jampol L.M., Sieving P.A., Pugh D., Fishman G.A., Gilbert H. Multiple evanescent white dot syndrome. I. Clinical findings. Arch Ophthalmol. 1984;102(5):671–674. doi: 10.1001/archopht.1984.01040030527008. [DOI] [PubMed] [Google Scholar]
- 14.Sieving P.A., Fishman G.A., Jampol L.M., Pugh D. Multiple evanescent white dot syndrome. II. Electrophysiology of the photoreceptors during retinal pigment epithelial disease. Arch Ophthalmol. 1984;102(5):675–679. doi: 10.1001/archopht.1984.01040030531009. [DOI] [PubMed] [Google Scholar]
- 15.Gass J.D., Hamed L.M. Acute macular neuroretinopathy and multiple evanescent white dot syndrome occurring in the same patients. Arch Ophthalmol. 1989;107(2):189–193. doi: 10.1001/archopht.1989.01070010195021. [DOI] [PubMed] [Google Scholar]
- 16.Holz F.G., Kim R.Y., Schwartz S.D. Acute zonal occult outer retinopathy (AZOOR) associated with multifocal choroidopathy. Eye (Lond) 1994;8(Pt 1):77–83. doi: 10.1038/eye.1994.15. [DOI] [PubMed] [Google Scholar]
- 17.Bryan R.G., Freund K.B., Yannuzzi L.A., Spaide R.F., Huang S.J., Costa D.L. Multiple evanescent white dot syndrome in patients with multifocal choroiditis. Retina. 2002;22(3):317–322. doi: 10.1097/00006982-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Kuznetcova T., Jeannin B., Herbort C.P. A case of overlapping choriocapillaritis syndromes: multimodal imaging appraisal. J Ophthalmic Vis Res. 2012;7(1):67–75. [PMC free article] [PubMed] [Google Scholar]
- 19.Schaal S., Schiff W.M., Kaplan H.J., Tezel T.H. Simultaneous appearance of multiple evanescent white dot syndrome and multifocal choroiditis indicate a common causal relationship. Ocul Immunol Inflamm. 2009;17(5):325–327. doi: 10.3109/09273940903043923. [DOI] [PubMed] [Google Scholar]
- 20.Neri P., Ricci F., Giovannini A. Successful treatment of an overlapping choriocapillaritis between multifocal choroiditis and acute zonal occult outer retinopathy (AZOOR) with adalimumab (Humira) Int Ophthalmol. 2014;34(2):359–364. doi: 10.1007/s10792-013-9801-z. [DOI] [PubMed] [Google Scholar]
- 21.Chen C.L., Wang R.K. Optical coherence tomography based angiography [Invited] Biomed Opt Express. 2017;8(2):1056–1082. doi: 10.1364/BOE.8.001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara D., Waheed N.K., Duker J.S. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res. 2016;52:130–155. doi: 10.1016/j.preteyeres.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Gorczynska I., Migacz J.V., Zawadzki R.J., Capps A.G., Werner J.S. Comparison of amplitude-decorrelation, speckle-variance and phase-variance OCT angiography methods for imaging the human retina and choroid. Biomed Opt Express. 2016;7(3):911–942. doi: 10.1364/BOE.7.000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torczynski E., Tso M.O. The architecture of the choriocapillaris at the posterior pole. Am J Ophthalmol. 1976;81(4):428–440. doi: 10.1016/0002-9394(76)90298-1. [DOI] [PubMed] [Google Scholar]
- 25.Braun R.D., Dewhirst M.W., Hatchell D.L. Quantification of erythrocyte flow in the choroid of the albino rat. Am J Physiol. 1997;272(3 Pt 2):H1444–H1453. doi: 10.1152/ajpheart.1997.272.3.H1444. [DOI] [PubMed] [Google Scholar]
- 26.Fixler D., Duadi H., Ankri R., Zalevsky Z. Determination of coherence length in biological tissues. Laser Surg Med. 2011;43(4):339–343. doi: 10.1002/lsm.21047. [DOI] [PubMed] [Google Scholar]
- 27.Spaide R.F., Fujimoto J.G., Waheed N.K. Image artifacts in optical coherence tomography angiography. Retina. 2015;35(11):2163–2180. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang A.A., Zhu M., Billson F. The interaction of indocyanine green with human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2005;46(4):1463–1467. doi: 10.1167/iovs.04-0825. [DOI] [PubMed] [Google Scholar]
- 29.Aleman T.S., Sandhu H.S., Serrano L.W. Acute zonal cone photoreceptor outer segment loss. JAMA Ophthalmol. 2017;135(5):487–490. doi: 10.1001/jamaophthalmol.2017.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalam K.V., Sambhav K. Optical coherence tomography angiography in retinal diseases. J Ophthalmic Vis Res. 2016;11(1):84–92. doi: 10.4103/2008-322X.180709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia Y., Bailey S.T., Hwang T.S. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A. 2015;112(18):E2395–E2402. doi: 10.1073/pnas.1500185112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Ameen A., Herbort C.P., Jr. Serpiginous choroiditis imaged by optical coherence tomography angiography. Retin Cases Brief Rep. 2018;12(4):279–285. doi: 10.1097/ICB.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 33.Deutman A.F. Acute multifocal ischaemic choroidopathy and the choriocapillaris. Int Ophthalmol. 1983;6(2):155–160. doi: 10.1007/BF00127644. [DOI] [PubMed] [Google Scholar]