Abstract
Cenderitide is a novel designer natriuretic peptide (NP) composed of C-type natriuretic peptide (CNP) fused to the C-terminus of Dendroaspis natriuretic peptide (DNP). Cenderitide was engineered to co-activate the two NP receptors, particulate guanylyl cyclase (pGC)-A and pGC-B. The rationale for its design was to achieve the renal-enhancing and anti-fibrotic properties of dual receptor activation, but without clinically significant hypotension. Here, we review the biology of the NPs and the rationale for their use in heart failure. Most importantly, we present the key studies related to the discovery of Cenderitide. Finally, we review the key clinical studies that have advanced this first-in-class dual NP receptor activator for heart failure.
Keywords: Designer natriuretic peptide, drug development, heart failure, natriuretic peptide, receptors
INTRODUCTION
The staggering growth of heart failure (HF) was recently underscored by the Heart Disease and Stroke Statistics – 2017 Update from the American Heart Association.1 This report stated that “projections show that the prevalence of HF will increase 46% from 2012 to 2030, resulting in >8 million people 18 years of age and older with HF.” Clearly the challenge in our efforts to reduce the burden of HF is to now better understand the mechanisms of HF, increase the emphasis on prevention, educate the community about HF and most importantly develop innovative new drugs for the treatment of HF.
An important advance in HF therapeutics has been the recent approval of the small molecule, Entresto which has provided fresh momentum for drug discovery in HF and related fields.2 Also, the demonstration of paracrine acting peptides released from cell therapies, as well as the role of peptides such as the natriuretic peptides (NPs) in the beneficial actions of Entresto via inhibition of neprilysin (NEP), have focused on the importance of peptide therapeutics for HF.3,4 Indeed, the use of peptides permit more targeted strategies through well-characterized receptors and signaling pathways as well as avoiding off-target actions associated with small molecules.5 Further, peptides possess larger surface area than small molecules which may optimize receptor activation and efficacy. Nonetheless, a major limitation to peptide is enzymatic degradation, as over 600 different proteases exist in humans limiting the bioavailability of peptides as compared to small molecules.6
NATRIURETIC PEPTIDES AND THE HEART AS AN ENDCRINE ORGAN
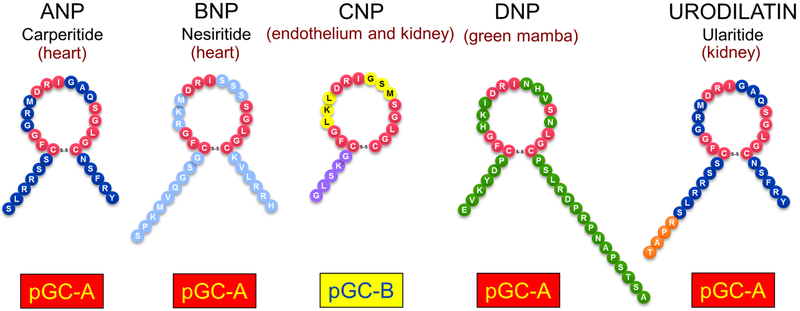
1981 marked the landmark discovery of the heart as an endocrine organ based on the seminal investigations of DeBold and co-workers that the heart synthesizes and releases atrial natriuretic peptide (ANP) with natriuretic and blood pressure lowering properties (Figure 1).7 Later studies established a second cardiac peptide, B-type NP (BNP) which shared similar biological actions to ANP.8 We and others reported that a third member of the NP family existed which was C-type NP (CNP) which is abundantly produced in the endothelium.9,10 More recently, studies have established that posttranslational modification of ANP results in a peptide called Urodilatin (URO) which is processed from proANP in the kidney.11 Finally, our group has advanced the existence of a novel peptide called Dendroaspis NP (DNP) which is derived from the green mamba snake and also shares potent biological actions similar to ANP.12,13 Finally, the concept of the heart as an endocrine organ was solidified by the work of Murad’s group which identified 3’,5’ cyclic guanosine monophosphate (cGMP) as the second messenger of ANP.14
Figure 1:
The natriuretic peptides. Atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP), urodilatin, and Dendroaspis natriuretic peptide (DNP) bind to guanylyl cyclase A (GC-A). C-type natriuretic peptide (CNP) binds to GC-B, ANP, BNP, CNP, and urodilatin are found in humans; DNP is found in the venom of the green mamba snake.
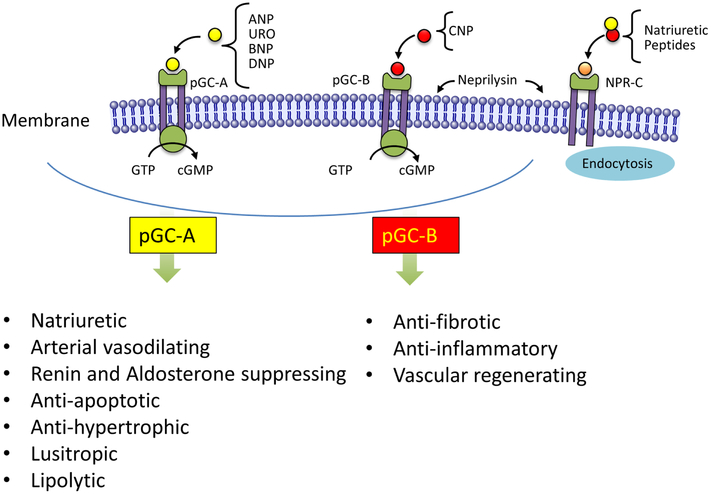
The continuing growing studies of the biology of the NP system supports its pivotal role in body fluid homeostasis by regulating intravascular volume and arterial pressure including functioning as an endogenous inhibiting mechanism to the renin-angiotensin-aldosterone system (RAAS) (Figure 2)15,16 A role for the NPs in metabolic homeostasis has also been advanced.17,18,19 Studies have established that the NPs function as ligands for a family of membrane bound receptors: CNP, evolutionarily the oldest of the NPs, binds to the particulate guanylyl cyclase B receptor (pGC-B), while all other NPs bind to the pGC-A receptor.20,21 These two pGC receptors are expressed in various tissues, including heart, vasculature, kidney, adrenals, and adipocytes (Figure 2). The respective biological actions include via pGC-A and pGC-B natriuresis, vasodilation, renin and aldosterone inhibition, suppression of apoptosis, and hypertrophy, inhibition of fibrosis, positive lusitropism and lipolysis with browning of adipocytes, anti-inflammatory properties and endothelial regenerating capacity.15,16 NPR-C, also called the NP clearance receptor, eliminates NPs from the circulation using endocytosis,22 although NPR-C may also mediate the microvascular actions of CNP.23 Clearance of the NPs is further mediated by the enzyme NEP, which is widely expressed in endothelium and lung with the highest abundance in the kidney.4 NEP is an almost unsaturable degrading enzyme which limits the biological activity of the NPs as does NPR-C. Differences in local pGC expression, degradation and clearance rates, and NP binding affinity cause all 5 NPs to have unique and specific properties.
Figure 2:
Specific biological actions for pGC-A activation by ANP, BNP, URO and DNP. Biological actions specific for pGC-B activation by CNP.
RATIONAL FOR THE THERAPEUTIC USE OF THE NATRIURETIC PEPTIDES IN HEART FAILURE
The pleotropic actions of the NP system make this humoral pathway attractive as a therapeutic strategy for HF. Indeed both ANP (carperitide) and BNP (nesiritide) have been approved in Japan and the US for acute decompensated HF (ADHF) respectively. Further, URO (Ularitide) also recently was tested in a large clinical trial in ADHF.24
While that NP system may directly mediate cardiorenal protective actions, the fact that the NP system inhibits RAAS also provides a powerful rationale for the development of NP-based HF therapy. Increasing the activity of NP or reducing their degradation may achieve a more favorable balance between RAAS and the NP system and therefore mediate beneficial actions on cardiorenal function and structure positively impacting outcomes as was seen in the PARADIGM-HF Trial.2 While the short-term use of NP such BNP (Nesiritide) and URO (Ularitide) has not been superior to conventional therapy, use of long-term NP therapy has resulted in positive outcomes in chronic HF. Specifically, Chen et al reported that 8 weeks of twice daily administration of subcutaneously (SQ) administered BNP to patients with NYHA Class III HF resulted in symptom improvement, reduced plasma renin activity and improved myocardial structure and function with preservation of renal function.25 Further studies of chronic SQ BNP in preclinical HFrEF and HFpEF have also resulted in positive results.26, 27 Most recently, a novel designer NP, Cenderitide, has emerged which goes beyond the properties of the naturally occurring NPs and represents a contemporary and innovative strategy for HF and this is reviewed below.
CENDERITIDE: A FIRST-IN-CLASS DESIGNER NATRIURETIC FOR HEART FAILURE
Designer Natriuretic Peptides.
Peptide engineering is a growing field in drug discovery which creates innovative peptides that go beyond endogenous peptides in molecular mechanisms of action, specificity and stability.28 Such advances in engineered designer peptides have occurred more widely outside of cardiovascular disease especially in the treatment of diabetes, cancer and HIV. In our program of designer NPs, we have set five goals.5 First, safety of a designer peptide is of highest priority especially in regard to immunogenicity. Second, enhanced receptor activation is a major goal which may be achieved through defining which amino acids in a structure are either key mediators of receptor binding and/or limit full receptor activation. Third, as a foremost mechanism limiting bioavailability is enzymatic degradation, addition of unique amino acids or amino acid substitutions may render a designer peptide more immune to degradation resulting in enhanced and/or more long acting biological actions. Such modifications may also reduce binding to NPR-C to bias the peptide to pGC-A or pGC-B. Four, an additional goal is to design peptides that have properties unique to a specific syndrome thus personalizing the peptide (e.g. limiting hypotension in HF, or augmenting adipocyte activation in obesity). Five, with progress in sustained release delivery systems, developing delivery platforms which permit daily, weekly or even monthly delivery of a peptide which may enhance efficacy and compliance has emerged as a key component in peptide engineering.
Cenderitide (CD-NP) for Heart Failure: Preclinical Drug Discovery.
The rational drug design strategy for Cenderitide (CD-NP) was based upon two objectives. First was the concept that by engineering a peptide which would co-activate both pGC-A (like ANP, BNP, DNP and URO) as well as pGC-B (like CNP), one could optimize activation of the NP system as can be appreciated in Figure 2. Second, the design of Cenderitide also was in part motivated from results from the ASCEND-HF and ROSE-HF Trials that demonstrated excessive hypotension after treatment with the pGC-A activator Nesiritide.29,30 During the development of Cenderitide, we sought to engineer a NP that still possessed the beneficial renal actions of pGC-A activation, but without unwanted hypotensive properties. As CNP lacks significant blood pressure lowering properties,31 we engineered a peptide that consisted of CNP, a pGC-B activator which is anti-fibrotic, anti-proliferative and vascular regenerating32, and the C-terminus of DNP recognizing DNP to be a potent pGC-A activator.33
The structure of Cenderitide is illustrated in Figure 3. Specifically, Cenderitide is a 37 amino acid NP which is a first-in-class designer NP that co-targets both pGC-A and pGC-B receptors. Toward this goal of dual pGC-A/pGC-B activation which does not exist in nature, Dickey et al elegantly demonstrated that Cenderitide clearly co-activates both pGC-A and pGC-B in HEK293 cells selectively overexpressing each receptor type.34 In a follow-on study, Dickey et al also reported that Cenderitide is more resistant to NEP degradation compared to the native ANP, BNP and CNP.35 In vitro, Cenderitide, and a Cenderitide analogue, also has antiproliferative, anti-fibrotic, and anti-hypertrophic properties.33,36,37 In vivo in normal canines, Cenderitide is a potent cGMP stimulator that has renal enhancing and cardiac unloading effects, with less reduction of blood pressure than BNP.33
Figure 3:
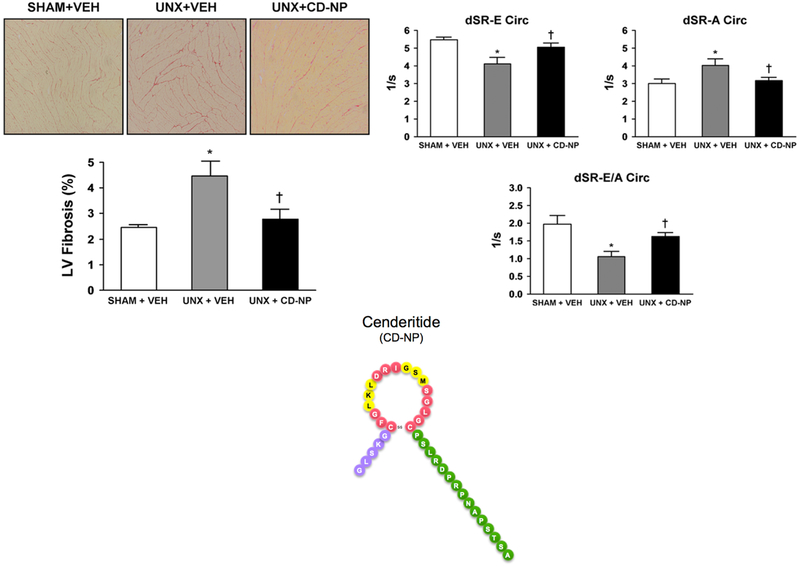
Left panels: LV Fibrosis. Representative histology images at 10× objective magnification of the LV stained with picrosirius red (upper panels) and the quantification of LV fibrosis staining (lower graph) in Sham+Vehicle, UNX+Vehicle and UNX+CD-NP rats. Values are mean 6 SEM. *P<0.05 vs Sham+Vehicle and P<0.05 vs UNX+Vehicle. Right panels: Diastolic Function. Effect of CD-NP treatment on preserving diastolic function in UNX+CD-NP rats compared to UNX+Vehicle rats. Values are mean 6 SEM. *P<0.05 vs Sham+Vehicle and P<0.05 vs UNX+Vehicle. The 37 amino acid structure of Cenderitide (CD-NP) is illustrated in the center bottom of the Figure 3. Cenderitide consists of the 22 amino acid structure of CNP (5 amino acid N-terminus in purple and 17 amino acid ring structure in red and yellow) together the 15 amino C-terminus of DNP in green. (Adapted from reference #41).
The importance of the unique structure of Cenderitide was recently reported by, Lee et al.38 Specifically, Lee and co-workers addressed the hypothesis that the 15-AA carboxyl-terminus of DNP, which is fused to CNP, uniquely facilitates dual pGC-A and pGC-B activation produced by Cenderitide. The actions of Cenderitide on cGMP activation in vitro in HEK293 cells selectively overexpressing human pGC-A or pGC-B were compared to native CNP and three variants of Cenderitide with altered carboxyl-termini. Specifically, for the variants, the carboxyl-terminus of Cenderitide was replaced by the carboxyl-terminus of ANP (CA-NP), BNP (CB-NP) or MANP (C-MANP), the latter a designer pGC-A activator which is currently in clinical trials for resistant hypertension.39,40 These studies also defined the renal actions of Cenderitide compared to CNP in vivo and ex vivo using normal canines and freshly harvested glomeruli.
These studies of Cenderitide and CNP confirmed the ability of Cenderitide to co-activate both pGC-A and pGC-B receptors in vitro. Importantly, these studies demonstrated that the carboxyl-terminus of DNP, derived from venom of the eastern green mamba snake, optimized dual pGC-A and pGC-B activation, which cannot be mimicked by carboxyl-termini from other pGC-A activators. Moreover, in freshly isolated canine glomeruli, free of the influence of systemic hemodynamics or circulating hormones, Cenderitide was 8 fold greater than CNP in increasing glomerular production of cGMP, which is consistent with the concept that the carboxyl-terminus of DNP successfully transforms CNP into a renal enhancing peptide. In vivo studies in normal canines demonstrated Cenderitide increased plasma cGMP 5 fold greater than CNP and markedly increased urinary cGMP excretion and net renal generation of cGMP. This renal activation of the cGMP system was associated with natriuresis and diuresis and an increase in GFR with Cenderitide. These studies established the important structural requirements for this first-in-class dual pGC-A/pGC-B activating designer NP engineered for the treatment of HF.
While the first clinical target for Cenderitide is HFrEF in the post acute HF period, a potential target which is emerging is HFpEF based upon the cGMP activating, RAAS inhibiting and anti-proliferative and anti-fibrotic properties of Cenderitide. This rational was strengthened by the recent study of Ichiki et al. which reported up-regulation of the pGC-B receptor together with reduced production of CNP in studies in human failing myocardium.36 Ichiki further found that Cenderitide, compared to either BNP or CNP, was superior in inhibiting collagen production in human cardiac fibroblasts.
Relevant to HFpEF, Martin and co-workers determined the ability of chronic SQ delivered Cenderitide (CD-NP) to prevent the development of cardiac fibrosis and preserve diastolic function in an experimental rat model of cardiac fibrosis produced by unilateral nephrectomy (UNX), to mimic CKD.41 Here we administered SQ Cenderitide for two weeks beginning at the time of UNX. To assess the sustained actions of Cenderitide upon the prevention of cardiac fibrosis and diastolic impairment, the heart was assessed by echocardiography two weeks after discontinuation of Cenderitide infusion, after which cardiac fibrosis was quantified and the profibrotic hormone aldosterone was determined.
Using this experimental model of cardiac fibrosis, we demonstrated that a two-week SQ infusion of Cenderitide significantly attenuated the development of cardiac fibrosis and preserved diastolic function (Figure 3). This attenuation of cardiac fibrosis is likely due properties involving activation of both the pGC-A and pGC-B receptors. Such mechanisms include 1) pGC-A mediated suppression of the potent pro-fibrotic factor aldosterone, which has been increasingly noted to induce cardiac fibrosis, and 2) inhibition of fibroblast proliferation and collagen synthesis linked to pGC-B activation. Importantly, the attenuation of cardiac fibrosis by Cenderitide treatment resulted in a preservation of diastolic and systolic function as documented by the maintenance of EF, sS, sSR, dSR-E, dSR-A, dSR-E/A. Thus, this study importantly demonstrated for the first time the potent anti-fibrotic properties of Cenderitide together with preservation of diastolic function, in vivo, using an experimental rat model of early cardiac fibrosis with impaired diastolic function.
Future in vitro and in vivo investigations are needed to assess the mechanisms by which this novel peptide exerts its anti-fibrotic effects and to also assess its therapeutic properties in other models of fibrotic disease. It also supports HFpEF as well as HFrEF as targets for Cenderitide.
Cenderitide (CD-NP) for Heart Failure: Clinical Studies.
A program for the clinical development of Cenderitide is underway especially motivated by a tremendous unmet need for therapy for this rapidly increasing cardiovascular syndrome, which has poor outcomes, and new drugs have repeatedly failed in clinical development.
Building on the promising preclinical findings discussed above, we launched in 2009 the clinical development of Cenderitide. Specifically, we sought to evaluate for the first time in healthy subjects the safety in normal humans to determine if the cGMP activating and renal enhancing properties with minimal blood pressure lowering properties of Cenderitide which were observed in preclinical studies would be translated to the human.42
In this first-in-human study, we observed that in normal humans that a 4 hour intravenous infusion of Cenderitide compared to placebo activated NP receptors linked to cGMP as demonstrated by the increase in both plasma cGMP and urinary cGMP excretion. Thus, this designer peptide is capable in humans in vivo to interact with human NP receptors relying on cGMP as a second messenger demonstrating ligand-receptor interactions. This observation also demonstrates that the use of the canine proved predictive of the actions of Cenderitide in humans, which may be helpful information when considering animal species for further evaluation of designer NPs.
Cenderitide induced natriuresis and diuresis in the absence of a change in creatinine clearance in normal humans. It is likely that the mild decrease in blood pressure in the Cenderitide compared to placebo group is only of statistical but not clinical significance. The natriuretic response in the absence of a change in creatinine clearance would indicate a Cenderitide-mediated reduction in sodium reabsorption within the human nephron. Considering studies in the canine, we speculate that the reduction in sodium reabsorption occurs at the level of the proximal and distal tubules. Further in-depth renal studies are warranted to determine the exact nephron segments involved and the response of the renal circulation.
A hallmark of the NPs, especially ANP, is the suppression of aldosterone thought secondary to activation of the pGC-A receptor in the adrenal gland. In this first-in-human study in normal humans with physiological concentrations of aldosterone, Cenderitide but not placebo reduced aldosterone. Further investigations will need to address the question of Cenderitide-mediated reduction of aldosterone in states in which aldosterone is activated, such as HF. Nonetheless, the ability to suppress aldosterone in the setting of natriuresis and diuresis is a feature that distinguishes Cenderitide from conventional diuretics, which are associated with activation of RAAS.
As discussed above, in normal human volunteers Cenderitide was well tolerated, without adverse actions and activated cGMP consistent with NP receptor activation. There was minimal blood pressure BP reduction together with natriuresis and diuresis, while GFR was preserved.
Based on the safety and tolerability of Cenderitide in healthy subjects and its important cardiorenal profile, we recently performed and reported the first prospective, randomized, placebo-controlled trial to determine for the first time the overall safety and tolerability of Cenderitide as well as the ability of Cenderitide to activate plasma and urinary cGMP in patients with stable chronic HF.43 This study tested the hypothesis that Cenderitide can be safely administered to HF subjects and that the activation of cGMP observed in preclinical studies and in normal subjects would be translated to HF. A total of 18 stable HF patients participated in the study. Of them, 12 were randomized to Cenderitide and 6 to placebo. The major findings of this study showed that a 4 hour infusion of Cenderitide was safe, well tolerated with increases in plasma cGMP, and urinary cGMP excretion in the absence of hypotension. Importantly, GFR was preserved and increased in the presence of a reduced GFR.
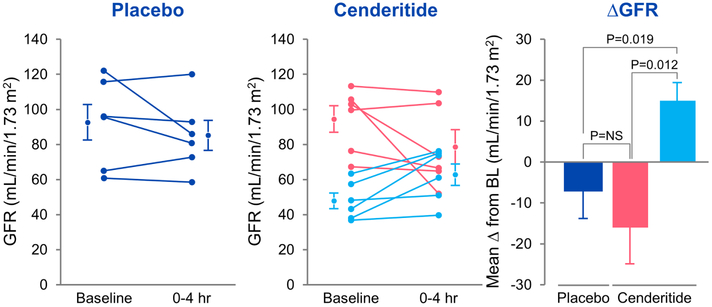
Impaired renal function characterized by a reduction in GFR and congestion secondary to sodium and water retention is more predictive of poor HF outcomes than LV ejection fraction. In normal canines, intravenous infusion of Cenderitide increased GFR and in freshly isolated canine glomeruli increased cGMP generation 33,38. In this recent study in stable HF subjects, intravenous infusion of Cenderitide preserved GFR with an increase in plasma cGMP and urinary excretion of cGMP, with no change in blood pressure or sodium excretion. The lack of blood pressure and natriuretic response may be due to reduced pGC-A and/ or pGC-B receptor activity. Thus, in future studies a dose-ranging study of longer duration is warranted to address this issue. Alternatively, the lack of natriuresis and diuresis may be related to the short duration of infusion or the holding of diuretics on the day of the study. Importantly, in this small study we did note that GFR increased in subjects with GFR below 65 ml/min (Figure 4), which needs confirmation in a larger trial. These renal actions especially related to GFR are consistent with pGC-A action, as supported by previous studies in isolated glomeruli. 38
Figure 4:
Individual GFR responses in placebo groups (a) and Cenderitide groups (b). Comparison of changes from baseline in GFR at 4 h between placebo, Cenderitide with baseline GFR 65 ml/min/1.73m2, and Cenderitide with baseline GFR<65 ml/min/1.73m2 groups (c). P value in box is from ANOVA for comparison of groups. Data are mean (SEM). NS, not significant. (Adapted from reference #43).
Nearly all etiologies of heart disease involve pathological myocardial remodeling characterized by excessive deposition of extracellular matrix proteins by cardiac fibroblasts, which reduces tissue compliance and accelerates the progression to HF leading to poor prognosis in HF patients. Indeed, left ventricular myocardium in both end stage HF and in LVAD patients is characterized by the presence of fibrosis.36 Thus, pathological fibrosis has emerged as an important target for pharmacological intervention in HF, yet approved drugs for this specific pathology are lacking.
Cenderitide, as a dual pGC-A/ pGC-B activator that may optimize antifibrotic properties of the NP/cGMP pathway, could represent a significant advance in targeting cardiac fibrosis. Supporting the potential clinical target of fibrosis in HF is our demonstration of significant increases in plasma cGMP in this recent study. Also as discussed above in preclinical investigations, Cenderitide possesses robust anti-fibrotic, ant-proliferative and aldosterone suppressing actions. Thus its potential to suppress cardiac fibrosis in both HFrEF and HFpEF is a credible hypothesis. Our major objective in this study in HF was to establish that Cenderitide was safe and well tolerated. In this study in stable HF, there were no adverse effects observed with Cenderitide. Specifically, there was no hypotension, tachycardia, dizziness, or other adverse effects.
To optimize chronic Cenderitide therapy in HF, we will advance a strategy of SQ delivery as is used for insulin. Such a delivery strategy is supported by our success with chronic delivery of BNP in patients with HF and our SQ pump delivery of Cenderitide in a rodent model of cardiac fibrosis and diastolic impairment.25,41 In experimental models of cardiovascular disease, novel nanoparticle gel polymer strategies are being tested.44 Further, a novel film delivery system in which Cenderitide is released from a patch-like device around the heart is also being tested.45 An oral delivery platform is favored but remains a challenge to achieve acceptable levels of bioavailability.
CONCLUSIONS
The NPs have always been regarded as attractive targets in the treatment of HF. Although their use was initially hampered by limited clinical efficacy - mainly the result of limitations of endogenous NPs such as rapid enzyme degradation or unwanted properties such as excessive hypotension, the therapeutic use of NPs remain a high sought after goal. Progress in this field is advancing with the use of designer NPs with Cenderitide representing the most clinically advanced as an innovative first-in-class dual pGC-A/pGC-B activator. This novel chimeric peptide is a product technological advances that have been made in drug development over the years, incorporating new and innovative peptide engineering strategies including novel delivery platforms. The era of the designer NPs continues to evolve with the promise of exciting therapeutics for cardiovascular disease and beyond.
Highlights.
Review of the biology of the natriuretic peptide system and the rationale for the use of natriuretic peptides in the treatment of heart failure.
Review of the principles of peptide engineering in rationale drug design.
Review of the discovery and preclinical research of Cenderitide – a first-in-class dual natriuretic peptide receptor designer peptide.
Review of the key two clinical trials advancing Cenderitide for the treatment of heart failure.
Acknowledgments
Funding from the National Institutes of Health (RO1 HL36634-29 and PO1 HL76611).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Benjamin E, Blaha M, Chiuve S et al. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim A, Marban E. Exosomes: Fundemental biology and roles in cardiovascular physiology. Ann Rev Physiol. 2016;78:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Burnett JC Jr. Biochemistry, therapeutics and biomarker implications of neprilysin in cardiorenal disease. Clin Chem. 2017;63:(1) 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meems LMG & Burnett JC Jr. Innovative therapeutics: designer natriuretic peptides. JACC Basic Transl. Sci. 2016;1;557–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Dis. 2015;20:122–128. [DOI] [PubMed] [Google Scholar]
- 7.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 1981;28:89–94. [DOI] [PubMed] [Google Scholar]
- 8.Mukoyama M, Nakao K, Hosoda K, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 1991;87:1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stingo AJ, Clavell AL, Heublein DM, Wei CM, Pittelkow MR, Burnett JC, Jr. Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol. 1992:H1318–1321. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa Y, Nakao K, Nakagawa O, et al. Human C-type natriuretic peptide. Characterization of the gene and peptide. Hypertension. 1992;19:809–813. [DOI] [PubMed] [Google Scholar]
- 11.Schulz-Knappe P, Forssmann K, Herbst F, Hock D, Pipkorn R, Forssmann WG. Isolation and structural analysis of "urodilatin", a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klinische Wochenschrift. 1988;66:752–759. [DOI] [PubMed] [Google Scholar]
- 12.Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J Biol Chem. 1992;267:13928–13932. [PubMed] [Google Scholar]
- 13.Lisy O et al. Renal actions of synthetic Dendroaspis natriuretic peptide. Kidney Int. 1999;56:502–508. [DOI] [PubMed] [Google Scholar]
- 14.Waldman SA, Rapoport RM & Murad F Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984;265:4332–14334. [PubMed] [Google Scholar]
- 15.Von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Failure. 2013;6:594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. 2014;35(7):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannone V, Boerrigter G, Cataliotti A, et al. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J Am Coll of Card. 2011;58:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang TJ. The natriuretic peptides and fat metabolism. N Engl J Med. 2012;367:377–378. [DOI] [PubMed] [Google Scholar]
- 20.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endo Rev. 2006;27:47–72. [DOI] [PubMed] [Google Scholar]
- 21.Structure Kuhn M., regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93:700–709. [DOI] [PubMed] [Google Scholar]
- 22.Fuller F, Porter JG, Arfsten AE, et al. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem. 1988;263:9395–9401. [PubMed] [Google Scholar]
- 23.Moyes AJ, Khambata RS, Villar I, et al. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J Clin Invest. 2014;124:4039–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Packer M, O'Connor C, McMurray J et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. 2017;DOI: 10.1056/NEJMoa1601895 [DOI] [PubMed] [Google Scholar]
- 25.Chen HH, Glockner JF, Schirger JA, Cataliotti A, Redfield MM, Burnett JC Jr., Novel protein therapeutics for systolic heart failure: chronic subcutaneous B-type natriuretic peptide. J Am Coll Card. 2012;60:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKie P, Schirger J, Benike S, Harstad L, Slusser B, Hodge D, Redfield M, Burnett JC Jr, Chen H. Chronic subcutaneous BNP therapy in asymptomatic systolic heart failure. Eur J Heart Fail. 2016;18:433–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan SH, McKie P, Schirger J, Benike S, Harstad L, Slusser B, Hodge D, Redfield M, Burnett JC Jr, Chen H. Chronic peptide therapy with B-type natriuretic peptide in patients with preclinical diastolic dysfunction (Stage B Heart Failure). JACC Heart Fail. 2016;4(7): 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jay SM, Lee RT. Protein engineering for cardiovascular therapeutics: untapped potential for cardiac repair. Circ Res. 2013;113:933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 30.Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Igaki T, Itoh H, Suga SI, Hama N, Ogawa Y, Komatsu Y, Yamashita J, Doi K, Chun TH, Nakao K. Effects of intravenously administered C-type natriuretic peptide in humans: comparison with atrial natriuretic peptide. Hypertens Res. 1998;21:7–13. [DOI] [PubMed] [Google Scholar]
- 32.Doi K, Ikeda T, Itoh H, Ueyama K, Hosoda K, Ogawa Y, Yamashita J, Chun TH, Inoue M, Masatsugu K, Sawada N, Fukunaga Y, Saito T, Sone M, Yamahara K, Kook H, Komeda M, Ueda M, Nakao K. C-type natriuretic peptide induces rediffer-entiation of vascular smooth muscle cells with accelerated reendothelialization. Arterioscler Thromb Vasc Biol. 2001;21:930–936. [DOI] [PubMed] [Google Scholar]
- 33.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC Jr., Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Card. 2008;52:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickey DM, Burnett JC Jr., Potter LR. Novel bifunctional natriuretic peptides as potential therapeutics. J Biol Chem. 2008;283:35003–35009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickey DM, Potter LR. Dendroaspis natriuretic peptide and the designer natriuretic peptide, CD-NP, are resistant to proteolytic inactivation. J Mol Cell Card. 2011;51:67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichiki T, Schirger JA, Huntley BK, et al. Cardiac fibrosis in end-stage human heart failure and the cardiac natriuretic peptide guanylyl cyclase system: regulation and therapeutic implications. J Mol Cell Card. 2014;75:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilic A, Rajapurohitam V, Sandberg S, Zeidan A, Hunter J, Faruq N, Lee CY, JBurnett JC Jr, Karmazyn M. A novel chimeric natriuretic peptide reduces cardiomyocyte hypertrophy through the NHE-1–calcineurin pathway. Cardiov Res. 2010; 88:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CY, Huntley BK, McCormick DJ, et al. Cenderitide: structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions. Eur Heart J. CPT. 2016;2:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKie PM, Cataliotti A, Huntley BK, Martin FL, Olson TM, Burnett JC Jr. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure-lowering, renal-enhancing, and aldosterone-suppressing actions. J Am Coll Card. 2009;54:1024–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen HH, Neutel J, Smith D, Heublein D, Burnett J. a first-in-human trial of a novel designer natriuretic peptide zd100 in human hypertension. J Am Coll Card. 2016;67:1946–1946. [Google Scholar]
- 41.Martin FL, Sangaralingham SJ, Huntley BK, et al. CD-NP: a novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart. PloS One. 2012;7:e52422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CY, Chen HH, Lisy O, et al. Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharm. 2009;49:668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawakami R, Lee CYW, Scott C, Bailey KR, Schirger JA, Chen HH, Benike SL, Cannone V, Martin FL, Sangaralingham SJ, Ichiki T, Burnett JC Jr. A human study to evaluate safety, tolerability, and cyclic GMP activating properties of a novel designer natriuretic peptide, cenderitide, in subjects with stable chronic heart failure. Clin Pharmacol Ther. 2017;doi: 10.1002/cpt974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lim J, Clements MA, Dobson J. Delivery of short interfering ribonucleic acid-complexed magnetic nanoparticles in an oscillating field occurs via caveolae-mediated endocytosis. PloS One. 2012;7:e51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng XW, Huang Y, Chen HH, Burnett JC Jr., Boey FY, Venkatraman SS. Cenderitide-eluting film for potential cardiac patch applications. PloS One. 2013;8:e68346. [DOI] [PMC free article] [PubMed] [Google Scholar]