Abstract
Scope
Turmeric is a top selling dietary supplement (DS) in the United States with rapidly expanding usage. Therefore, turmeric DS formulations available for sale in an urban US retail marketplace were analyzed, and point of sale information was related to measures of quality relevant to safety.
Methods and Results
Eighty-seven unique turmeric DS were identified; a majority (94%) contained turmeric-derived curcuminoid extracts (TD-CE), which were combined with other bioactives in 47% of products, including piperine (24%), an additive that could alter the metabolism of concurrent medications. While curcuminoid content was within 80% of anticipated for a majority of products analyzed (n = 35), curcuminoid composition (% curcumin) did not meet USP criteria for TD-CE in 59% and was suggestive of possible unlabeled use of synthetic curcumin in some. Lead content was associated with inclusion of turmeric root and exceeded USP limits in one product. Residues of toxic class 1 or 2 solvents, which are not needed for TD-CE isolation, were present in 71% of products, although quantified levels were within USP-specified limits.
Conclusion
Assessment of turmeric DS quality at point of sale is difficult for consumers and may best be managed in partnership with knowledgeable health care professionals.
Keywords: Turmeric, curcuminoids, curcumin, dietary supplements, solvents, lead
INTRODUCTION
In the United States (US), the majority of herbal dietary supplements (DS) are purchased from conventional mainstream or natural retailers.[1, 2] In 2016 turmeric was the top selling herbal DS within the natural retailer channel for the fourth consecutive year with over $47 million in sales, an increase of 32% from the prior year.[2] Turmeric rose to tenth place in conventional retail sales in 2016 with $22 million in sales, an 86% increase from the preceding year.[1, 2] Turmeric is an easily recognizable, golden-hued spice composed of the dried ground rhizome of Curcuma longa L, a plant native to tropical South Asia with a long history of traditional medicinal use, particularly for its anti-inflammatory properties. Turmeric bioactivity is largely attributed to its curcuminoids, polyphenolic compounds that comprise approximately 3% of the dried rhizome by weight.[3] Because of the structural similarity of the most abundant curcuminoids (curcumin [CURC], demethoxycurcumin [DMC] and bis-demethoxycurcumin [BDMC]), polyphenol-enriched extracts prepared from turmeric root contain a mixture of these naturally occurring curcuminoids.[4–7] Interest in the health benefits of turmeric have largely focused on curcuminoids, whose anti-inflammatory properties have been attributed to their impact on various molecular targets including NF-κB, TNF-α, IL-1, IL-6 and COX-2,[5, 8, 9] rather than turmerone-enriched volatile essential oils, which also have demonstrable anti-inflammatory effects.[10–12]
Despite their therapeutic potential, turmeric DS in the US are categorized and regulated as dietary supplements, as defined by the Dietary Supplement Health and Education Act of 1994 (DSHEA), with oversight of content and quality assurance left largely to the manufacturer, and federal regulation primarily limited to product removal from the marketplace if adverse effects occur and are reported.[13, 14] To help fill what has been seen by some as a regulatory void,[15] several independent organizations (e.g. Consumer Lab, NSF International, US Pharmacopeia [USP], UL International) evaluate the quality of DS in the US, providing content information to consumers either by subscription or, if directed by the manufacturer, via product labeling. Thus, at point of sale, consumers do not necessarily have complete information available regarding turmeric DS product quality. Because purified curcuminoids have a notoriously low bioavailability, consumers also face additional choices at point of sale regarding the use of enhanced bioavailability products and/or the type of enhancement employed, including the addition of other plant-derived substances, such as turmeric-derived essential oils [16] or piperine,[17] an alkaloid extracted from the fruits of black pepper (Piper nigrum L)[18] that inhibits drug metabolizing enzymes in the liver,[19] or the use of other proprietary means of complexing curcuminoids with agents such as glycerin, vegetable gum, phospholipids and/or other lipid formulations.[16, 20–29]
Given the nature of the DS regulatory environment in the US, the popularity of turmeric DS, the potential complexity of their content, and published reports of possible untoward effects associated with turmeric DS or curcuminoid use, including diarrhea, abnormal liver function, alteration of iron absorption and worsening of heart conduction defects,[30–33] reasonable concerns about the safe use of turmeric DS can arise. Our interest in this topic was stimulated by a case of turmeric DS-induced autoimmune hepatitis wherein the particular dietary supplement ingested could not be identified,[30] as commonly occurs with herbal supplement related adverse events.[34] Therefore, we undertook a survey to evaluate the types of turmeric DS formulations available for sale in the US, focusing on urban retail channels frequently used by consumers. We performed an evaluation of labeled content and pricing and analyzed the relationship of this point of sale information with product quality and safety, as determined by independent analysis of the curcuminoid, lead, and solvent content of a representative sample of turmeric DS.
MATERIALS AND METHODS
Turmeric DS identification
A comprehensive inventory of turmeric DS available for sale at 21 retailers was undertaken in Tucson, AZ between January - February 2017. Retailers included: 1) conventional large-chain national retailers (n= 5); 2) natural retailers, defined as full-format supermarkets with ≥50% of sales from natural/organic products[1] (n= 6; 3 national and 3 local); and 3) specialty shops (n= 5; 2 national and 2 local vitamin retailers and 1 local botanica, a culturally appropriate healthcare resource for US Latinos[35]). Additionally, because practitioners account for a small but unique portion of the market,[1] turmeric DS available at local naturopathic offices (n= 5) were included. DS were included in the survey if turmeric was the primary ingredient indicated in the product name or description. A total of 87 unique turmeric DS were identified from an original 228 surveyed turmeric DS, after excluding identical products available at multiple retailers (n= 99) or containing different capsule counts (n= 42). Labeling information for these 87 DS was recorded, including: 1) the type, amount and recommended daily dosing of turmeric; 2) the inclusion of additional bioactives (natural products, vitamins, or minerals) and 3) the use of piperine/black pepper extract or proprietary formulations to enhance curcuminoid bioavailability.
Calculated turmeric DS (n = 87) curcuminoid content
Capsular curcuminoid content was calculated based on labeled information, manufacturer’s websites and/or previously published information on the curcuminoid content of proprietary formulations[20, 21, 24, 27, 28, 36–38] or dried turmeric rhizomes.[3–5] In a minority of products (7/87) where labels did not provide sufficient information to allow for clear estimate of capsular curcuminoid content (e.g. combinations of multiple turmeric extracts without information on relative amounts of each), authors estimated content based on comparison with similarly composed formulations. Product-specific daily recommended curcuminoid doses were calculated as the product of the maximum number of daily capsules recommended and calculated capsular curcuminoid content, which was corrected for bioequivalence for enhanced bioavailability proprietary or piperine-containing products (curcuminoid content multiplied by fold-increase in curcuminoid bioavailability, published or claimed[16, 17, 20–28])
Selection of turmeric DS (n = 35) for chemical analyses
A subset (n= 35) of the 87 unique turmeric DS (Supplement Table 1) was selected for analysis of curcuminoid, solvent and lead content using the following criteria: 1) cost per capsule (<5th or >95th percentile, using median priced retailers and 60-count bottles); 2) commercial availability (sold at ≥3 retailers and/or store-specific generic brands); 3) type of turmeric content (root, proprietary enhanced bioavailability formulations, curcuminoid-enriched extracts); 4) inclusion of other bioactives (piperine and/or ≥3 additional bioactives). Supplements were purchased in April 2017 and stored at room temperature protected from light prior to shipment to laboratories for analyses. For products with outlying values upon initial testing, assays were repeated to confirm initial results, and, where indicated, a different lot of outlying products were purchased (ending in August 2017) and sent for identical testing to assess product versus lot specific trends.
Curcuminoid Analysis
Because a validated, standardized assay for curcuminoids (CURC, DMC and BDMC) has yet to be established, selected turmeric DS were sent to 2 independent laboratories for analysis blinded to brand and labeled content: a commercial laboratory that provides nutraceutical manufacturers with analytical curcuminoid testing of raw ingredients or finished products (Chromadex, Boulder, CO, USA) and the academic laboratory of Dr. Claus Schneider (Vanderbilt University, Nashville, TN), which routinely assays curcuminoid levels for research purposes.[39] In both laboratories, capsular content weight was calculated by subtracting the shell weight from the total capsule weight, and capsular curcuminoid content (CURC, DMC and BDMC) was analyzed via high performance liquid chromatography (HPLC). In the commercial laboratory, CURC, DMC and BDMC standards were weighed, diluted with ethanol prior to sonication for 5 minutes, re-equilibrated to room temperature and diluted to final volume with methanol prior to analysis. Turmeric DS samples were diluted with ethanol prior to sonication for 30 minutes, re-equilibrated to room temperature, diluted to volume with ethanol and filtered prior to analysis. HPLC analysis was performed at 35°C using an Agilent 1290 II UHPLC System and an isocratic flow of 0.01% phosphoric acid (60%) and acetonitrile (40%) at a rate of 1.0 mL/min with UV detection at 425 nm to quantify chromatographic peaks corresponding to CURC, DMC, and BDMC (ChromaDex Analytical Method: 99.1–03-3.0–000280). In the academic laboratory, CURC, DMC, and BDMC were synthesized and quantified using the extinction coefficients reported previously.[40] Calibration curves (0.75 – 400 μM) were constructed for CURC, DMC and BDMC. Stock solutions of samples, assayed in duplicate, were prepared by dissolving 20 mg of capsule content in methanol to make a solution of 1 mg/ml. Five μl of each solution were analyzed by autosampler injection on an Agilent 1200 series HPLC system with diode array detection. An Agilent column (Eclipse XDB-C18, 3.5 μm, 3 × 100 mm) was eluted with a linear gradient of acetonitrile/water/acetic acid 40/60/0.01 to 80/20/0.01 (by volume) in 15 min at 0.6 ml/min flow rate. Peaks areas corresponding to CURC, DMC, and BDMC were quantified using chromatograms recorded at 430 nm. Curcuminoid composition (% CURC) values for products containing turmeric –derived curcuminoid extract were compared with USP recommended standards.[41] The presence or absence of piperine was assessed in all DS assayed for curcuminoid content by comparison with an authentic standard in the HPLC analyses of the academic laboratory as a peak with absorbance maximum at 340 nm eluting between DMC and BDMC.
Residual Solvent Analysis
Quantification of 51 residual solvents (class 1, class 2 and class 3)[42] in selected turmeric DS was performed by Covance Laboratories (Madison, WI) using gas chromatography mass spectrometry (GC-MS) and published methods validated by USP.[43] Limits of quantification varied by solvent (range, 0.5 – 500 ppm), with results compared with USP recommended concentration limits for each solvent.[43]
Lead Analysis
Analysis of lead in selected turmeric DS was completed by Covance Laboratories (Madison, WI) using methods published and available from AOAC International.[44] Quantification was determined via inductively coupled plasma with mass spectrometric detection (ICP-MS), with comparison to known standards. The limit of detection (LOD) was 0.005 ppm. Values, reported as half the LOD if undetectable, were compared to allowable lead limits by weight (1 μg/g = 1 ppm) as recommended by USP.[45]
Statistical Analysis
Descriptive statistics are reported, as indicated, as median or as mean ± standard error (SE) or standard deviation (SD). Paired t-tests and graphical inspection were used to compare absolute (g) and relative amounts (%) of total and/or individual curcuminoids detected for each product using the two assay methods. To determine whether point of sale information could predict turmeric DS quality, the assayed products were classified as to: 1) turmeric content category (any root, enhanced bioavailability “proprietary” formulation, or “other” [primarily curcuminoid extract only]); 2) inclusion of non-turmeric bioactives, including piperine; 3) price, expressed as US dollars per gram of labeled total curcuminoid content (Supplement Table 1). Relationships between point of sale information and assayed measures of quality, including curcuminoid content and composition, lead content, and evidence of class 1 or 2 residual solvents were determined by: 1) Chi-squared or Fisher’s exact testing for categorical measures, 2) t test or ANOVA with post hoc testing for continuous measures, or 3) logistic regression. Associations between continuous variables were assessed by Pearson’s correlation. To reduce skewness and improve distributional characteristics, price, curcuminoid content (total and %CURC) and lead content were log transformed for analysis. The significance level for all analyses was set at α=0.05. Statistical analyses were performed using either Prism 6.0 (Graphpad, San Diego, CA, USA) or STATA 14.2 (StataCorp LP, College Station, TX, USA). The Tukey IQR method was used to identify unusual observations. Products with unusual values were flagged for subsequent follow-up, including re-assay to verify results. All observations were used in statistical data analysis.
RESULTS
Turmeric DS Labeled Content (n = 87)
As per labeling, most turmeric DS were formulated to contain turmeric-derived curcuminoid extracts, which were present as proprietary enhanced-bioavailability formulations in almost one third (n = 27 of 87), with turmeric root being a sole or additional component in 28% (n = 24 of 87) (Figure 1A). More than half of products were enhanced bioavailability (n = 48 of 87), with piperine being used for this purpose in 24% (n = 21 of 87), although only a minority of piperine products (19%, n = 4 of 21) were labeled as having increased bioavailability (vs. 78% [ n = 21 of 28] of proprietary formulations) (Figure 1B). One third of products contained additional bioactives other than piperine (n = 29 of 87), (Figure 1B). Daily recommended curcuminoid doses were highest for piperine-containing products (median, 9.0 g/d) and intermediate for enhanced bioavailability formulations (median, 3.1 g/d) (Figure 1B). Compared to mainstream retailers, health food or supplement specialty stores offered a larger selection of products, including more proprietary formulations and products with additional bioactives, for a higher average price (Supplement Table 2). Naturopathic practices tended to sell a single product, typically a proprietary formulation, with the highest average price.
Figure 1.
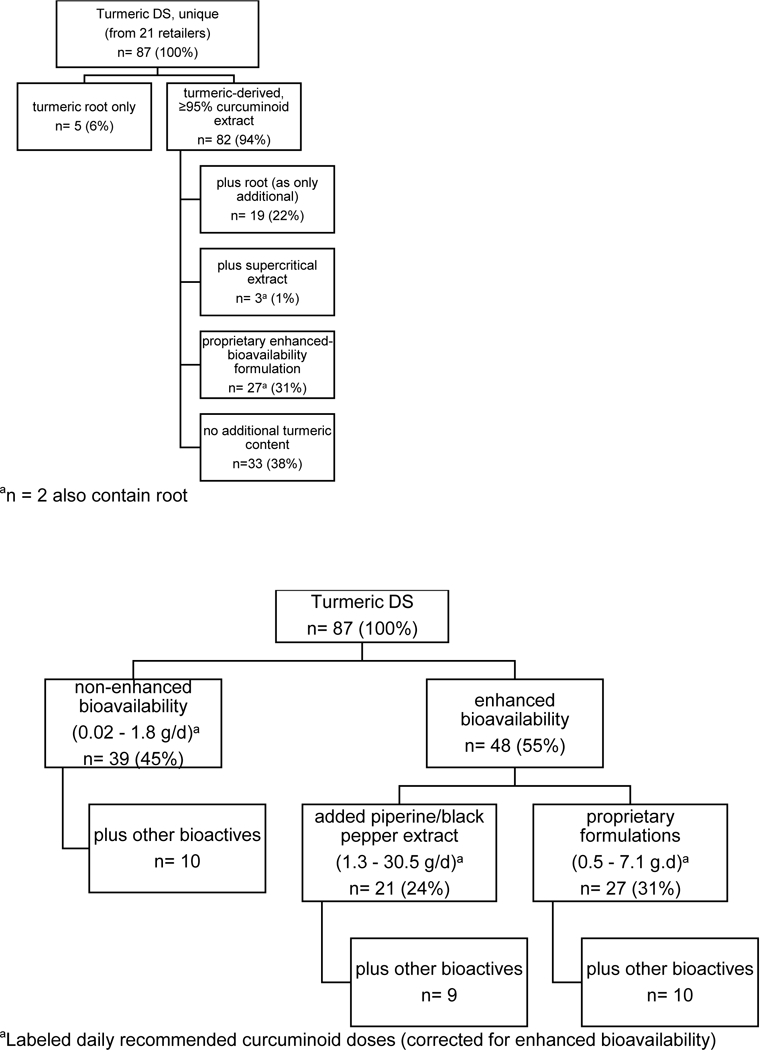
Unique Turmeric DS Formulations (n= 87). (A) Labeled turmeric-derived content (root, >95% curcuminoid extract, supercritical extract and/or proprietary enhanced bioavailability curcuminoid formulation). (B) Labeled non-turmeric content and/or method of enhancing curcuminoid bioavailability (piperine additive vs. proprietary formulation).
Assayed Curcuminoid Content
The two methods used to analyze relative amounts of CURC, DMC and BDMC were in close agreement (Table 1, curcuminoid composition). However, the absolute amount (mg/capsule) of total curcuminoids, CURC or DMC were higher upon testing by the academic laboratory (Table 1), as were capsular fill weights used to calculate these values (product of capsular weight and % composition) (Table 1). Limited blinded replicate testing for total curcuminoids suggested greater intra-assay precision by the academic laboratory (data not shown). Although capsular total curcuminoid content was statistically different between the two laboratories, the average amount of anticipated total curcuminoids detected, expressed as the ratio of assayed to calculated total curcuminoids, was not (Figure 2A, p= 0.08). Total curcuminoid content was within 20% of anticipated values for 80–83% (n = 28 or 29) of the products, depending on the assay. For the sole product whose assayed curcuminoid content was > 30% less than anticipated (Figure 2A, #19), curcuminoid content remained low upon testing of a second lot (33% of anticipated). There was no statistically significant relationship between the amount of anticipated total curcuminoids detected and point of sale information related to cost or content (turmeric or other bioactives). Piperine was detected in all 11 samples that disclosed its presence in labeling information, and it was not detected in any of the other samples. There was a 13-fold difference in the amount of detectable piperine between the turmeric DS (vs. 3-fold difference in labeled piperine content [Supplemental Table 1]).
Table 1.
Assay Comparison: Curcuminoids
| CAPSULE CONTENT (n = 35 products) | ASSAY 1 (academic) | ASSAY 2 (commerical) | Mean Difference (± SEM) | p-valuesa |
|---|---|---|---|---|
| Curcuminoid Composition (% of total curcuminoids) | % (mean) | % (mean) | % | |
| % curcumin | 80.9 | 80.9 | 0.09 ± 0.22 | 0.73 |
| % Demethoxycurcumin | 14.6 | 14.6 | 0.09 ± 0.14 | 0.54 |
| % bis-demethoxycurcumin | 4.6 | 4.4 | -0.17 ± 0.15 | 0.31 |
| Capsule Fill Weight, mg | mg (mean) | mg (mean) | mg | |
| 661.2 | 646.6 | 14.2 ± 5.4 | 0.01 | |
| Curcuminoids, mg per capsule | ||||
| Total | 207.9 | 190.3 | 17.6 ± 5.8 | 0.005 |
| Curcumin | 172.3 | 157.5 | 14.9 ± 5.1 | 0.006 |
| Demethoxycurcumin | 29.6 | 27.2 | 2.4 ± 0.8 | 0.004 |
| bis-Demethoxycurcumin | 5.9 | 5.7 | 0.3 ± 0.1 | 0.051 |
p-values by paired t-test
Figure 2.
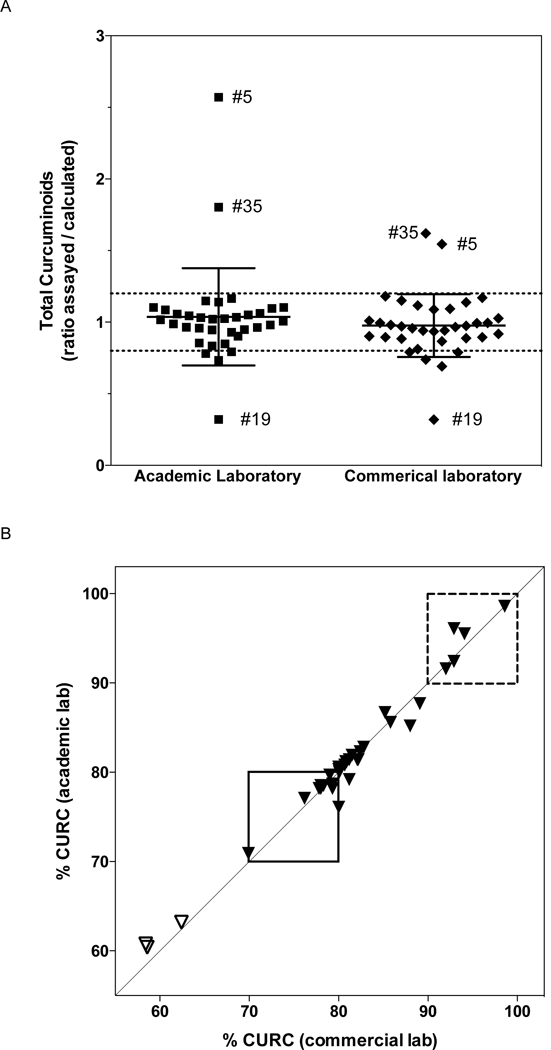
Assayed turmeric DS curcuminoid content (n= 35). (A) Total curcuminoid content (g/capsule), as determined by the commercial or academic laboratories, is expressed as a ratio of measured vs. calculated content (based on label information). Scatterplot includes mean ± SD, with dashed lines indicating ratios 20% different than anticipated. Means are not statistically different (p = 0.08). For products with outlying values, sample identification numbers are indicated. (B) Composition of curcuminoids, expressed as % curcumin [CURC], as determined by commercial vs. academic laboratories. Dashed box indicates products with >90% CURC, as assayed by both laboratories. Closed box indicates products meeting USP curcuminoid extract specifications (70–80% CURC), as assayed by both laboratories. Open triangles indicate root-only products, which do not contain curcuminoid extracts.
The relative amounts of the three curcuminoids, CURC, DMC and BDMC, were analyzed to test whether they were present within ranges anticipated for turmeric root (i.e. CURC < 70%) or within USP defined ranges for turmeric-derived curcuminoid extracts, namely a content of 70–80% CURC, as indicated by the closed box in Figure 2B. Root-only products all had low %CURC content (Figure 2B, open triangles). The remaining turmeric DS were all labeled as containing turmeric-derived curcuminoid extracts, although the USP-defined percent CURC range was exceeded by 59% of products, including 5 products where CURC content exceeded 90% (Figure 2B, dashed box). One >90% CURC sample also had lower than anticipated total curcuminoid content (Fig 2A, #19). There was no statistically significant relationship between curcuminoid composition (% CURC) and labeled content (turmeric or other bioactives), by ANOVA or t testing, respectively; however, there was a negative association with price (Pearson r= −0.35, p= 0.04), suggesting less expensive products were associated with a higher percent CURC.
Residual Solvents
Solvents detected were below USP recommended limits in all products tested (n = 35). Median number of detectable residual solvents per product was 2, with n=5 (14%) having >4 residual solvents (Figure 3A), a finding maintained across lots for 2 of the 5 products. Residual levels of carcinogenic class 1 or toxic class 2 solvents were detected in 71% of products; the median number of class 1 or 2 solvents per product was one (range 0 to 4) (Figure 3A). Toluene was the most commonly detected class 2 solvent (Figure 3B), being present in almost 70% of all products and found in combination with ethyl acetate, the most commonly used class 3 solvent, in approximately half. Of particular note, residuals of a class 1 solvent (1,2-dicholorethane [EDC]) were detected in one product (Figure 3A), in combination with 4 other solvents; EDC was not detected upon testing of a different lot where only residues of the two most commonly identified solvents, toluene and ethyl acetate, remained. Evidence of class 1 or 2 solvents was not associated with price or bioactive content, but was associated with turmeric content category. Using logistic regression analysis to calculate odds ratios (OR), the odds of selecting a product with a class 1 or 2 solvent was 23-fold less for proprietary formulations (solvents in 1 of 6 products) versus those containing any root (14 of 17; OR=0.043, p= 0.013) or 25-fold less versus “other” products containing non-proprietary curcuminoid extracts (10 of 12; OR=0.040, p=0.016). There was no significant difference between “other” products (containing non-proprietary curcuminoid extracts) vs. products with any root (OR=1.07, p= 0.945).
Figure 3.
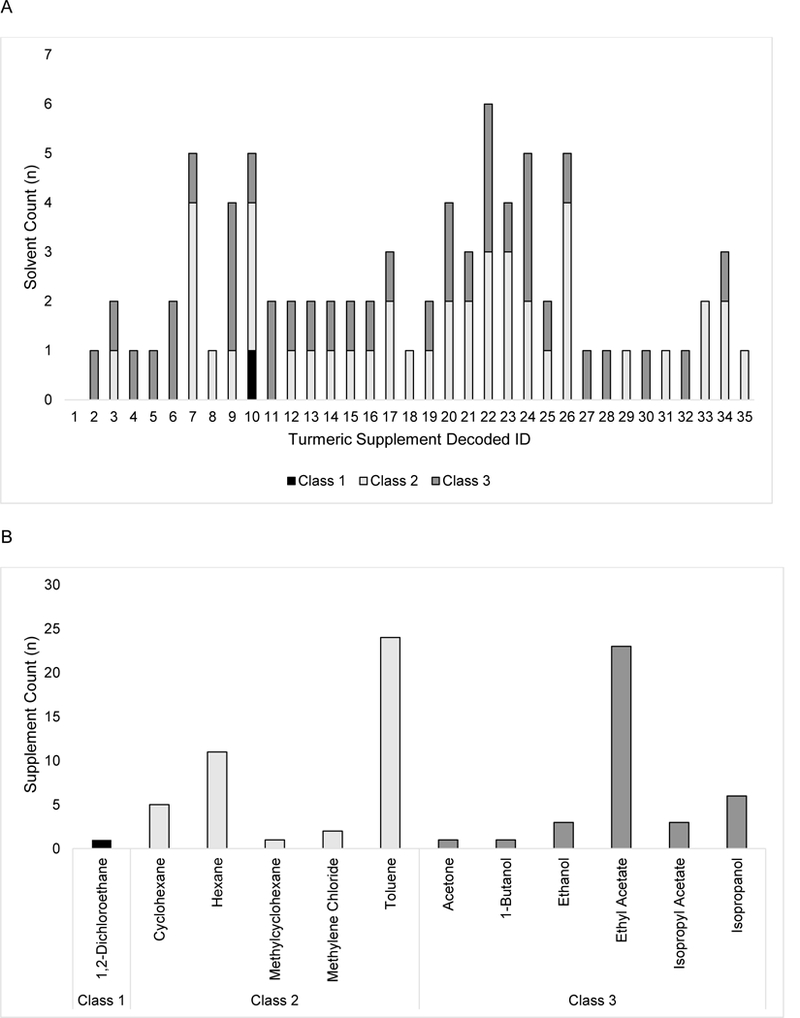
Assayed turmeric DS solvent content (n= 35). (A). The total number of residual class 1,2 or 3 solvents detected per product are indicated. (B) The number of products containing specific class 1,2 or 3 solvent residues are presented.
Lead Content
Lead was detected (≥0.005 ppm) in all but one product (Supplement Table 1). The average level of lead (± SD) was 0.12 ± 0.21 ppm (Figure 4A). Additional lots for all outlying products (n=5), which contained ≥0.2 ppm lead (Figure 4A), were tested. Lead content ≥0.2 ppm persisted between lots for two products (#19, #31), including one product (sample #19) with lead content consistently higher than acceptable USP levels (116 or 126% of USP). Lead content was associated with turmeric content category, but not bioactive content or cost. Lead content, as assessed by t test, was significantly higher in products containing any root vs. products without root (Figure 4B, p= 0.007), with average levels being 2.5-fold higher in root-containing products.
Figure 4.
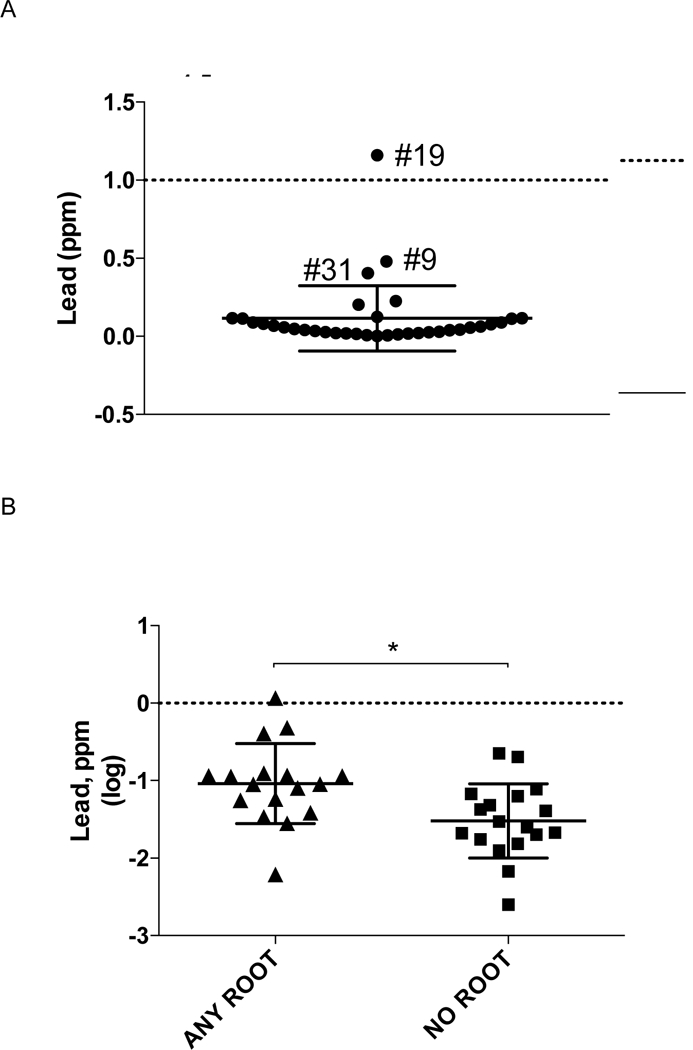
Turmeric DS lead content (n= 35). (A) Lead content, expressed as ppm, is indicated for products, including mean ± SD. For products with outlying values, sample identification numbers are indicated. Dashed line indicates USP specified limit for lead content (1 ppm). (B) Lead content, log transformed to normalize distribution, for products containing root vs no root. Scatter plots indicate individual product and mean (± SD) values. * P < 0.007, root vs no root. Dashed line indicates USP specified limit for lead content.
DISCUSSION
Heterogeneity of turmeric DS
The goal of this study was to assess the heterogeneity of turmeric DS offered for sale in the US, and to determine whether information available at point of sale was associated with safety-related measures of quality, including curcuminoid content and toxicologically relevant solvent and lead content. We identified 87 unique turmeric DS in the Tucson, AZ retail marketplace, which included 10 national chains, and considered this a representative sample as it was similar in size to the number of available turmeric DS concurrently listed in the National Institutes of Health (NIH) Dietary Supplement Label Database (n= 112), a detailed but non-comprehensive listing maintained by a joint effort of the Office of Dietary Supplements and US National Library of Medicine.[46] The turmeric DS marketplace appeared dynamic since only half of our turmeric DS were in the NIH DLSD database, and because some product formulations were no longer available when we attempted to re-purchase additional product lots. This implies a rapid turnover of certain products, which could limit consumers’ use of a consistent product of their choice over an extended period of time. Another layer of heterogeneity was identified due to variations in the type of turmeric content used, the inclusion of non-turmeric bioactives, and the labeled or unlabeled use of methods to enhance curcuminoid bioavailability in different formulations.
Curcuminoid content and implications for effective and safe dosing
Total curcuminoid content in the capsules was within 80% of anticipated values for a majority of turmeric DS tested here, a finding that agrees well with similar testing of 19 turmeric DS by Consumer Laboratories.[47] Turmeric DS were formulated to deliver vastly different amounts of curcuminoids, an attribute that could not always be easily deduced by consumers from information provided on the label. Although defined doses for health management or disease treatment are not well established, using a bench mark of 1–2 g curcuminoids/d that is supported by studies of arthritis,[8, 48] a traditional ethnobotanical use, some root-only products containing <15 mg curcuminoids may not deliver sufficient doses to achieve medicinal benefits. At the other extreme, some piperine-containing products could deliver curcuminoid doses exceeding those found to be well tolerated in short term clinical trials,[49] should curcuminoid bioavailability actually be increased 30-fold by piperine, as has been reported in the literature for a single dosing combination (100:1 ratio of curcumin to piperine) that is not reflective of all piperine-containing products.[17] Additional safety concerns related to use of piperine-containing products (24% of turmeric DS identified) could theoretically also occur in consumers taking concurrent medications, since piperine is thought to enhance curcuminoid bioavailability via inhibition of CYP3A, a hepatic enzyme responsible for the metabolism of 50% of all drugs.[50]
Possible indication of unlabeled use of synthetic curcumin
The lower percent CURC values in root-only products (n= 3) were consistent with the lower relative abundance of curcumin in turmeric oleoresins prepared by solvent extraction of dried turmeric rhizomes (38–63% CURC).[4, 5, 7] However, the fact that percent CURC values exceeded USP standards [41] that are characteristic of commercial curcuminoid extracts purified from turmeric oleoresins[5, 7] in the majority of products labeled as containing turmeric-derived curcuminoid extracts (59%) was a potential concern. The high percent CURC composition in these turmeric DS, particularly for the 16% of products where CURC constituted >90% of total curcuminoids, was suggestive of 1) possible substitution of chemically synthesized curcumin for plant-derived curcuminoids, a practice forbidden by US federal regulation,[51] or 2) the unlabeled use of curcumin-only extracts further purified from turmeric-derived curcuminoid.[52, 53] Because turmeric-derived curcumin-only extracts are not readily available commercially,[52, 53] while surreptitious substitution of synthetic curcumin for turmeric-derived curcuminoids is a practice of unknown scope that has been reported by the nutraceutical industry,[54] it seems likely that products with high percent CURC may contain synthetic curcumin. However, such a conclusion would require curcuminoid isotope distribution testing to determine with certainty,[55] a practice that has now been adopted by some turmeric DS manufacturers.[54] While the medicinal significance of substituting (synthetic) curcumin for naturally occurring curcuminoids is not known, the lower stability of pure curcumin in biological fluids[56] and potential for phenol-specific differences in active metabolite formation,[57] raises the possibility that purified curcumin versus turmeric-derived curcuminoid mixtures may not be bioequivalent. The negative association of turmeric DS cost with high percent CURC suggests that the likelihood of purchasing a product that may contain synthetic rather than turmeric–derived curcuminoids may be higher with less expensive products.
Toxicological considerations: organic solvent residues and lead
A second previously documented safety issue related to turmeric DS is the unreported use of class 1 and 2 solvents to prepare curcuminoid extracts.[58] Nutraceutical manufacturers are legally only required to verify acceptable levels of solvents that they have reason to suspect may be present in materials used to formulate DS, including known or suspected carcinogens (class 1 solvents) or solvents that could cause irreversible toxicity (class 2 solvents).[27] Class 3 solvents with low toxic potential and no health-based exposure limits can be effectively used for curcuminoid extraction and purification from turmeric root (e.g. acetone, ethyl acetate, ethanol, isopropyl alcohol),[3, 7, 59] eliminating the need for class 1 or 2 solvent use. Thus, while residual solvent levels were all below USP specified levels, it was notable that 71% of the turmeric DS tested contained traces of class 1 or 2 solvents, independent of the inclusion of additional bioactives. Traces of EDC, a toxic, class 1 solvent whose surreptitious use has been previously reported for the preparation of commercial curcuminoid extracts,[58, 60] was detected in only one lot of a single product, which contained curcuminoids as the only bioactive. In general, proprietary enhanced bioavailability curcuminoid preparations were much less likely to contain class 1 or 2 solvent residuals.
There have been multiple reports of unsafe lead levels in turmeric root sold for culinary use (turmeric spice) in the US, a contaminant that has been attributed to the use of lead chromate to polish rhizomes at harvest to enhance the weight or color of ground root sold in bulk for commercial use.[61] Consistent with contamination of culinary turmeric root products, average lead content was higher in turmeric DS products containing turmeric root, which represented one third of the turmeric DS in this survey. For a single product tested here, lead levels in multiple lots exceeded USP recommended limits. A Freedom of Information Act request to the FDA for reported adverse events associated with turmeric DS also identified one additional report, by Consumer Labs, of high lead levels in a different turmeric DS product (data not shown).
CONCLUSION
Turmeric DS comprise a $70 million segment of the dietary supplement market and are one of the most commonly sold DS in the US. They are notable for a diversity in formulations with potential relevance to safety and efficacy, illustrating the difficulties that consumers face when choosing turmeric DS from a complex and dynamic marketplace. While some information available at point of sale can aid consumers in maximizing their choice of a high-quality supplement to meet their health care goals, safe use of turmeric DS may best be accomplished by a partnership between patients and health care providers that includes consideration of possible product-specific content.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the National Center for Complementary and Integrative Health at the National Institutes of Health (NIH-NCCIH awards R34AT007837 to JF and R01AT006896 to CS). PBL was supported by postdoctoral fellowship award 16POST27250138 from the American Heart Association. CA received support from the NIH National Heart, Lung and Blood Institute (T35HL007479). Views expressed here do not necessarily represent those of the NIH.
ABBREVIATIONS
- DS
dietary supplements
- CURC
curcumin
- DMC
demethoxycurcumin
- BDMC
bis-demethoxycurcumin
Footnotes
CONFLICT OF INTEREST None of the authors have any conflict of interest related to the work reported here.
REFERENCES
- [1].Smith T, Kawa K, Eckl V, Johnson J, Sales of Herbal Dietary Supplements in Us Increased 7.5% in 2015. Consumers Spent $6.92 Billon on Herbal Supplements in 2015, Marking the 12th Consectuive Year of Growth. HerbalGram 2016, 67–73. [Google Scholar]
- [2].Smith T, Kawa K, Eckl V, Morton C, Stredney R, Herbal Supplement Sales in Us Increase 7.7% in 2016. Consumer Preferences Shifting toward Ingredients with General Wellness Benefits, Driving Growth of Adaptogens and Digestive Health Products. HerbalGram 2017, 56–65. [Google Scholar]
- [3].Pawar H, Karde M, Mundle N, Jadhav P, Mehra K, Phytochemical Evaluation and Curcumin Content Determination Ofturmeric Rhizomes Collected from Bhandara District of Maharashtra(India). Medicinal Chemistry 2014, 4. [Google Scholar]
- [4].Ashraf K, Mujeeb M, Ahmad A, Ahmad N, Amir M, Determination of Curcuminoids in Curcuma Longa Linn. By Uplc/Q-Tof-Ms: An Application in Turmeric Cultivation. J Chromatogr Sci 2015, 53, 1346–1352. [DOI] [PubMed] [Google Scholar]
- [5].Funk JL, Frye JB, Oyarzo JN, Kuscuoglu N, Wilson J, McCaffrey G, Stafford G, Chen G, Lantz RC, Jolad SD, Solyom AM, Kiela PR, Timmermann BN, Efficacy and Mechanism of Action of Turmeric Supplements in the Treatment of Experimental Arthritis. Arthritis Rheum 2006, 54, 3452–3464. [DOI] [PubMed] [Google Scholar]
- [6].Jiang H, Timmermann BN, Gang DR, Use of Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry to Identify Diarylheptanoids in Turmeric (Curcuma Longa L.) Rhizome. J Chromatogr A 2006, 1111, 21–31. [DOI] [PubMed] [Google Scholar]
- [7].Revathy S, Elumalai S, Benny M, Antony B, Isolation, Purification and Identification of Curcuminoids from Turmeric (Curcuma Longa L.) by Column Chromatography. Journal of Experimental Sciences 2011, 2, 21–25. [Google Scholar]
- [8].Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, Solyom AM, Timmermann BN, Turmeric Extracts Containing Curcuminoids Prevent Experimental Rheumatoid Arthritis. J Nat Prod 2006, 69, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhou H, Beevers CS, Huang S, The Targets of Curcumin. Curr Drug Targets 2011, 12, 332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hasegawa T, Nakatani K, Fujihara T, Yamada H, Aroma of Turmeric: Dependence on the Combination of Groups of Several Odor Constituents. Natural Product Communications 2015, 10, 1047–1050. [PubMed] [Google Scholar]
- [11].Funk JL, Frye JB, Oyarzo JN, Zhang H, Timmermann BN, Anti-Arthritic Effects and Toxicity of the Essential Oils of Turmeric (Curcuma Longa L.). J Agric Food Chem 2010, 58, 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lantz RC, Chen GJ, Solyom AM, Jolad SD, Timmermann BN, The Effect of Turmeric Extracts on Inflammatory Mediator Production. Phytomedicine 2005, 12, 445–452. [DOI] [PubMed] [Google Scholar]
- [13].Dietary Supplement Health and Education Act of 1994. Public Law 103–417. Silver Spring, MD, USA: US Food and Drug Administration; 1994. [Google Scholar]
- [14].Sax JK, Dietary Supplements Are Not All Safe and Not All Food: How the Low Cost of Dietary Supplements Preys on the Consumer. Am J Law Med 2015, 41, 374–394. [DOI] [PubMed] [Google Scholar]
- [15].Job KM, Kiang TK, Constance JE, Sherwin CM, Enioutina EY, Herbal Medicines: Challenges in the Modern World. Part 4. Canada and United States. Expert Rev Clin Pharmacol 2016, 9, 1597–1609. [DOI] [PubMed] [Google Scholar]
- [16].Douglass BJ, Clouatre DL, Beyond Yellow Curry: Assessing Commercial Curcumin Absorption Technologies. J Am Coll Nutr 2015, 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS, Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta medica 1998, 64, 353–356. [DOI] [PubMed] [Google Scholar]
- [18].Kanaki N, Dave M, Padh H, Rajani M, A Rapid Method for Isolation of Piperine from the Fruits of Piper Nigrum Linn. J Nat Med 2008, 62, 281–283. [DOI] [PubMed] [Google Scholar]
- [19].Srinivasan K, Black Pepper and Its Pungent Principle-Piperine: A Review of Diverse Physiological Effects. Crit Rev Food Sci Nutr 2007, 47, 735–748. [DOI] [PubMed] [Google Scholar]
- [20].Kanai M, Imaizumi A, Otsuka Y, Sasaki H, Hashiguchi M, Tsujiko K, Matsumoto S, Ishiguro H, Chiba T, Dose-Escalation and Pharmacokinetic Study of Nanoparticle Curcumin, a Potential Anticancer Agent with Improved Bioavailability, in Healthy Human Volunteers. Cancer Chemother Pharmacol 2012, 69, 65–70. [DOI] [PubMed] [Google Scholar]
- [21].Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ, Togni S, Dixon BM, Comparative Absorption of a Standardized Curcuminoid Mixture and Its Lecithin Formulation. J Nat Prod 2011, 74, 664–669. [DOI] [PubMed] [Google Scholar]
- [22].Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG, Safety and Pharmacokinetics of a Solid Lipid Curcumin Particle Formulation in Osteosarcoma Patients and Healthy Volunteers. J Agric Food Chem 2010, 58, 2095–2099. [DOI] [PubMed] [Google Scholar]
- [23].Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S, A Pilot Cross-over Study to Evaluate Human Oral Bioavailability of Bcm-95cg (Biocurcumax), a Novel Bioenhanced Preparation of Curcumin. Indian J. Pharm Sci 2008, 70, 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].US Patent, US20170072004 A1, Compositions and methods for improving the bioavailability of curcuminoids, filed 9/10/2015 (Howe B, Prasad K, inventors).
- [25].US Patent US 20160151440 A1, A Novel Composition of Curcumin with Enhanced Bioavailability, filed 8/19/2014 (Gopi S, inventor).
- [26].Jurenka JJS, Properties of Curcumin, a Major Constituent of Curcuma Longa: A Review of Preclinical and Clinical Research. Altern Med Rev 2009, 14, 141–153. [PubMed] [Google Scholar]
- [27].Jäger R LR, Calvanese AV, Joy JM, Purpura M, Wilson JM, Comparative Absorption of Curcumin Formulations. Nutr J 2014, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schiborr CKA, Behnam D, Jandasek J, Toelstede S, Frank J, The Oral Bioavailability of Curcumin from Micronized Power and Liquid Micelles Is Significantly Increased in Healthy Humans and Differs between Sexes. Mol Nutr Food Res. 2014, 58, 516–526. [DOI] [PubMed] [Google Scholar]
- [29].Sasaki H SY, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T, Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol Pharm Bull 2011, 35, 660–665. [DOI] [PubMed] [Google Scholar]
- [30].Funk J, Alfafara C, McEvoy S, Strom M, Lukefahr A, Turmeric Dietary Supplement-Induced Autoimmune Hepatitis: A Case Report. The FASEB Journal 2017, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Esatbeyoglu T, Huebbe P, Ernst IM, Chin D, Wagner AE, Rimbach G, Curcumin--from Molecule to Biological Function. Angew Chem (Intl Ed Engl) 2012, 51, 5308–5332. [DOI] [PubMed] [Google Scholar]
- [32].Chin D, Huebbe P, Frank J, Rimbach G, Pallauf K, Curcumin May Impair Iron Status When Fed to Mice for Six Months. Redox Bio 2014, 2, 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee SW, Nah SS, Byon JS, Ko HJ, Park SH, Lee SJ, Shin WY, Jin DK, Transient Complete Atrioventricular Block Associated with Curcumin Intake. Int J Cardiol, 2011, 150, e50–52. [DOI] [PubMed] [Google Scholar]
- [34].Teschke R, Bahre R, Genthner A, Fuchs J, Schmidt-Taenzer W, Wolff A, Suspected Black Cohosh Hepatotoxicity--Challenges and Pitfalls of Causality Assessment. Maturitas 2009, 63, 302–314. [DOI] [PubMed] [Google Scholar]
- [35].Gomez-Beloz A, Chavez N, The Botanica as a Culturally Appropriate Health Care Option for Latinos. J Altern Complement Med 2001, 7, 537–546. [DOI] [PubMed] [Google Scholar]
- [36].Ramachandran CRA, Escalon E, Aviram A, Melnick SJ, Potentiation of Gemcitabine by Turmeric Force in Pancreatic Cancer Cell Lines. Oncol Rep 2010, 23, 1529–1535. [DOI] [PubMed] [Google Scholar]
- [37].Braga MELP, Carvalho JE, Meireles MA, Comparison of Yield, Composition, and Antioxidant Activity of Turmeric (Curcuma Longa L.) Extracts Obtained Using Various Techniques. Agric Food Chem 2003, 51, 6604–6611. [DOI] [PubMed] [Google Scholar]
- [38].Begum ANJM, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA, Curcumin Structure-Function, Bioavailability, and Efficacy in Models of Neuroinflammation and Alzheimer’s Disease. J Pharmacol Exper Ther 2008, 326, 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Luis PB, Gordon ON, Nakashima F, Joseph AI, Shibata T, Uchida K, Schneider C, Oxidative Metabolism of Curcumin-Glucuronide by Peroxidases and Isolated Human Leukocytes. Biochem Pharmacol 2017, 132, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hoehle SI, Pfeiffer E, Solyom AM, Metzler M, Metabolism of Curcuminoids in Tissue Slices and Subcellular Fractions from Rat Liver. J Agric Food Chem 2006, 54, 756–764. [DOI] [PubMed] [Google Scholar]
- [41].Sharaf M, Curcuminoids. Pharmacopeial Forum 2008, 33, 1215. [Google Scholar]
- [42].Guidance for Industy: Q3C-Tables and List. 2 ed. Silver Spring, MD, USA: U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER); 2012. [Google Scholar]
- [43].Residual Solvents <467>. The United States Pharmacopeia 38/ The National Formulary 33; Chemical Tests. Rockville, MD: The United States Pharmacopeial Convention, Inc.;2012. [Google Scholar]
- [44].Offical Methods of Analysis. Method 2011.19 and 993.14: AOAC International. [Google Scholar]
- [45].Elemental Contaminants in Dietary Supplements <2232>. The United States Pharmacopeia 38/ The National Formulary 33; General Chapter Vol The United States Pharmacopeial Convention, Inc. Rockville, MD, 2012. [Google Scholar]
- [46].Dietary Supplement Label Database. Quick Search Results “Turmeric” https://dsld.nlm.nih.gov/dsld/rptQSearch.jsp?item=turmeric&db=adsld-ingred. Accessed August 3, 2017.
- [47].ConsumerLab. Product Review: Turmeric and Curcumin Supplements and Spices. 2017. https://www.consumerlab.com/reviews/turmeric-curcumin-supplements-spice-review/turmeric/. Accessed May 4, 2017.
- [48].Chandran B GA, A Randomized, Pilot Study to Assess the Efficacy and Safety Of curcumin in Patients with Active Rheumatoid Arthritis. Phytother Res 2012, 26, 1719–1725. [DOI] [PubMed] [Google Scholar]
- [49].Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY, Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer Res 2001, 21, 2895–2900. [PubMed] [Google Scholar]
- [50].Rezaee MM, Kazemi S, Kazemi MT, Gharooee S, Yazdani E, Gharooee H, Shiran MR, Moghadamnia AA, The Effect of Piperine on Midazolam Plasma Concentration in Healthy Volunteers, a Research on the Cyp3a-Involving Metabolism. Daru 2014, 22, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Part 201(ff)(1)(c) Food and Drug Cosmetic Act. In: Food Drug Administration HHS, ed. Code of Federal Regulations (CFR) Title 21.
- [52].Song W, Qiao X, Liang WF, Ji S, Yang L, Wang Y, Xu YW, Yang Y, Guo DA, Ye M, Efficient Separation of Curcumin, Demethoxycurcumin, and Bisdemethoxycurcumin from Turmeric Using Supercritical Fluid Chromatography: From Analytical to Preparative Scale. J Sep Sci 2015, 38, 3450–3453. [DOI] [PubMed] [Google Scholar]
- [53].Heffernan C, Ukrainczyk M, Gamidi RK, Hodnett BK, Rasmuson ÅC, Extraction and Purification of Curcuminoids from Crude Curcumin by a Combination of Crystallization and Chromatography. Org. Process Res. Dev. 2017, 21, 821–826. [Google Scholar]
- [54].Grebow J, Curcumin Suppliers “Guaranteeing” Natural Curcumin Is Not Synthetic by Using Carbon Dating: Supplyside West Report. Nutritional Outlook Magazine 2016, 19. [Google Scholar]
- [55].ISO 16620–2:2015(en), Plastics- Biobased content- Part 2: Determination of biobased carbon content. https://www.iso.org/obp/ui/-iso:std:iso:16620:−2:ed-1:v1:en. Accessed October 2, 2017.
- [56].Kharat M, Du Z, Zhang G, McClements DJ, Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of Ph, Temperature, and Molecular Environment. J Agric Food Chem 2017, 65, 1525–1532. [DOI] [PubMed] [Google Scholar]
- [57].Gordon ON, Luis PB, Ashley RE, Osheroff N, Schneider C, Oxidative Transformation of Demethoxy- and Bisdemethoxycurcumin: Products, Mechanism of Formation, and Poisoning of Human Topoisomerase Iialpha. Chem Res Toxicol 2015, 28, 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liva R, Toxic Solvent Found in Curcumin Extract. Integrative Medicine 2010, 9, 50–54. [Google Scholar]
- [59].Priyadarsini KI, The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kadu PWDPP, Extraction, Isolation, Purification and Identification of Curcumin: A Review Article. European J Biomed Pharm Sci 2015, 2, 108–123. [Google Scholar]
- [61].Cowell W, Ireland T, Vorhees D, Heiger-Bernays W, Ground Turmeric as a Source of Lead Exposure in the United States. Public health reports (Washington, D.C. : 1974) 2017, 132, 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


