Abstract
Carriers of a pathogenic germline mutations in the PTEN gene, a well-known tumor suppressor gene, are at increased risk of multiple benign and malignant tumors, e.g. breast, thyroid, endometrial and colon cancer. This is called PTEN Hamartomous Tumor Syndrome (PHTS). PHTS patients may also have an increased risk of immunological dysregulation, such as autoimmunity and immune deficiencies. The effects of PTEN on the immune system have been studied in murine knockout models demonstrating that loss of PTEN function leads to dysregulation of the immune response. This results in susceptibility to autoimmunity, impaired B cell class switching with subsequent hypogammaglobulinemia. Additionally, a decreased ability of dendritic cells to prime CD8+ T cells was observed, leading to impaired tumor eradication. Immune dysfunction in PHTS patients has not yet been extensively studied but might be a manageable contributing factor to the increased cancer risk in PHTS.
Introduction
The role of the immune system in carcinogenesis and cancer progression has been widely established, but an association between hereditary tumor syndromes and immunological dysfunction has not yet been demonstrated. Hereditary tumor syndromes are caused by germline mutations in tumor suppressor genes or proto-oncogenes, one of such tumor syndromes is PTEN hamartoma tumor syndrome (PHTS). PHTS is an autosomal dominant tumor syndrome, caused by loss of function mutations in the phosphatase and tensin homolog gene (PTEN). PTEN is a negative regulator of the PI3K/Akt pathway, thereby acting as a tumor suppressor gene. Through the PI3K/Akt pathway, PTEN has a regulating effect on cell proliferation, cell metabolism, cell survival and angiogenesis [1], [2], [3].
PHTS is a collective term that replaces previously used syndrome names, such as; Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome and Proteus-like syndrome. It is associated with an increased risk of benign and malignant tumors of the breast, thyroid, endometrium, colon and other forms of tumors. Additionally, PHTS patients may have many different features ranging from macrocephaly, developmental delay and autism specter disorders to benign skin and organ lesions such as trichilemmomas and dysplastic gangliocytoma [4], [5], [6], [7]. The current estimate is that 1 in 200,000 individuals has PHTS. However, because several PHTS features are quite common in the general population, such as benign lesions of the breast uterus and skin, these patients may not have been recognized as PHTS. This means that the incidence of PHTS in the general population may be higher than the earlier estimates [8]. Clinical characteristics of PHTS show high penetrance and it is estimated that at age 30, nearly 100% of germline PTEN mutation carriers exhibit some features associated with PHTS. De novo mutations make up for 10–40% of diagnosed cases [4].
Involvement of the immune system in carcinogenesis is widely known and accepted. During cancer development, regulatory cellular processes are lost and genetic alterations accumulate [9]. This leads to the expression of neoantigens by cancer cells that can eventually be recognized by the immune system as foreign and consequently elicit a CD8+ T cell mediated response [10]. In recent years, evidence of immune dysregulation in PHTS patients has emerged with the publication of small case series as well as more extensive cohorts [11], [12], [13], [14], [15], [16], [17]. Nevertheless, a possible relationship between immune dysregulation and cancer risk has not been established in PHTS. This review aims to elaborate on known immunological phenomena in PHTS individuals and PTEN knockout mice and to identify directions for future research. We hypothesize that intervening in the immunological dysregulation may lead to new treatment options for PHTS patients.
Immunological Phenomena in PHTS Patients
In spite of the heterogeneous phenotypes of PHTS individuals, the immunological symptoms reported in literature seem quite consistent. Recurring upper respiratory tract infections are reported in multiple case reports and case series [11], [12], [18]. In one of these case reports, one individual developed a skin manifestation compatible with a reactive cutaneous lymphocytic vasculitis that fully resolved after tonsillectomy [12]. A larger retrospective study with 34 PHTS patients, did not report any susceptibility to infections [14].
In all of the cases with recurrent upper respiratory tract infections, hyperplasia of the adenoids and/or tonsils were present [11], [12], [18]. In one individual diagnosed with PHTS, extreme pharyngeal papillomatosis and tonsillar hypertrophy triggered by Epstein–Barr viral infection caused extensive airway obstruction necessitating tracheotomy. Observations from biopsies revealed only benign lymphofollicular hyperplasia without malignancy. The son of this individual carried the same PTEN mutation and presented himself at the age of 4 with sleep apnea due to extensive tonsillar enlargement. Pathological examination revealed papillomatous changes with lymphofollicular hyperplasia, similar to the findings in the father's case [16].
Lymphoid hyperplasia in PHTS patients is not restricted to adenoid and tonsillar lymphoid tissue, Tsujita et al. used PET-scans to determine lymphoid hyperplasia in two PHTS individuals and demonstrated increased cervical and abdominal lymph nodes [18]. Gastrointestinal polyps with follicular lymphoid hyperplasia has been a reported finding in independent case series. In 34 PHTS patients, 16 (50%) had gastro-intestinal lymphoid hyperplasia, located in the colon and rectosigmoid without signs of mucosa-associated lymphoid tissue (MALT) lymphoma. Investigation of MALT tissue in controls and PHTS patients revealed reduced apoptosis and increased proliferation of CD10+ pre-B cells. There were no differences detected between control and PHTS T cell populations [14]. In a more recent publication, 7 out of 12 (58%) PTEN patients with confirmed PTEN mutations had hamartomous polyps with hyperplastic lymphoid follicles [19]. It must be stressed, however, that polyposis is a possible feature of PHTS that has not been studied extensively and large studies have not yet been published.
Abnormalities in the humoral response of the adaptive immune system have also been reported. Hypogammaglobulinemia has been reported in several publications [11], [20]. Further analysis of the immunoglobulin subtypes in PHTS patients show impaired class switch recombination (CSR) leading to a disrupted IgG and IgA subclass distribution with increased IgG1 and decreased IgG2 concentrations. Similar results were described for IgA [20]. In a larger study, hypogammaglobulinemia was not observed in 34 PHTS patients, although PHTS immunoglobulin levels were reported to be in the lower level of normal [14]. In one case report of a 5- year old boy, within 15 months long-term humoral response to Haemophilus Influenzae B and pneumococcal vaccination declined to nearly baseline levels [11]. Further study of this phenomenon is warranted.
Lymphopenia has also been reported in a few case series [11], [12], [13], [18]. Increases in the absolute number of peripheral transitional B cell subsets combined with a reduction of circulating CD4+ T cells with subsequent inversion of the CD4+/ CD8+ ratio is shown in multiple studies [13], [14], [20]. The increase of transitional B cells appears to be more pronounced in patients with hypogammaglobulinemia [20].
In patients with PHTS, dysregulation of the immune system is also reflected by hyperinflammation leading to an increased incidence of disorders. In 34 patients described by Heindl et al., 7 (21%) displayed autoimmune disorders such as autoimmune lymphocytic thyroiditis and autoimmune haemolytic anemia [14]. More recently, autoimmunity related phenomena were seen in 27% of 79 PHTS patients, including thyroiditis, colitis, celiac disease, haemolytic anemia and pernicious anemia [13]. These results imply that autoimmunity may be a feature of PHTS and therefore, it warrants further investigation. An overview of the immunological features previously observed in PHTS patients is depicted in Table 1.
Table 1.
Immunological clinical features in PHTS patients.
| Study (number of cases reported) | Described clinical features (number of cases with features) |
|---|---|
| Heindl et al.(34) [14] | -lymphoid hyperplasia (26/34) |
| -Autoimmunity (11/34)⁎ | |
| Browning et al. (2) [11] | -Recurrent (upper) respiratory tract infections (2/2) |
| -Panhypogammaglobulinemia (1/2) | |
| -Decreased long term antibody response to specific vaccines (1/2) | |
| -Lymphoid hyperplasia (2/2) | |
| -Increased amount of transitional B cells | |
| Driessen et al.(9) [20] | -Hypogammaglobulinemia(3/9) |
| -Increased absolute number of transitional B cells | |
| -Affected class switch recombination, increasing IgG1, and decreasing IgG2 | |
| Mauro et al.(1) [12] | -Recurrent upper respiratory tract infections |
| -Reactive cutaneous lymphocytic vaculitis | |
| -Lympohopenia | |
| Sharma et al.(2) [16] | -Recurrent upper respiratory tract infections in childhood (2/2) |
| -Lymphoid hyperplasia (2/2) | |
| Tsujita et al. (4) [18] | -High serum IgM (1/4) |
| -Recurrent pulmonary opportunistic infections(2/4) | |
| -Lymphopenia (1/4) | |
| -CD4+/CD8+ ratio inversion (1/4) | |
| -Lymphoid hyperplasia(2/4) | |
| Shaco-Levy et al. (12) [19] | -Hamartomas with lymphoid follicles 7/12 |
| -Juvenile hamartoma inflammatory intestinal polyps(12) | |
| Boccone et al. (1) [17] | -Lymphoid hyperplasia |
| Chen et al. (79) [13] | -Lymphoid hyperplasia (18/79) |
| -Autoimmunity (21/79) | |
| -Significant reduction of peripheral blood lymphocytes | |
| -Increased number transitional B-cells | |
| -CD4+/CD8+ ratio inversion |
Autoimmunity includes Hashimoto's thyroiditis and autoimmune haemolytic anemia.
In some primary immunodeficiency syndromes, the pathway in which PTEN has a function, the PI3K/Akt1/mTOR pathway, is known to be upregulated and is a well-known causal factor of immunodeficiency. Activated PI3Kδ syndrome (APDS) leads to a plethora of immunological phenomena such as recurrent sinopulmonary infections, inability to clear viral infections, benign lymphadenopathy, and autoimmune diseases [21], [22]. It is caused by gain-of-function mutations in the phosphoinositide 3 kinase (PI3Kδ) gene, a leukocyte specific subunit of PI3K [23], [24]. Loss of function in the downregulating gene PIK3R1, coding for the regulatory PI3K subunit p85α, has also been reported to cause immune deficiencies [25]. Symptoms correlate with the symptoms observed in PHTS patients, although APDS symptoms tend to be more severe. The clinical similarities and implication of the same pathway underscore the involvement of PTEN in immune system function.
PTEN and the Immune System
In cancer development failure of the immune system to recognize and destroy malignant cells is mandatory for tumor survival. Tumors can employ numerous tactics to escape immune surveillance [26], [27]. By modulating their own micro-environment, tumors can evade the immunological anti-tumor response by the secretion of immunosuppressing cytokines and chemokines. Information on this so-called tumor micro-environment (TME) in PTEN deficient organisms comes almost exclusively from murine models, as gathering sufficient human PHTS related tumors is complex due the fact that PHTS is a rare disease. The interplay between different cells of the immune system has not been studied extensively in PTEN −/− mice.
Natural Killer Cells
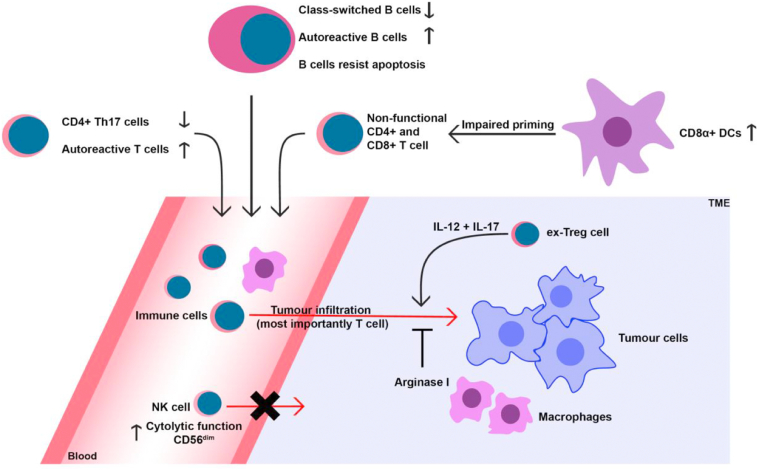
Natural killer (NK) cells have functions in the finding and destruction of infected, foreign or malignant cells [28], [29]. The role of PTEN and the PI3K/Akt pathway in NK function and maturation has been a point of interest for some time [30], [31], [32], [33]; Briercheck and colleagues reported that an NK cell lineage specific deletion of PTEN gives rise to NK cells with increased cytolytic function [34]. Additionally, the migration of NK cells towards distal tumors in PTEN-deleted NK cells in mice is impaired and the migration of NK cells from the bone marrow to the bloodstream is increased. Tumor cells that were introduced into the peripheral bloodstream were cleared more effectively than in wild type mice [35]. If these findings are translatable to human PHTS physiology, this would imply a decreased ability of NK cells to target cancer cells in tissue and thus an increased cancer risk.
Macrophages
Macrophages are important players in the TME, and in certain tumors as much as 50% of tumor mass can be tumor associated macrophages (TAMs) [36]. Infiltration of tumors by TAMs is generally associated with poorer prognosis [37], [38], [39]. To describe their functional programming and activation status, macrophages have classically been divided in the proinflammatory M1 type macrophage and the anti-inflammatory, proliferation supporting M2 type. M1 macrophages are equipped for the “eradication phase” of the immune response, while M2 macrophages are essential in the tissue healing process [40]. Nevertheless, macrophages are characterized by a high functional plasticity and therefore a wide range of variety with respect to their pro- and anti-inflammatory activity is observed. Studies in myeloid PTEN deficient mice report an increase in M2-like peritoneal and bone marrow-derived macrophages with an increased arginase I activity [41]. Importantly, the TAMs infiltrating the TME of many malignant tumors, including breast cancers which are prevalent in PHTS patients, show a preponderance of M2-like macrophages that are regarded as tumor promotors [42]. Increased arginase production by TAMs can lead to impaired T cell function due to arginine depletion in the TME, thereby reducing immune surveillance [41]. Moreover, the PI3K/AKT/mTOR is one of the key regulators of the cell metabolism. Emerging evidence indicates that functional reprogramming of macrophages/TAMs is highly dependent on changes in the immune cell metabolism linked to activation of the Akt/mTOR pathway, which is in turn essential for reshaping the epigenetic landscape and functional program of the cell [43], [44], [45], [46], [47].
Myeloid Derived Suppressor Cells (MDSCs)
MDSCs comprise a heterogeneous group of immune cells with suppressive functions in chronic inflammatory conditions [48], [49]. Infiltration of tumors with MDSCs is associated with attenuated T cell function and decreased effect of immune checkpoint inhibition therapy (ICI) [50], [51], [52]. By selectively inhibiting the myeloid specific PI3Kɣ in murine models, De Heneau et al. restored sensitivity for ICI in tumors with high MDSC infiltration [53]. Sensitisation to ICI was also achieved in head and neck cancers by selective inhibition of PI3Kδ and PI3Kɣ isoforms [54]. With PTEN as a negative regulator of the PI3K/Akt pathway, (partial) loss of PTEN function may contribute to MDSC dependent suppression of T cells and inhibit tumor surveillance.
Dendritic Cells
Dendritic cells (DCs) are the professional antigen presenting cells and have a pivotal role in the priming of the adaptive immune system [55]. Dysfunction of DCs could theoretically lead to decreased tumor immune surveillance through impaired activation of CD8+ cytotoxic T cells. In a PTEN−/− myeloid lineage murine model, PTEN deletion led to an increased colon cancer tumor load and decreased survival. However, an increased number of CD8α+ DCs was found in the spleen. CD8α+ cross-presenting DCs are a unique DC subset specialized in cross-priming of CD8+ cytotoxic T cells and essential for tumor immune-surveillance [56]. However, in myeloid PTEN−/− mice this cross-priming of exogenous antigens was deficient. If these findings are translatable to PHTS, deficient priming of CD8+ cytotoxic T cells may lead to decreased tumor cell killing activity, promoting tumor growth. Additionally, PTEN−/− DCs with increased programmed death-ligand 1(PD-L1) and PD-L2 expression were observed. These molecules are known to induce anergy in T cells at ligation [57], providing an additional means for the tumor to escape surveillance.
B Cells
B cells are vital for mounting a humoral response against infection and tumors. By recognition of (neo)antigens they can produce antigen specific immunoglobulins. In B cell-specific PTEN−/− mice, B cells show increased proliferation and decreased apoptosis in the marginal zones of the spleen. Additionally, they have an increased number of peritoneal B cells [58]. These cells produce polyreactive IgM that can react weakly to autoantigens [59]. A deficient class switch recombination was observed in another B cell specific PTEN−/− mouse model, with decreased IgG and IgA levels and a fourfold increase in IgM [60]. Dysfunction of a B cell antibody response leads to decreased tumor surveillance and an increase in peritoneal B cells could result in autoimmune disease.
T cells
The role of T cells in counteracting tumor growth by recognition of tumor-expressed antigens and subsequent activation of effector T cells has been widely established [61], [62]. The PI3K/Akt pathway has a central role in T cell development [63]. In thymocyte restricted PTEN−/−, CD3ɣ−/− mice models, thymocytes missing the functional β-chain receptor were not adequately removed during β-selection [64]. The failure to induce apoptosis in these TCRβ- cells could explain lymphoid hyperplasia in PHTS patients. Lymphoid hyperplasia and autoimmunity was also demonstrated in PTEN+/− mice and was attributed to a decreased response to CD95 (Fas) induced apoptosis [65]. Binding to the Fas receptor is a major pathway for CD8+ cells to induce cell death in tumor cells. Decreased response to Fas-induced apoptosis is often seen in cancer, promoting tumor growth [66].
In T helper (Th) cell specific PTEN knockout mice, Th cells show an improved stimulatory function and increased excitability by sole TCR activation. Production of proinflammatory IL-2 by these PTEN −/− T helper cells, and proliferation, is increased [63], [67]. Although not reported in all studies with T cell specific knockout models, the downside of this increased immune response is the loss of self-tolerance and the induction of auto-reactive T cells [68]. The pathophysiological link between autoimmunity remains largely unclear, but autoimmune disease is associated with increased cancer risk [69], [70], [71]. The increased proliferation of T cells in T cell specific murine knockout models does not apply to the Th17 subset, Th17 cells are associated with autoimmune disorders such as arthritis. Downregulation of IL-2 by PTEN is required for Th17 cells to develop. Loss of PTEN reduces severity of Th17-associated autoimmunity disorders [72]. In antigen presenting cells specific PTEN −/− mice, there was a marked decrease in autoimmune arthritis and autoimmune encephalomyelitis. IL-17 and IL-22 production was also reduced compared to wild-type mice. Direct administration of arthritogenic serum, thereby circumventing the adaptive immune response, did not lead to a diminished phenotype. Most likely, in mice, PTEN is essential in antigen presenting cells to induce functional programming towards Th17 cell development [73], [74].
CD4+ FoxP3+ regulatory T cells (Tregs) have an important role in the creation of an immunologically suppressed tumor environment. Tumors actively recruit Tregs to create an immunosuppressive TME. The ablation of Tregs in mice drastically decreases tumor load that may come at a price of lethal autoimmunity [75], [76]. In tumors, Tregs maintain their immunosuppressive phenotype by interaction with the immunoregulatory enzyme indoleamine 2,3,-dioxygenase (IDO) (over)expressed in tumor cells and in immune cells often present in the TME, such as DCs [77]. Downregulation of the Akt pathway is important for Tregs to maintain stability. Consequently, PTEN was shown to be an important regulator of Treg function [77]. In Treg specific PTEN knockout mice, phosphorylation of Akt was increased after IDO stimulation leading to a switch in phenotype from Tregs to hyperinflammatory ex-Tregs. As a result, proinflammatory cytokines such as IL-2 and IL-17 were expressed in the TME and facilitated an effective anti-tumor response. This suggests that loss of PTEN in Tregs induces an immunogenic TME [77]. Other studies have also demonstrated this effect in murine Foxp3+ PTEN−/− models [78], [79]. A recent study in PHTS individuals, using biopsies of mucosa associated lymphoid tissue, shows that Tregs have a normal phenotype. Suggesting that residual PTEN activity in PHTS is sufficient to sustain the immunosuppressive Treg phenotype or that there is compensatory phosphatase activity [13].
The effects of PTEN deletion on the adaptive immune system are well documented in mouse models (Figure 1). These models show changes to B- and T cells that can result in hypogammaglobulinemia and autoreactivity. Changes to the TME in Treg PTEN−/− mice demonstrate the important regulatory role PTEN has in regulating the anti-cancer immune response.
Figure 1.
Overview of effect of loss of PTEN on the murine immune system.
PTEN-Deficient Tumors
Limited data is available on the immune cell populations in PTEN-deficient tumors. Peng and colleagues report that human melanoma samples lacking PTEN contain less tumor infiltrating lymphocytes and achieve less tumor reduction with anti-PD1 antibodies compared to PTEN-expressing tumors [80], [81]. Similar results were reported in a case report of a female with metastatic uterine leiomyosarcoma who achieved near total remission after anti-PD1 monotherapy. There was one treatment resistant lesion which was removed surgically. The resistant lesion showed post treatment biallelic PTEN mutations and significant up-regulation of immunomodulatory molecules such as VEGF and CCL2 was observed in PTEN null tumors [80]. VEGF has been known to contribute to an immunosuppressive TME by recruiting MDSCs, Tregs and immature DCs [82]. These data show that PTEN deletion in tumors may lead to an immunotolerant TME by the inhibition of lymphocyte infiltration and the upregulation of immunomodulatory molecules.
Conclusion and Perspective
Current studies suggest that immune dysregulation is one of the features of PHTS. This dysregulation is reflected in autoimmune disorders, lymphoid hyperplasia, hypogammaglobulinemia and changes in T- and B- cell subsets. The role of immune dysregulation in carcinogenesis in PHTS patients has not yet been extensively explored. The effects of PTEN-loss have almost exclusively been investigated in mouse knockout models. In murine PTEN knockout models, PTEN-null myeloid cells show dysfunction that could be conducive to an increased cancer risk and a less immunogenic TME. In these mouse models, the effect of different cell types carrying PTEN mutations interacting with each other cannot be studied adequately. Additional models need to be developed to study the effect of PTEN mutations on human physiology (e.g. organoids).
Targeting dysfunctional immune cell types could lead to new treatment strategies for cancer in PHTS patients. A viable candidate cell type to target could be the CD141+ DC subset, improving tumor antigen cross presentation to CD8+ T cells [83]. Tumor characteristics, such as up-regulated VEGF expression, can also be a treatment target. Future investigation may focus on studying tumors of PHTS patients and translating aforementioned findings from animal models to humans. This could achieve greater insight in PHTS pathophysiology and help identify possible targets for therapy.
By means of this review, we have demonstrated that signs of immune dysregulation have been reported consistently in PHTS patients. Clinical phenomena differ among patients, but three clinical hallmarks can be identified from current available literature. Firstly, an increased susceptibility to viral and bacterial infection is observed [11], [12], [16], [18]. Secondly, an increased frequency of autoimmune disorders is reported in PHTS patients, ranging from autoimmune thyroiditis to haemolytic anemia [13], [14].
PHTS has previously been characterized as a genetic tumor risk syndrome with macrocephaly caused by a germline mutation in the PTEN gene. Literature suggests that immune dysregulation is also a feature caused by pathogenic germline PTEN mutations. Combined with the knowledge that the immune system has an instrumental role in carcinogenesis, immune dysregulation and increased cancer risk could be considered as two features of PHTS that are not merely co-occurring but interdependent.
Linking immunological dysregulation to pathological germline mutations in PTEN and increased cancer risk might have implications for future treatment of PHTS patients. Uncovering the ways by which the immune system contributes to carcinogenesis in PHTS may provide manageable targets for further treatment of this grave disease.
Footnotes
Declarations of interest: None.
Funding: None.
Conflict of Interest Statement: All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 2.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins BD, Hodakoski C, Barrows D, Mense SM, Parsons RE. PTEN function: the long and the short of it. Trends Biochem Sci. 2014;39:183–190. doi: 10.1016/j.tibs.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mester J, Charis E. PTEN hamartoma tumor syndrome. Handb Clin Neurol. 2015;132:129–137. doi: 10.1016/B978-0-444-62702-5.00009-3. [DOI] [PubMed] [Google Scholar]
- 5.Mester JL, Tilot AK, Rybicki LA, Frazier TW, II, Eng C. Analysis of prevalence and degree of macrocephaly in patients with germline PTEN mutations and of brain weight in Pten knock-in murine model. Eur J Hum Genet. 2011;19:763–768. doi: 10.1038/ejhg.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607–1616. doi: 10.1093/jnci/djt277. [DOI] [PubMed] [Google Scholar]
- 7.Lachlan KL, Lucassen AM, Bunyan D, Temple IK. Cowden syndrome and Bannayan Riley Ruvalcaba syndrome represent one condition with variable expression and age-related penetrance: results of a clinical study of PTEN mutation carriers. J Med Genet. 2007;44:579–585. doi: 10.1136/jmg.2007.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngeow J, Eng C. PTEN hamartoma tumor syndrome: clinical risk assessment and management protocol. Methods. 2015;77-78:11–19. doi: 10.1016/j.ymeth.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Tian T, Olson S, Whitacre JM, Harding A. The origins of cancer robustness and evolvability. Integr Biol (Camb) 2011;3:17–30. doi: 10.1039/c0ib00046a. [DOI] [PubMed] [Google Scholar]
- 10.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 11.Browning MJ, Chandra A, Carbonaro V, Okkenhaug K, Barwell J. Cowden's syndrome with immunodeficiency. J Med Genet. 2015;52:856–859. doi: 10.1136/jmedgenet-2015-103266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauro A, Omoyinmi E, Sebire NJ, Barnicoat A, Brogan P. De novo PTEN mutation in a young boy with cutaneous vasculitis. Case Rep Pediatr. 2017;2017:9682803. doi: 10.1155/2017/9682803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HH, Handel N, Ngeow J, Muller J, Huhn M, Yang HT, Heindl M, Berbers RM, Hegazy AN, Kionke J. Immune dysregulation in patients with PTEN hamartoma tumor syndrome: Analysis of FOXP3 regulatory T cells. J Allergy Clin Immunol. 2017;139:607–620.e615. doi: 10.1016/j.jaci.2016.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heindl M, Handel N, Ngeow J, Kionke J, Wittekind C, Kamprad M, Rensing-Ehl A, Ehl S, Reifenberger J, Loddenkemper C. Autoimmunity, intestinal lymphoid hyperplasia, and defects in mucosal B-cell homeostasis in patients with PTEN hamartoma tumor syndrome. Gastroenterology. 2012;142:1093–1096.e1096. doi: 10.1053/j.gastro.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Hodge D, Misbah SA, Mueller RF, Glass EJ, Chetcuti PA. Proteus syndrome and immunodeficiency. Arch Dis Child. 2000;82:234–235. doi: 10.1136/adc.82.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma MR, Petty EM, Lesperance MM. Airway obstruction caused by PTEN hamartoma (Bannayan-Riley-Ruvalcaba) syndrome. Arch Otolaryngol Head Neck Surg. 2007;133:1157–1160. doi: 10.1001/archotol.133.11.1157. [DOI] [PubMed] [Google Scholar]
- 17.Boccone L, Dessi V, Zappu A, Piga S, Piludu MB, Rais M, Massidda C, De Virgiliis S, Cao A, Loudianos G. Bannayan-Riley-Ruvalcaba syndrome with reactive nodular lymphoid hyperplasia and autism and a PTEN mutation. Am J Med Genet A. 2006;140:1965–1969. doi: 10.1002/ajmg.a.31396. [DOI] [PubMed] [Google Scholar]
- 18.Tsujita Y, Mitsui-Sekinaka K, Imai K, Yeh TW, Mitsuiki N, Asano T, Ohnishi H, Kato Z, Sekinaka Y, Zaha K. Phosphatase and tensin homolog (PTEN) mutation can cause activated phosphatidylinositol 3-kinase delta syndrome-like immunodeficiency. J Allergy Clin Immunol. 2016;138:1672–1680.e1610. doi: 10.1016/j.jaci.2016.03.055. [DOI] [PubMed] [Google Scholar]
- 19.Shaco-Levy R, Jasperson KW, Martin K, Samadder NJ, Burt RW, Ying J, Bronner MP. Gastrointestinal polyposis in Cowden syndrome. J Clin Gastroenterol. 2017;51:e60–e67. doi: 10.1097/MCG.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 20.Driessen GJ, I.J. H, Wentink M, Yntema HG, van Hagen PM, van Strien A, Bucciol G, Cogulu O, Trip M, Nillesen W. Increased PI3K/Akt activity and deregulated humoral immune response in human PTEN deficiency. J Allergy Clin Immunol. 2016;138:1744–1747.e1745. doi: 10.1016/j.jaci.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Coulter TI, Chandra A, Bacon CM, Babar J, Curtis J, Screaton N, Goodlad JR, Farmer G, Steele CL, Leahy TR. Clinical spectrum and features of activated phosphoinositide 3-kinase delta syndrome: A large patient cohort study. J Allergy Clin Immunol. 2017;139:597–606.e594. doi: 10.1016/j.jaci.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas CL, Chandra A, Nejentsev S, Condliffe AM, Okkenhaug K. PI3Kdelta and primary immunodeficiencies. Nat Rev Immunol. 2016;16:702–714. doi: 10.1038/nri.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angulo I, Vadas O, Garcon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C. Phosphoinositide 3-kinase delta gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342:866–871. doi: 10.1126/science.1243292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucas CL, Zhang Y, Venida A, Wang Y, Hughes J, McElwee J, Butrick M, Matthews H, Price S, Biancalana M. Heterozygous splice mutation in PIK3R1 causes human immunodeficiency with lymphoproliferation due to dominant activation of PI3K. J Exp Med. 2014;211:2537–2547. doi: 10.1084/jem.20141759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deau MC, Heurtier L, Frange P, Suarez F, Bole-Feysot C, Nitschke P, Cavazzana M, Picard C, Durandy A, Fischer A. A human immunodeficiency caused by mutations in the PIK3R1 gene. J Clin Invest. 2014;124:3923–3928. doi: 10.1172/JCI75746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 27.Klein G. Immune surveillance--a powerful mechanism with a limited range. Natl Cancer Inst Monogr. 1976;44:109–113. [PubMed] [Google Scholar]
- 28.Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The broad spectrum of human natural killer cell diversity. Immunity. 2017;47:820–833. doi: 10.1016/j.immuni.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tassi I, Cella M, Gilfillan S, Turnbull I, Diacovo TG, Penninger JM, Colonna M. p110gamma and p110delta phosphoinositide 3-kinase signaling pathways synergize to control development and functions of murine NK cells. Immunity. 2007;27:214–227. doi: 10.1016/j.immuni.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 32.Guo H, Samarakoon A, Vanhaesebroeck B, Malarkannan S. The p110 delta of PI3K plays a critical role in NK cell terminal maturation and cytokine/chemokine generation. J Exp Med. 2008;205:2419–2435. doi: 10.1084/jem.20072327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mace EM. Phosphoinositide-3-kinase signaling in human natural killer cells: new insights from primary immunodeficiency. Front Immunol. 2018;9:445. doi: 10.3389/fimmu.2018.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briercheck EL, Trotta R, Chen L, Hartlage AS, Cole JP, Cole TD, Mao C, Banerjee PP, Hsu HT, Mace EM. PTEN is a negative regulator of NK cell cytolytic function. J Immunol. 2015;194:1832–1840. doi: 10.4049/jimmunol.1401224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leong JW, Schneider SE, Sullivan RP, Parikh BA, Anthony BA, Singh A, Jewell BA, Schappe T, Wagner JA, Link DC. PTEN regulates natural killer cell trafficking in vivo. Proc Natl Acad Sci U S A. 2015;112:E700–E709. doi: 10.1073/pnas.1413886112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahmar Q, Keirsse J, Laoui D, Movahedi K, Van Overmeire E, Van Ginderachter JA. Tissue-resident versus monocyte-derived macrophages in the tumor microenvironment. Biochim Biophys Acta. 2016;1865:23–34. doi: 10.1016/j.bbcan.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 38.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 40.Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32:463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 41.Sahin E, Haubenwallner S, Kuttke M, Kollmann I, Halfmann A, Dohnal AM, Chen L, Cheng P, Hoesel B, Einwallner E. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J Immunol. 2014;193:1717–1727. doi: 10.4049/jimmunol.1302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 43.Rabold K, Netea MG, Adema GJ, Netea-Maier RT. Cellular metabolism of tumor-associated macrophages – functional impact and consequences. FEBS Lett. 2017;591:3022–3041. doi: 10.1002/1873-3468.12771. [DOI] [PubMed] [Google Scholar]
- 44.Arts RJ, Plantinga TS, Tuit S, Ulas T, Heinhuis B, Tesselaar M, Sloot Y, Adema GJ, Joosten LA, Smit JW. Transcriptional and metabolic reprogramming induce an inflammatory phenotype in non-medullary thyroid carcinoma-induced macrophages. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1229725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stienstra R, Netea-Maier RT, Riksen NP, Joosten LAB, Netea MG. Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab. 2017;26:142–156. doi: 10.1016/j.cmet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Netea-Maier RT, Smit JWA, Netea MG. Metabolic changes in tumor cells and tumor-associated macrophages: A mutual relationship. Cancer Lett. 2018;413:102–109. doi: 10.1016/j.canlet.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 48.Mantovani A. The growing diversity and spectrum of action of myeloid-derived suppressor cells. Eur J Immunol. 2010;40:3317–3320. doi: 10.1002/eji.201041170. [DOI] [PubMed] [Google Scholar]
- 49.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol. 2014;41:174–184. doi: 10.1053/j.seminoncol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L, Beckhove P, Sucker A. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res. 2015;21:5453–5459. doi: 10.1158/1078-0432.CCR-15-0676. [DOI] [PubMed] [Google Scholar]
- 53.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539:443–447. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis RJ, Moore EC, Clavijo PE, Friedman J, Cash H, Chen Z, Silvin C, Van Waes C, Allen C. Anti-PD-L1 efficacy can be enhanced by inhibition of myeloid-derived suppressor cells with a selective inhibitor of PI3Kdelta/gamma. Cancer Res. 2017;77:2607–2619. doi: 10.1158/0008-5472.CAN-16-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 56.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 57.Kuttke M, Sahin E, Pisoni J, Percig S, Vogel A, Kraemmer D, Hanzl L, Brunner JS, Paar H, Soukup K. Myeloid PTEN deficiency impairs tumor-immune surveillance via immune-checkpoint inhibition. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2016.1164918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murakami M, Tsubata T, Okamoto M, Shimizu A, Kumagai S, Imura H, Honjo T. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature. 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- 59.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5:403–408. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125:3413–3421. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 63.Buckler JL, Liu X, Turka LA. Regulation of T-cell responses by PTEN. Immunol Rev. 2008;224:239–248. doi: 10.1111/j.1600-065X.2008.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Juntilla MM, Koretzky GA. Critical roles of the PI3K/Akt signaling pathway in T cell development. Immunol Lett. 2008;116:104–110. doi: 10.1016/j.imlet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 66.Peter ME, Hadji A, Murmann AE, Brockway S, Putzbach W, Pattanayak A, Ceppi P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015;22:885–886. doi: 10.1038/cdd.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soond DR, Garcon F, Patton DT, Rolf J, Turner M, Scudamore C, Garden OA, Okkenhaug K. Pten loss in CD4 T cells enhances their helper function but does not lead to autoimmunity or lymphoma. J Immunol. 2012;188:5935–5943. doi: 10.4049/jimmunol.1102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 69.Egiziano G, Bernatsky S, Shah AA. Cancer and autoimmunity: Harnessing longitudinal cohorts to probe the link. Best Pract Res Clin Rheumatol. 2016;30:53–62. doi: 10.1016/j.berh.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Volkers N. Do autoimmune diseases raise the risk of cancer? J Natl Cancer Inst. 1999;91:1992–1993. doi: 10.1093/jnci/91.23.1992. [DOI] [PubMed] [Google Scholar]
- 71.Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev. 2017;16:1049–1057. doi: 10.1016/j.autrev.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 72.Kim HS, Jang SW, Lee W, Kim K, Sohn H, Hwang SS, Lee GR. PTEN drives Th17 cell differentiation by preventing IL-2 production. J Exp Med. 2017;214:3381–3398. doi: 10.1084/jem.20170523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sahin E, Brunner JS, Kral JB, Kuttke M, Hanzl L, Datler H, Paar H, Neuwinger N, Saferding V, Zinser E. Loss of phosphatase and tensin homolog in APCs impedes Th17-mediated autoimmune encephalomyelitis. J Immunol. 2015;195:2560–2570. doi: 10.4049/jimmunol.1402511. [DOI] [PubMed] [Google Scholar]
- 74.Bluml S, Sahin E, Saferding V, Goncalves-Alves E, Hainzl E, Niederreiter B, Hladik A, Lohmeyer T, Brunner JS, Bonelli M. Phosphatase and tensin homolog (PTEN) in antigen-presenting cells controls Th17-mediated autoimmune arthritis. Arthritis Res Ther. 2015;17:230. doi: 10.1186/s13075-015-0742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhances radiotherapy. J Exp Med. 2013;210:2435–2466. doi: 10.1084/jem.20130762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 2010;70:7800–7809. doi: 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- 77.Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, Wolchok JD, Mautino MR, Celis E, Sharpe AH, Francisco LM. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv. 2015;1 doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16:188–196. doi: 10.1038/ni.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bucheit AD, Chen G, Siroy A, Tetzlaff M, Broaddus R, Milton D, Fox P, Bassett R, Hwu P, Gershenwald JE. Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clin Cancer Res. 2014;20:5527–5536. doi: 10.1158/1078-0432.CCR-14-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Voron T, Marcheteau E, Pernot S, Colussi O, Tartour E, Taieb J, Terme M. Control of the immune response by pro-angiogenic factors. Front Oncol. 2014;4:70. doi: 10.3389/fonc.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]