Abstract
Cystic lung diseases are a group of disorders that appear similar on radiological studies on chest computed tomography. Each disorder is characterized by its own etiology, pathophysiology, course of progression and manifestation. Lymphangioleiomyomatosis (LAM) is one of the cystic lung diseases that can either be hereditary or sporadic. The sporadic form is a rare disease with no accurate prevalence reported but is believed to be less than 10 per million. LAM is associated with inappropriate activation of mammalian target of rapamycin (mTOR) signaling which regulates cellular growth. The sporadic form is almost confined to premenopausal female population and estrogen is believed to play an important role in the pathogenesis. Pregnancy and use of estrogen based oral contraceptives can aggravate symptoms of already existing LAM. Here we describe a case of LAM that was previously treated as asthma and was diagnosed after exacerbation of respiratory symptoms after pregnancy. We offer a review of the medical literature regarding the etiology, clinical course, diagnosis and treatment of LAM.
Keywords: LAM, Lymphangioleiomyomatosis, sLAM, Sporadic, tsLAM, Tuberous sclerosis
1. Introduction
Cystic lung diseases are a group of disorders that appear similar on radiological studies on chest computed tomography. Each disorder is characterized by its own etiology, pathophysiology, course of progression and manifestation. Lymphangioleiomyomatosis (LAM) is one of the cystic lung diseases that can either be hereditary or sporadic. The sporadic form is a rare disease with no accurate prevalence reported but is believed to be less than 10 per million. LAM is associated with inappropriate activation of mammalian target of rapamycin (mTOR) signaling which regulates cellular growth. The sporadic form is almost confined to premenopausal female population and estrogen is believed to play an important role in the pathogenesis. Pregnancy and use of estrogen based oral contraceptives can aggravate symptoms of already existing LAM. Here we describe a case of LAM that was previously treated as asthma and was diagnosed after exacerbation of respiratory symptoms after pregnancy. We offer a review of the medical literature regarding the etiology, clinical course, diagnosis and treatment of LAM.
2. Case
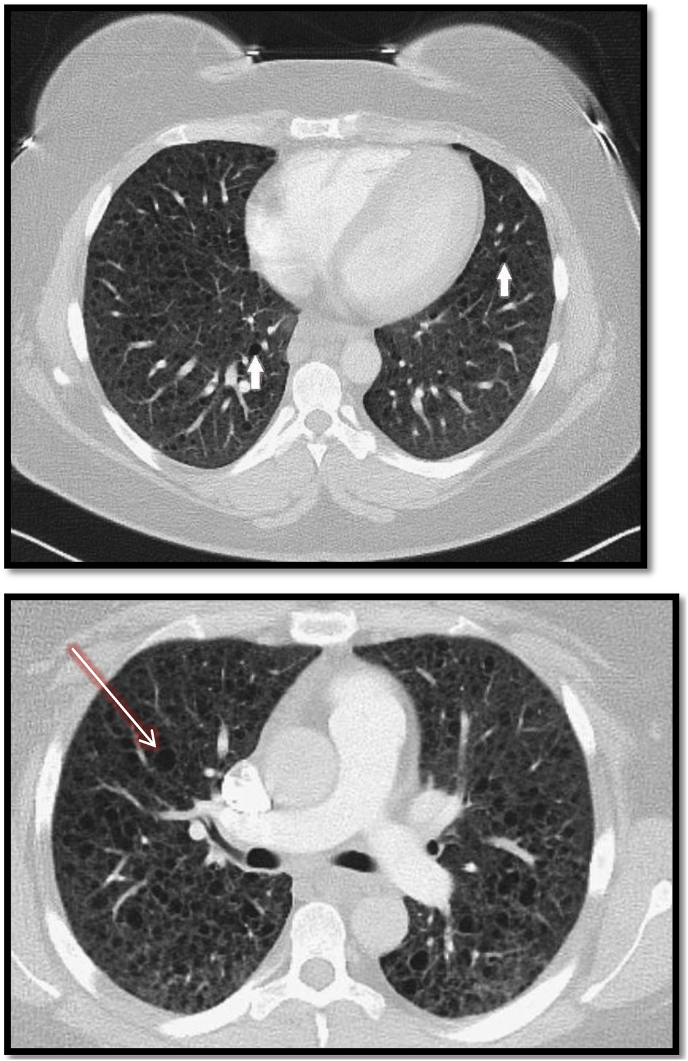
A 32 year old female G3P2A1 presented with the chief complaint of worsening dyspnea on exertion. Her history was significant for recent delivery three months prior and papillary thyroid carcinoma with partial thyroidectomy and radioactive iodine therapy (RAI). She had symptoms of intermittent dyspnea for one year which was diagnosed as mild intermittent asthma and was treated with bronchodilator inhalers as needed with good control of her dyspnea. However, she noticed worsening of symptoms postpartum after her third delivery without improvement with the use of inhalers, which prompted her to seek medical attention. She was on oral contraceptives (medroxyprogesterone intramuscular injection) after her last pregnancy. The symptoms were aggravated by minimal physical activity such as walking. She was a non-smoker with no prior history of lung disease or allergies other than her asthma. Her family history did not include any genetic or chronic lung diseases. Physical exam was normal except for mild wheezing which was prominent on the lower lung zones bilaterally. Initial chest x-ray was normal (Fig. 1). Pulmonary function tests showed obstructive pattern with FEV/FVC 64%, FEV1 86% of predicted values and DLCO of 42%. Six minute walking test showed requirements of 3 liters per minute of oxygen to maintain saturation above 88% and the patient was started on oxygen treatment. High Resolution Computed Tomography (HRCT) of the chest revealed numerous small cystic spaces bilaterally with diffuse distribution pattern (Fig. 2). There was mild septal thickening between the cyst spaces with no signs of pleural effusion, bronchiectatic or emphysematous changes. Laboratory work up was negative for rheumatoid factor, antinuclear antibody, anti-topoisomerase I, anti-citrulline antibodies. Vascular Endothelial Growth Factors-D was not available at our institution; however, serum levels of VEGF was 48pg/mL (Normal range is less than 96.2 pg/mL).
Fig. 1.
Chest X-Ray without significant findings. Normal lung architecture.
Fig. 2.
HCRT of the chest showing bilateral thin-walled numerous cysts (white arrows) which are scattered throughout the lungs with bleb formation (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
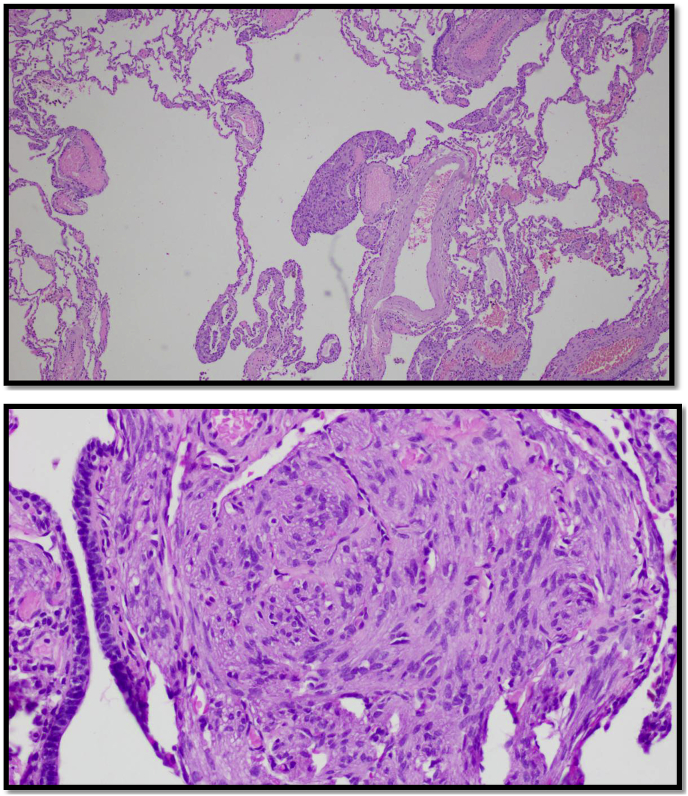
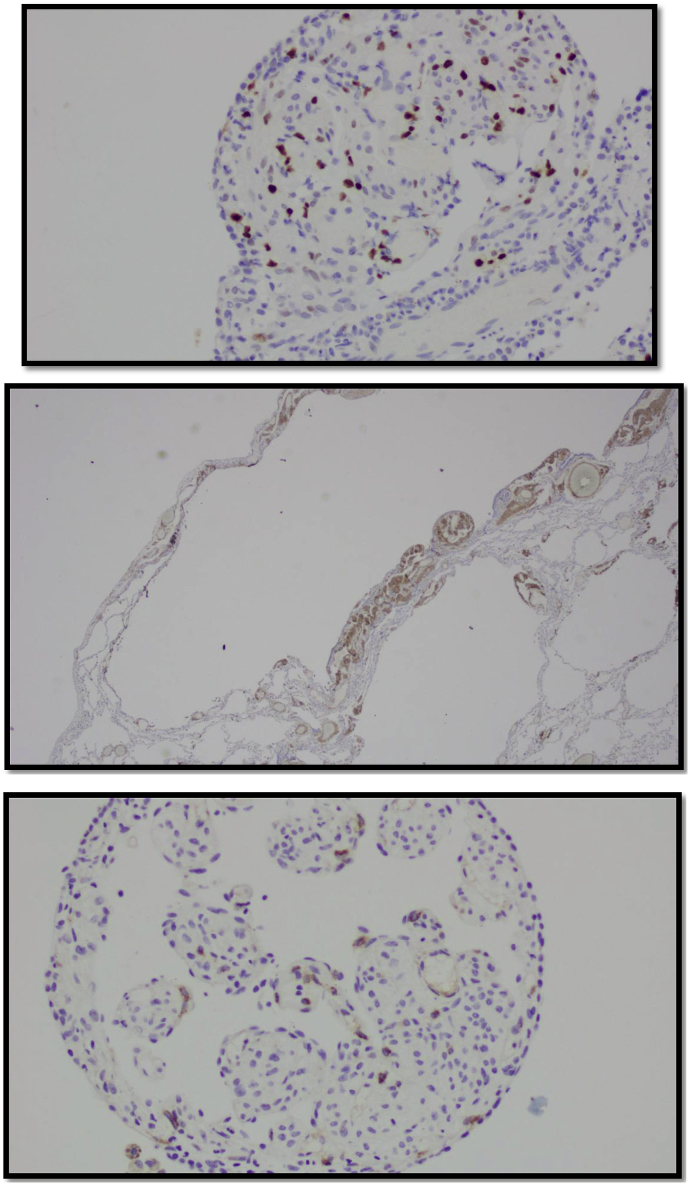
The patient underwent video assisted thoracoscopic surgery (VATS) with wedge resection for pathological studies. The specimens showed cyclically dilated air spaces with irregular proliferation of smooth muscle cells (Fig. 3). Immunostaining was positive for lesional smooth muscle cells, actin, desmin and focal positive estrogen and progesterone receptors with Ki-67 proliferation index were 5% (Fig. 4).
Fig. 3.
For H&E. H&E Stain of lung pathology showing plump of spindle-shaped lesional cells from nodules in walls of cystic air spaces. High Magnification of the previous specimen showing spindle-shaped myoid cells in the lung parenchymal wall.
Fig. 4.
For Immunohistochemical Staining. Lesional cells nuclear stain is focally positive for estrogen receptors. Immunohistochemical stain for smooth muscle myosin is positive in lesional cells. Positive immunohistochemically stain for HMB45.
Based on the radiological and histological findings the diagnosis was lymphangioleiomyomatosis. At that point we were able to order VEGF-D and the serum levels were 1038 pg/mL (Normal < 900 pg/mL). The patient was started on oral Sirolimus 2 mg daily which was titrated to achieve serum levels between 5-15 ng/mL and her Sirolimus trough levels ranged during first year of treatment between 4 and 14 ng/mL. The patient tolerated the medication well and the only adverse events were buccal aphthous and mild headache that resolved spontaneously. She also had pulmonary rehabilitation for three months. She was assured that she could continue her current oral contraceptives. Her repeated pulmonary function testing showed slight decline of DLCO on 6 months follow up that improved and stabilized later with subsequent measurements. Her respiratory symptoms improved although she was still requiring 3 L/m of oxygen during sleep and exertion. Patient received pneumococcal and annual influenza vaccination. She was advised to have annual ultrasounds of her kidneys.
3. Discussion
Cystic lung disease is a group of heterogeneous disease that differs in etiology but have common findings on CT scan.
LAM is a disease of abnormal proliferation, differentiation and migration of smooth muscle-like cells within the lung parenchyma that results in obstruction of small respiratory airway tract which produces a characteristic constellation of clinical, radiological and physiological findings [1]. LAM can be hereditary in which is associated with tuberous sclerosis or can occur sporadically which is the most common form of the disease. The sporadic form is predominantly found in premenopausal female population. The exact prevalence of sporadic Lymphangioleiomyomatosis (sLAM) is unknown but one study estimated the prevalence to range between 3 and 7 per million [2]. The hereditary form was shown to affect 1–3% of patients with tuberous sclerosis from a study that estimated the prevalence between 1939 and 1993 [3]. Another study found that the prevalence was much higher and could reach up to 80% in women with tuberous sclerosis and was higher in older women compared to younger female patients [4]. The hereditary form of LAM which is associated with TS was found to occur in males although females are still predominantly affected [5].
LAM was recently reclassified as a low-grade destructive locally invasive neoplasm after it was reported in multiple cases with a recurrence of the disease following total lung transplantation. The 2015 WHO classification of lung tumors considered LAM to be a mesenchymal tumor [6]. Moreover, the disease was proven to show evidence of clonal origin and potential ability to metastasize although the clinical significance of this remains negligible.
Pathophysiology in the hereditary and sporadic forms involve the loss of function of Tuberous Sclerosis Complex 2 (TSC2) gene which plays a role in inhibition of migration of cells and patients with tuberous sclerosis have a loss of the function of TSC2 which can lead to activation of the regulatory kinase mTOR (Mammalian Target of rapamycin) causing an increase in the levels of lymphangiogenic growth factor and a resultant abnormal growth of muscle like cells in the lung parenchyma that can lead to cyst formation and chylous effusions in the alveoli [7]. Kidneys could also be affected in 40–50% of sporadic LAM cases and present as angiomyolipoma which are vascular benign tumors. Another unique manifestation of the abnormal cell growth is perivascular epitheloid cell tumor (PEComa) which is a rare disease that affects the visceral organs. Moreover, estrogen plays a pivotal role of abnormal proliferation in sporadic LAM (sLAM). This is supported by predilection of the disease in premenopausal women, aggravation of symptoms with pregnancy and use of oral estrogen contraceptives. The mechanism is hypothesized to be due to the enhancement of genomic and non-genomic signaling pathways which was proven in vitro studies that showed estradiol to be able to cause enhancement of phosphorylation and activation of Erk1/2 pathway which leads to increased expression of c-myc and resultant expression of proliferative genes [8]. The role of estrogen has also been well established in the activation of mTOR pathway in different cell types like in the case of breast cancer [9].
Clinical manifestations are most commonly related to the effect of the disease on pulmonary parenchyma and rarely do patients present with symptoms of extra pulmonary involvement. Respiratory symptoms include dyspnea on exertion which is usually treated initially as asthma or COPD before LAM is diagnosed [10]. Chylous effusions are a common finding and are the result of lymphangiogenesis. Other symptoms that were less frequently reported include spontaneous pneumothorax, cough and fatigue [10,11].
Pulmonary function tests (PFTs) show an obstructive pattern and reduced diffusion capacity [12]. Hypoxemia during sleep is a common finding [13]. Chest X-Ray is normal in most patients with LAM unless there is a complication due to pleural effusions or pneumothorax. CT chest shows the hall mark of numerous thin-wall cysts that are diffuse and bilateral with a well-defined boarder [14]. Pulmonary nodules, hilar lymphadenopathy and pneumothorax were reported as well but are less common than cystic findings [15]. Some reports recommend abdominal CT in sporadic LAM to look for angiomyolipoma or lymphangiomyoma in the kidneys [16]. A cyst scoring system has been developed with HRCT that calculates the percentage of cyst volume to the total lung volume and was found to correlate with pulmonary function testing and can assess disease progression and severity [17].
Many reports found a correlation between vascular endothelial growth factor (VEGF-D) concentration in blood and the severity of the disease. VEGF-D is secreted by LAM cells and it acts on the endothelial vascular level causing angiogenesis and lymphangiogenesis [18] and levels of > 800 pg/mL in women with cystic lung disease on HRCT is sufficient for the diagnosis of LAM as they are very specific and can spare patients the need to have a biopsy [19]. One study done by Radzikowska E. et al. suggested that patients with sporadic sLAM and tuberous sclerosis TS/LAM with higher titers of VEGF-D had worse PFTs compared to patients who had low levels of VEGF-D which suggested that the serum levels correlate with disease severity and response to treatment [20]. Recently, CA-125 has been proposed as a marker that correlates with progression of the disease and responsiveness to treatment as high levels of this marker were associated with worsening PFTs [21].
Biopsy remains the gold standard for diagnosis of LAM. The biopsy can be done either through transbronchial or surgical approach. Surgical biopsy has a high yield but is associated with more complications. The American Thoracic Society and the Japanese Respiratory Society recommends transbronchial biopsy of the lung as a diagnostic tool given its good yield and reduced complications compared to surgical resection [22].
Histopathology shows multiple cysts and spindle shaped cells representing vascular smooth muscle cells that can be pleomorphic and has features of perivascular epithelial cells [23,24].
Immunohistochemistry can show co-expression of myogenic and melanocytic markers like HMB-45, HMSA-1 and a-smooth muscle actin which were positive in our patient [25]. Cytology with pleural fluid study can aid in diagnosis when effusion is present but the sensitivity is unknown, but some cases reports were diagnosed through cytology studies of pleural effusions [26].
In our patient there was no decline in DLCO with the continuous use of progesterone based contraceptives despite positive progesterone receptors seen in the tissue specimen. One retrospective study done by Taveira-DaSilva et al. showed that progesterone use did not alter PFTs in patients with LAM [27]. Although estrogen is more implicated in the pathogenicity and worsening of lymphangioleiomyomatosis, studies have shown that PR receptors are more expressed than ER in patients with LAM [[28], [29], [30]].
| At diagnosis | 6 months after treatment | 8 months after | 12 months after | |
|---|---|---|---|---|
| FEV1 | 86% | 88% | 88% | 90% |
| DLCO | 42% | 37% | 40% | 44% |
Treatment of LAM is aimed at controlling symptoms and supportive measures with smoking cessation, supplemental oxygen and bronchodilator therapy. The effectiveness of bronchodilator therapy has been debated. One study done by Taveira-DaSilva et al. found no difference in disease duration between patients who responded to bronchodilator therapy and those who did not [31], but in clinical practice bronchodilators are still being used for symptomatic relief.
Sirolimus is an mTOR inhibitor of inappropriate activation of mammalian target of rapamycin signaling pathway which is responsible for the proliferation of lymphangioleiomyomatosis cells and is the only FDA approved treatment for LAM. It has shown efficacy in the MILES trial in reducing symptoms, improving quality of life, stabilizing lung functions and decreasing levels of VGEF-D [32]. The MILES trial group recommends Sirolimus blood levels between 5-15 ng/mL for optimal efficacy and good control of LAM which is usually achieved by an oral dose of 2 mg daily. Another observational cohort study done on 15 patients with LAM in Japan assessed the efficacy of lower doses of Sirolimus with lower trough levels and found improvement of pulmonary function tests and resolution of chylous effusions with levels of the drug less than 5 ng/mL [33]. The dose is usually started at 2 mg and then titrated to achieve levels between 5-15 ng/mL. The medication is well tolerated and its adverse event profile is does dependent and less commonly seen with the lower doses that are used for the treatment of LAM. Given the potential of QT prolongation it is recommended to have a baseline ECG before initiating therapy and regularly during therapy. Monitoring also includes checking blood count, hepatic function and serum total cholesterol given that hypercholesterolemia is the most frequently side effect seen with treatment [34]. Also given the potential immunosuppressant effect of Sirolimus it is usually held if severe infection develops and prior to surgeries. No studies have been done to assess the safety of the medication in the long term and usually the strategy of treatment is to continue the medication at the lowest effective dose to achieve control of symptoms and stabilization of pulmonary function tests. Theoretically the concern with the use of Sirolimus is its immunosuppressive effect and the potential to increase the risk of infections and progression of latent malignancies if present and the only study to assess long term safety is an ongoing multicenter international trial (MIDAS Trial) that is expected to have results in 2020. However, one study found an increased risk of respiratory infection in patients with LAM treated with Sirolimus and concluded that there was no increased risk of respiratory infections with the medication and on the contrary that Sirolimus could have a protective effect [35]. In the same manner a new study suggested that mTOR inhibition has the potential to enhance immune function and decrease risk of infections in elderly population [36]. Everolimus is a drug of the same class that is used if there is an allergy to Sirolimus but does not have FDA approval. Pulmonary rehabilitation has shown to be beneficial to improve exercise capacity and quality of life with no effect on the PFTs [37].
Estrogen related therapies have been explored in some studies given the role of estrogen in the pathophysiology of the disease but have been of low yield [38].
Lung transplantation is indicated in the severe forms of LAM with severe respiratory functional decline despite medical therapy. There are no absolute contraindications for transplant and indications are yet to be established. It has been shown that lung transplantation improves lung function and quality of life [39]. Some anecdotal cases have been reported of recurrence of LAM after bilateral lung transplant although this recurrence did not seem to affect survival [40].
4. Conclusion
LAM is a rare cystic lung disease that can present as a part of tuberous sclerosis or sporadically. The sporadic form occurs almost exclusively in premenopausal women. There should be a high threshold of suspicion when premenopausal women present with asthma like symptoms that do not respond well to bronchodilators or when respiratory symptoms are aggravated after pregnancy or use of estrogen based oral contraceptives. Diagnosis is based on radiological findings, serum VGEF-D levels and pathology. Treatment is aimed at controlling and preventing progression of symptoms with Sirolimus. Current evidence together with reports of recurrence of LAM after transplantation suggests that LAM is a low-grade metastatic neoplasm that selectively targets the lungs.
Disclosure
The authors have nothing to disclose.
Contribution to the manuscript
Dr. Karam Khaddour contributed to production of the case report with the literature review discussion and collection of radiological imaging.
Dr. Wendy Ward contributed by providing pathology slides with description.
Dr. Maryna Shayuk, Dr. Dipesh Ludhwani, Dr. Sateesh Gowda contributed to review of the written article.
References
- 1.Kalassian K.G., Doyle R., Kao P., Ruoss S., Raffin T.A. Lymphangioleiomyomatosis: new insights. Am. J. Respir. Crit. Care Med. 1997;155:1183–1186. doi: 10.1164/ajrccm.155.4.9105053. [DOI] [PubMed] [Google Scholar]
- 2.Harknett E.C., Chang W.Y., Byrnes S., Johnson J. Use of Variability in national and regional data to estimate the prevalence of lymphangioleiomyomatosis. QJM. 2011;104:971–979. doi: 10.1093/qjmed/hcr116. [DOI] [PubMed] [Google Scholar]
- 3.Hancock E., Osborne J. Lymphangioleiomyomatosis: a review of the literature. Respir. Med. 2002;96:1–6. doi: 10.1053/rmed.2001.1207. [DOI] [PubMed] [Google Scholar]
- 4.Cudzilo C.J., Szczesniak R.D. Lymphangioleiomyomatosis screening in women with tuberous sclerosis. Chest. 2013;144:578–585. doi: 10.1378/chest.12-2813. [DOI] [PubMed] [Google Scholar]
- 5.Aubry M.C. Pulmonary lymphangioleiomyomatosis in a man. Am. J. Respir. Crit. Care Med. 2000;162:749–752. doi: 10.1164/ajrccm.162.2.9911006. [DOI] [PubMed] [Google Scholar]
- 6.Travis W.D. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015 Sep;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 7.Goncharova E.A., Goncharov D.A., Lim P.N., Noonan D., Krymskaya V.P. Modulation of cell migration and invasiveness by tumor suppressor TSC2 in lymphangioleiomyomatosis. Am. J. Respir. Cell Mol. Biol. 2006;34:473–480. doi: 10.1165/rcmb.2005-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J., Astrinidis A., Howard S., Henske E.P. Estradiol and Tamoxifen stimulate LAMassociated angiomyolipoma cell growth and activate both genomic and non genoming signaling pathways. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L694–L700. doi: 10.1152/ajplung.00204.2003. [DOI] [PubMed] [Google Scholar]
- 9.Yu J., Henske E.P. Estrogen-induced activation of mammalian target of rapamycin is mediated via tuberin and the small GTPase Ras homologue enriched in brain. Cancer Res. 2006 1;66:9461–9466. doi: 10.1158/0008-5472.CAN-06-1895. [DOI] [PubMed] [Google Scholar]
- 10.Ryu J.H., Moss J., Beck G.J., Lee J.C., Brown K.K. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am. J. Respir. Crit. Care Med. 2006 1;173:105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antón E., Casanova A., Xaubet A., Román A. Lymphangioleiomyomatosis a study of 72 patients from the Spanish registry. Sarcoidosis Vasc. Diffuse Lung Dis. 2009;26:85–91. [PubMed] [Google Scholar]
- 12.Baldi B.G., Freitas C.S., Araujo M.S., Dias O.M. Clinical course and characterisation of lymphangioleiomyomatosis in a Brazilian reference center. Sarcoidosis Vasc. Diffuse Lung Dis. 2014 8;31:129–135. [PubMed] [Google Scholar]
- 13.Medeiros P., Jr., Lorenzi-Filho G., Pimenta S.P. Sleep desaturation and its relationship to lung function, exercise and quality of life in LAM. Respir. Med. 2012;106:420–428. doi: 10.1016/j.rmed.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Chu S.C., Horiba K., Usuki J., Avila N.A. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115:1041–1052. doi: 10.1378/chest.115.4.1041. [DOI] [PubMed] [Google Scholar]
- 15.Tobino K., Johkoh T., Fujimoto K., Sakai F. Computed tomographic features of lymphangioleiomyomatosis: evaluation in 138 patients. Eur. J. Radiol. 2015;84:534–541. doi: 10.1016/j.ejrad.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Avila N.A., Kelly J.A., Chu S.C., Dwyer A.J., Moss J. Lymphangioleiomyomatosis. Abdominopelvic CT and US findings. Radiology. 2000;216:147–153. doi: 10.1148/radiology.216.1.r00jl42147. [DOI] [PubMed] [Google Scholar]
- 17.Schmithorst V.J. Automated algorithm for quantifying the extent of cystic change on volumetric chest CT: initial results in Lymphangioleiomyomatosis. AJR Am. J. Roentgenol. 2009;192:1037–1044. doi: 10.2214/AJR.07.3334. [DOI] [PubMed] [Google Scholar]
- 18.Seyama K. Vascular endothelial growth factor-D is increased in serum of patients with lymphangioleiomyomatosis. Lymphat. Res. Biol. 2006;4:143–152. doi: 10.1089/lrb.2006.4.143. [DOI] [PubMed] [Google Scholar]
- 19.Young L.R., Vandyke R. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138:674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radzikowska E., Jaguś P., Sobiecka M., Chorostowska-Wynimko J. Correlation of serum vascular endothelial growth factor-D concentration with clinical presentation and course of lymphangioleiomyomatosis. Respir. Med. 2015;109:1469–1475. doi: 10.1016/j.rmed.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Glasgow C.G., Pacheco-Rodriguez G. CA-125 in disease progression and treatment of lymphangioleiomyomatosis. Chest. 2018;153:339–348. doi: 10.1016/j.chest.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta N., Finlay G.A., Kotloff R.M., Strange C. Lymphangioleiomyomatosis diagnosis and management: high resolution chest CT, transbronchial lung biopsy, and pleural disease management. An official American Thoracic Society/Japanese Respiratory Society clinical practice guidelines. Am. J. Respir. Crit. Care Med. 2017 15;196:1337–1348. doi: 10.1164/rccm.201709-1965ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitaichi M., Nishimura K., Itoh H., Izumi T. Pulmonary lymphangioleiomyomatosis: a report of 46 patients including a clinicopathologic study of prognostic factors. Am. J. Respir. Crit. Care Med. 1995;151:527–533. doi: 10.1164/ajrccm.151.2.7842216. [DOI] [PubMed] [Google Scholar]
- 24.McCormack F.X., Travis W.D., Colby T.V., Henske E.P., Moss J. Lymphangioleiomyomatosis: calling it what it is: a low-grade, destructive, metastasizing neoplasm. Am. J. Respir. Crit. Care Med. 2012 15;186:1210–1212. doi: 10.1164/rccm.201205-0848OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martignoni G., Pea M., Reghellin D. Molecular pathology of lymphangioleiomyomatosis and other perivascular epithelioid cell tumors. Arch. Pathol. Lab Med. 2010;134:33–40. doi: 10.5858/2008-0542-RAR1.1. [DOI] [PubMed] [Google Scholar]
- 26.Rivera G., Gokaslan T., Kurian E.M. Lymphangioleiomyomatosis diagnosed by effusion cytology: a case report. J. Cytol. 2015;32:287–289. doi: 10.4103/0970-9371.171256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taveira-DaSilva A.M. Decline in lung function in patients with lymphangioleiomyomatosis treated with or without progesterone. Chest. 2004;126:1867–1874. doi: 10.1378/chest.126.6.1867. [DOI] [PubMed] [Google Scholar]
- 28.Yano S. Exacerbation of pulmonary lymphangioleiomyomatosis by exogenous oestrogen used for infertility treatment. Thorax. 2002;57:1085–1086. doi: 10.1136/thorax.57.12.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberstein E.M. Pulmonary lymphangioleiomyomatosis (LAM): examining oral contraceptive pills and the onset of disease. J. Womens Health (Larchmt) 2003;12:81–85. doi: 10.1089/154099903321154176. [DOI] [PubMed] [Google Scholar]
- 30.Gao L. In pulmonary lymphangioleiomyomatosis expression of progesterone receptor is frequently higher than that of estrogen receptor. Virchows Arch. 2014;464:495–503. doi: 10.1007/s00428-014-1559-9. [DOI] [PubMed] [Google Scholar]
- 31.Taveira-DaSilva Angelo M. Reversible airflow obstruction in lymphangioleiomyomatosis. Chest. 2010;137:744. doi: 10.1378/chest.09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCormack F.X., Inoue Y., Moss J. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 2011;28:1595–1606. doi: 10.1056/NEJMoa1100391. 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ando K. Efficacy and safety of low-dose sirolimus for treatment of lymphangioleiomyomatosis. Respir. Investig. 2013;51:175–183. doi: 10.1016/j.resinv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Yao J., Taveira-DaSilva A.M. Sustained effects of sirolimus on lung function and cystic lung lesions in lymphangioleiomyomatosis. Am. J. Respir. Crit. Care Med. 2014 1;190:1273–1282. doi: 10.1164/rccm.201405-0918OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Courtwright A.M., Goldberg H.J., Henske E.P., El-Chemaly S. The effect of mTOR inhibitors on respiratory infections in lymphangioleiomyomatosis. Eur. Respir. Rev. 2017 17:26. doi: 10.1183/16000617.0004-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannick Joan B., Morris Melody. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 2018;10(449) doi: 10.1126/scitranslmed.aaq1564. eaaq1564. [DOI] [PubMed] [Google Scholar]
- 37.Araujo M.S., Baldi B.G., Freitas C.S. Pulmonary rehabilitation in lymphangioleiomyomatosis: a controlled clinical trial. Eur. Respir. J. 2016;47:1452–1460. doi: 10.1183/13993003.01683-2015. [DOI] [PubMed] [Google Scholar]
- 38.Lu C., Lee H.S., Pappas G.P. A phase II clinical trial of an aromatase inhibitor for postmenopausal women with lymphangioleiomyomatosis. Ann. Am. Thorac. Soc. 2017;14:919–928. doi: 10.1513/AnnalsATS.201610-824OC. [DOI] [PubMed] [Google Scholar]
- 39.Maurer J.R. Lung transplantation in the management of patients with lymphangioleiomyomatosis: baseline data from the NHLBI LAM Registry. J. Heart Lung Transplant. 2007;26:1293–1299. doi: 10.1016/j.healun.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zaki Khawaja S., Aryan Zahra, Mehta Atul C., Akindipe Olufemi, Budev Marie. Recurrence of lymphangioleiomyomatosis: nine years after a bilateral lung transplantation. World J. Transplant. 2016 24;6:249–254. doi: 10.5500/wjt.v6.i1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]