Abstract
Background:
Patients with Duchenne muscular dystrophy (DMD) are at high risk of endocrine and bone health complications resulting from the high glucocorticoid (GC) doses used to treat this condition. There are limited data characterizing the clinical management of these complications.
Objective:
To determine the frequency of bone health screening, endocrinologist evaluation, and use of endocrine and bone health pharmacotherapy in the clinical care of males with DMD.
Methods:
A population based cohort study using data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet) was conducted. Clinical data was abstracted from the medical records of 683 males with DMD at five surveillance sites across the US.
Results:
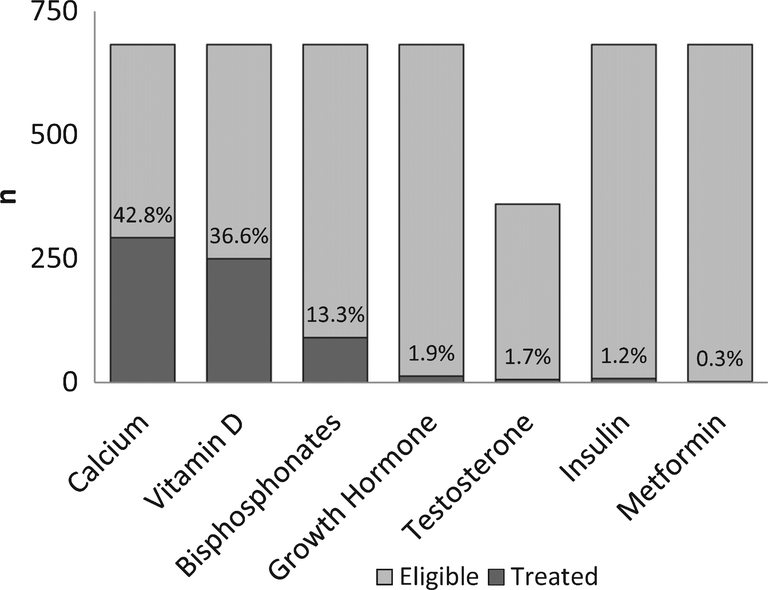
A DXA scan had been documented in 24% of cases; the percentage of cases with DXA varied across surveillance sites from 13% to 43%, p < 0.001. History of fracture and greater disease duration were associated with greater odds of having a DXA. Only 4.7% of cases had documentation of an endocrinologist evaluation. The frequency of documented endocrine and bone health pharmacotherapy use included calcium (42.8%), vitamin D (36.6%), bisphosphonates (13.3%), growth hormone (1.9%), testosterone (1.7%), insulin (1.2%), and metformin (0.3%)
Conclusions:
A low percentage of DMD males had record of DXA scan, endocrinologist evaluation, or treatment with endocrine or bone health pharmacotherapy. Endocrine and bone health care may represent an unmet need in the DMD population.
Keywords: Muscular dystrophy, duchenne, osteoporosis, glucocorticoids, bisphosphonates, hypogonadism
INTRODUCTION
Duchenne muscular dystrophy (DMD; OMIM 310200) is a progressive, incurable neuromuscular disease resulting from loss-of-function mutations in the dystrophin gene. It is the most common form of pediatric muscular dystrophy, with an estimated prevalence of 1.4 per 10,000 US males aged 5–24 years [1]. DMDhas an X-linked recessive inheritance pattern; therefore the majority of severely affected individuals are male. Patients typically present early in childhood with signs of muscle weakness including delayed motor milestones, abnormal gait, and difficulty rising to stand [2]. Later complications include scoliosis, cardiomyopathy, respiratory insufficiency, and limb contractures [3]. If left untreated, loss of ambulation occurs by age 12 years, on average, and survival beyond 20 years of age without ventilatory support is uncommon [4].
Over the past two decades, the use of high dose glucocorticoid (GC) therapy to treat DMDhas gained widespread acceptance and is now recommended as the standard of care [5]. GC therapy has been shown to improve muscle function, prolong ambulation, reduce scoliosis, and delay progression of cardiac and respiratory disease [6]. Chronic GC treatment is not without risks, however. The endocrine system is adversely affected by GC exposure, and patients with DMD who are treated with GC therapy are at high risk of endocrine complications including osteoporosis [7], fracture [8], growth failure [9], hypogonadism [10], obesity and related complications including glucose intolerance and diabetes [11], and secondary adrenal insufficiency.
In 2010 a working group for the US Centers for Disease Control and Prevention published the first “Care Considerations for DMD” designed to aid practitioners in the clinical management DMD [5, 12], which were subsequently updated in 2018 [13–15]. The 2010 recommendations provided a framework for the screening and management of bone health and endocrine complications of DMD, but acknowledged the lack of evidence-based research in this area. Key recommendations relating to bone health included: assessment of bone mineral density by dual energy X-ray absorptiometry (DXA) starting at diagnosis and/or initiation of GC therapy; supplementation with calcium and/or vitamin D in deficient patients; and consideration of bisphosphonates for patients with vertebral compression fractures. The Care Considerations also recommended routine assessment of height, weight, and nutritional status but provided no specific recommendations regarding the further assessment or treatment of patients with complications such as obesity, short stature, or pubertal delay.
Despite the potentially negative effect of endocrine complications on the health and quality of life of patients living with DMD, relatively little has been reported on the evaluation and treatment of these conditions in clinical practice. The primary objectives of this study were to characterize the use of DXA, endocrinologist referral, and bone health and endocrine pharmacotherapies in males with DMD followed by the multi-site Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). The MD STARnet project was designed to collect health information directly from medical record abstraction on all patients with childhood-onset dystrophinopathies living in specific regions of the US, and is therefore well-suited for studies describing the utilization of clinical screening tests and therapies [16].
METHODS
Data source
MD STARnet, funded by the US Centers for Disease Control and Prevention, is the largest population-based surveillance program of cases with childhood-onset dystrophinopathies in the US. MD STARnet includes data on cases born after January 1, 1982, diagnosed before age 21 years, and resided in Arizona (AZ), Colorado (CO), Georgia (GA), Hawaii (HI), Iowa (IA), and a 12 county region of Western New York (WNY). Data were abstracted from medical records by trained abstractors [17]. Cases identified as having a dystrophinopathy were classified by a systematic clinician review process as “definite”, “probable”, “possible”, “affected female” or “asymptomatic” [18]. A classification of “definite” required clinical symptoms compatible with a dystrophinopathy, increased CK, an X-linked family history and confirmation of dystrophin mutation or abnormal dystrophin in the individual or an affected family member; classification as “probable” required clinical symptoms compatible with a dystrophinopathy, increased CK and an X-linked family history. Cases were further classified into DMD and Becker muscular dystrophy phenotypes using criteria including age at loss of ambulation, steroid use, and genetic testing results. Data collection in AZ, CO, IA, and WNY started in 2004, followed by GA in 2005, and HI in 2008. Data from 1982 to each site’s start date were collected retrospectively; data after the start date were collected annually through December of 2011. Participants were followed until death, out-migration, or end of data collection period. Surveillance data collection was approved at all sites under public health surveillance regulations (CO, IA, GA, WNY) or institutional review board (AZ, HI).
Study sample
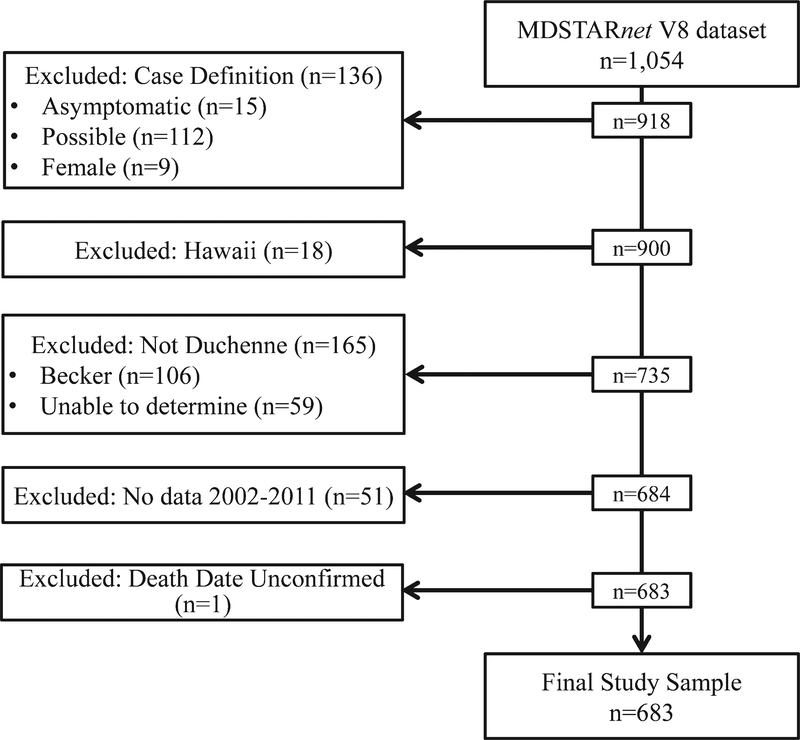
The sample selection for this study is illustrated in Fig. 1. Inclusion criteria included case classification as “definite” or “probable” and DMD phenotype. Exclusion criteria included female sex, and case classification as “possible” or “asymptomatic”. Cases from HI were excluded based upon incomplete follow-up. Because of low frequencies of outcomes prior to 2002, analyses were restricted to cases with clinic visits from January 1, 2002 to December 31, 2011.
Fig. 1.
Study flow chart. Flow diagram illustrating the criteria applied to derive the evaluable cohort.
Study variables
Documentation of a DXA scan in the medical record during the study period was the primary outcome used to assess bone health screening; documentation of a spine radiograph was secondarily assessed. The use of endocrine and bone health pharmacotherapies during the study period was ascertained based upon presence of a prescription or other documentation of medication use in the medical record. The following pharmacotherapies were evaluated: calcium, vitamin D, bisphosphonates, growth hormone, testosterone, insulin, and metformin. Evaluation by an endocrinologist was determined by documentation of an endocrinologist visit in the medical record. Other variables of interest included demographics (age at first and last clinic visit, racial/ethnic group), disease duration (time since diagnosis), health insurance, anthropometrics [height, weight, body mass index (BMI)], fracture, spine radiograph, and surveillance site. Diagnosis age was calculated as the first occurrence of the following events, in descending order of priority: abnormal creatinine kinase, abnormal DNA results, abnormal muscle biopsy, signs and symptoms noted by a clinician. GC exposure was defined as a cumulative use of GCs for≥six months prior to or during the study period. Clinically, many boys with DMD are seen on a twice yearly basis, therefore six months was felt to be the shortest reliable measure of GC exposure. This definition has also been used to determine steroid exposure in recent clinical trials [19] and other clinical studies within the MD STARnet cohort [20]. Variables of interest were assessed at time of first occurrence of outcome (DXA, pharmacotherapy, etc) or at time of most recent clinic visit within the study period (in cases with no documentation of outcome) unless otherwise specified. Loss of ambulation was defined at the first mention of full time wheel chair usage or cessation of mobility. Deaths during the study period were confirmed using vital records data. A case was considered lost to follow-up if the last known visit occurred more than two years prior to December 31, 2011 (among living) or if the last known visit occurred more than two years prior to death date (if deceased).
Statistical analysis
Standard descriptive statistics were used to characterize cases and report frequencies. Distributions of variables were assessed prior to analyses. For continuous variables, means were compared using Satterthwaite t-tests; for categorical variables, proportions were assessed using Pearson chi-squared test; and for ordinal variables, trends were assessed using the Cochran-Armitage trend test. Primary analyses included data from the full study period (2002–2011); secondary analyses were performed using annual data to assess for trends over time. To be considered eligible (i.e. to be listed in the denominator) for the annual analyses, cases were required to have documentation of at least one clinic visit in the given calendar year. Multiple logistic regression was used to identify clinical factors associated with presence of one or more DXA scans in the medical record during the study period. Variables of interest with bivariate association at p < 0.10 were included as candidates for the multiple logistic regression model. Backwards variable selection was then applied, subject to the same p-value threshold. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Clinical characteristics
A total of 683 cases from five surveillance sites were included in this study. Case characteristics are shown in Table 1. There were 107 deaths during the study period and 109 cases were lost to follow-up. The mean (±SD) age at diagnosis was 4.5±2.9 years, the mean age at first and last clinic visit during study period was 8.6±5.1 and 14.4±6 years, respectively. The majority of cases (73.3%) were ambulatory at first visit; 40.4% lost ambulation over the study period. A total of 161 cases (23.6%) had been exposed to cumulative GC therapy for ≥6 months. A total of 455 fractures were documented in 251 cases (36.7%). The first documented fracture was at the femur in 33.5% and spine in 4.4% of cases, respectively; the full distribution of fracture site is provided in Supplemental Table 1. History of fracture was more common in GC exposed individuals (50.3%) compared to those with no GC exposure (33%), p < 0.0001. Age at first visit and prevalence of GC exposed cases did not differ across surveillance sites; however differences were seen in age at last visit, racial distribution, ambulatory status, and fracture history (Supplemental Table 2).
Table 1.
Characteristics of boys with Duchenne muscular dystrophy from MD STARnet 2002–2011
| Eligible Sample, n | 683 |
|---|---|
| Age at first clinic visit, y | 8.6 ± 5.1 |
| Age at last clinic visit, y | 14.4 ± 6 |
| Age at diagnosis, y | 4.5 ± 2.9 |
| Racial Group | |
| White, n (%) | 397 (58.1) |
| Hispanic, n (%) | 158 (23.3) |
| Black, n (%) | 47 (6.9) |
| Other/multiple, n (%) | 37 (5.4) |
| BMI Z-score1,2,3 | 0.3 ± 2.7 |
| Height Z-score2 | −1 ± 1.6 |
| Weight Z-score2 | –0.3 ± 1.8 |
| Ambulatory1,4, n (%) | 459 (73.3) |
| Loss of ambulation5, n (%) | 185 (40.4) |
| Glucocorticoid exposure6, n (%) | 161 (23.6) |
| Fracture6, n (%) | 251 (36.7) |
| Duration of clinical data in study period, y | 6.2 ± 3.2 |
| Death during study period, n (%) | 107 (15.7) |
At first clinic visit during study period.
n = 608.
Determined using US reference data [39].
n = 675.
Percentage of cases who were ambulatory at first visit and lost ambulation over study period.
During or prior to study period.
DXA and spine radiograph utilization
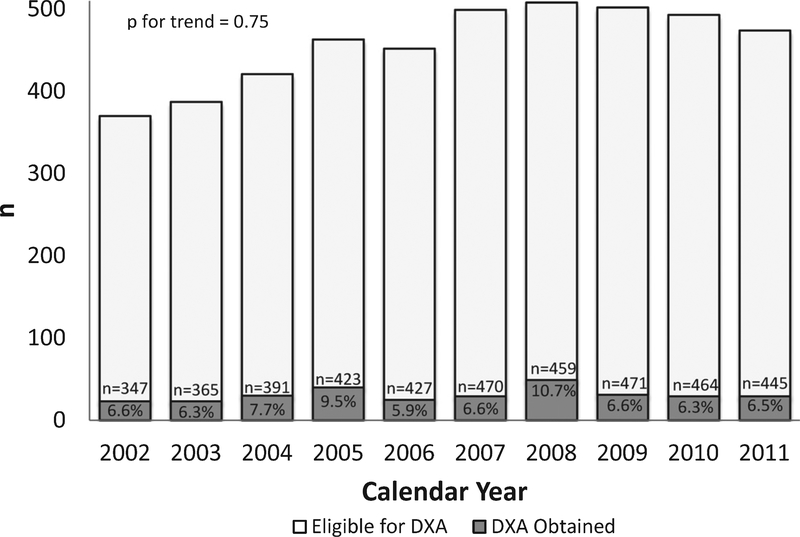
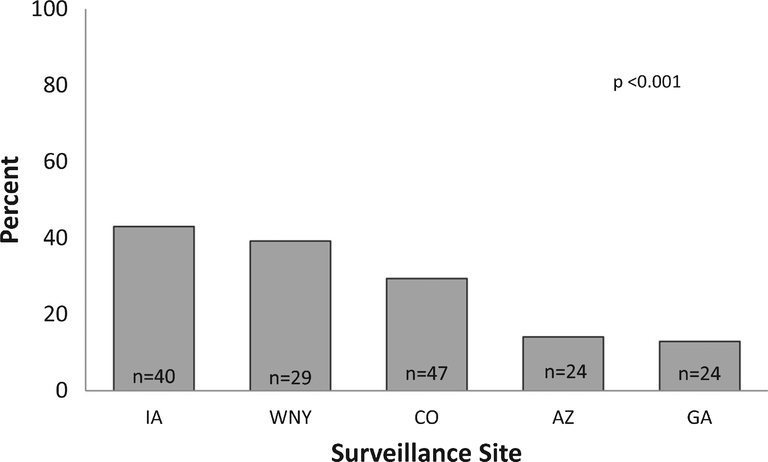
A total of 343 DXA scans were documented in 164 cases (24% of the cohort; 25% of the cohort ≥ five years of age). The number of DXA scans per case ranged from 1 to 8 (mean 2.1); only one case had a DXA scan documented in each year in which they were eligible. The percentage of cases with a documented DXA scan in a given year ranged from a minimum of 5.9% in 2006 to a maximum of 10.7% in 2008 (Fig. 2). There was no evidence of a trend toward increasing or decreasing DXA utilization over the study period (p =0.75). The percentage of cases with a documented DXA varied by surveillance site, ranging from 12.9% to 43%, p < 0.001 (Fig. 3).
Fig. 2.
Annual frequency of DXA utilization in boys with Duchenne muscular dystrophy from MD STARnet 2002–2011. The percentage of cases with DXA ranged from 5.9% to 10.7%, with no evidence of a trend over the study period. Total number (n) of cases eligible for a DXA in a given year based upon documentation of least one clinic visit in the given calendar year.
Fig. 3.
Frequency of DXA utilization by surveillance site in boys with Duchenne muscular dystrophy from MD STARnet 2002–2011. The percentage of cases who received one or more DXA scans across sites during the study period ranged from 12.9% to 43%, p < 0.001. Total number (n) of cases with DXA is shown in bars.
At time of first documented DXA scan, cases had a mean age of 11.6±3.9 years, and were 69.5% white, 3.1% black, and 12.8% Hispanic/Latino; 31.1% were GC exposed, and 40.8% had a history of fracture. Compared to individuals with no documentation of DXA, those with≥one documented DXA scan had greater disease duration (12.2±5 vs 9.2±6.1 years, p < 0.01), were more likely to have fractured (60.4% vs 29.3%, p < 0.0001), been exposed to GCs (35.4% vs 19.8%, p < 0.0001) and were more likely to be white (69.5% vs 54.5%, p < 0.001). Given the potential for variability in demographics and medical practice by surveillance site, multiple logistic regression analysis was performed to identify characteristics of cases with at least one documented DXA scan vs those with no documentation of DXA, after adjustment by variables of interest including surveillance site (Table 2). Documentation of DXA was associated with greater odds of fracture [OR 2.76 (95% CI:1.83–4.17)] and disease duration [1.05 (1.01–1.09)] and decreased odds of being of Hispanic/Latino racial group compared to white [0.46 (0.26–0.8)]. There was a trend toward greater odds for GC exposure [1.46 (0.93–2.28)], and lesser odds for black racial group [0.41(0.13–1.05)] in cases with a documented DXA.
Table 2.
Adjusted odds ratios from multiple logistic regression analysis to identify variables associated with presence of DXA scan in boys with Duchenne muscular dystrophy from MD STARnet 2002–2011
| Variables of Interest | Adjusted OR (95% CI) | p |
|---|---|---|
| Fracture | 2.76 (1.83–4.16) | <0.001 |
| Glucocorticoid exposure1 | 1.46 (0.93–2.28) | 0.10 |
| Disease duration2, y | 1.05 (1.01–1.09) | 0.01 |
| Diagnosis age, y | 0.97 (0.9–1.04) | 0.39 |
| Race | ||
| White | Ref | |
| Black | 0.41 (0.13–1.05) | 0.09 |
| Hispanic/Latino | 0.46 (0.26–0.8) | <0.01 |
| Other/unknown | 1.34 (0.74–2.42) | 0.33 |
| Surveillance Site | ||
| GA | Ref | |
| AZ | 1 (0.52–1.93) | 0.99 |
| CO | 2.35 (1.31–4.3) | <0.01 |
| IA | 3.9 (2.08–7.45) | <0.001 |
| WNY | 2.96 (1.51–5.84) | <0.01 |
Cumulative use for≥six months prior to or during the study period.
At time of last study visit – (date of last visit - date of diagnosis). Abbreviations: AZ, Arizona; CO, Colorado; GA, Georgia, IA, Iowa, WNY; Western New York.
A total of 1221 spine radiographs were documented in 371 (54.3%) cases. The stated indication was “scoliosis” for the majority (95.4%) of radiographs, with an indication of “fracture” listed for 2.1% of radiographs. Mean age at first spine radiograph did not differ from that of first DXA (12±3.4 vs 12.2±3.4 years, p = 0.69). Cases with documentation of≥one DXA were more likely to have had a spine radiograph (71.9% vs 48.7%, p < 0.01) and the indication for spine radiograph was more likely to be “fracture” (3.5% vs 1.3%, p = 0.01).
Endocrinologist evaluation
A total of 32 cases (4.7% of the cohort, 5.6% of GC exposed cohort) had documentation of an endocrinologist evaluation. The percentage of cases with an endocrinologist evaluation ranged from 2.4% to 9.7% across surveillance sites (p = 0.08). Mean age at time of first endocrine visit was 14.6±4.4 years; 28% had a history of GC exposure and 66% had a history of fracture. Mean height, weight, and BMI Z-scores were –1.89±2.1, –0.25±2.16, and 1±1.34, respectively. Cases with a documented endocrinology evaluation had lower height Z-score (–2.3±2.3 vs –1.1±1.8, p < 0.01), were more likely to have a history of fracture (65% vs 25%, p < 0.001) and were more likely to be white (81% vs 57%) compared to those with no documentation of an endocrinology evaluation.
By comparison, 319 cases (46.7% of the cohort) had documentation of an orthopedic surgery evaluation. The mean age at time of first documented orthopedic visit was 11.7±4.9 years; cases with documentation of orthopedic visit had greater disease duration (11.1±5.2 vs 9±6.4 years, p < 0.001), were more likely to have fractured (46.1% vs 28.6%, p < 0.001) and been exposed to GCs (29.8% vs 18.1%, P < 0.01) compared those with no documentation of orthopedic visit.
Bone health and endocrine pharmacotherapy
The frequency of documented bone health and endocrine pharmacotherapy use is shown in Fig. 4. Calcium and vitamin D were the most common bone health medications reported in the medical record, documented for 42.8% and 36.6% of cases, respectively. Bisphosphonate use was documented in 13.3% of cases. The frequency of bisphosphonate use varied significantly across surveillance sites, ranging from 9.9% to 34.1%, p < 0.0001. The mean age at time of first bisphosphonate was 13.3±3.4 years; 75.8% had a history of fracture and 38.5% had been exposed to GCs. Calcium and vitamin D use was documented in 74.7% and 80.2% of participants treated with bisphosphonates, respectively. Those with documentation of bisphosphonate therapy had greater disease duration (13.7±4.2 vs 9.4±6, p < 0.0001), lower height Z-score (–1.8±2 vs –1±1.8, p < 0.001) and were more likely to have fractured (75.8% vs 30.7%, p < 0.0001), been exposed to GCs (38.5% vs 21.3%, p < 0.001), and be of white racial group [76.2% vs 1.1 % (black) and 13.2% (Hispanic), p < 0.001]. The documented use of other endocrine pharmacotherapy was low, including growth hormone (1.9%), testosterone (1.7% of males aged 14 or older), insulin (1.2%) and metformin (0.3%). Sensitivity analyses limited to GC exposed cases found the frequency of pharmacotherapy use to be: calcium (54.8%) vitamin D (44.1%), bisphosphonates (21.7%), growth hormone (0%), testosterone (1.2%), insulin (1.9%), and metformin (0.6%).
Fig. 4.
Frequency of endocrine and bone health pharmacotherapy in boys with Duchenne muscular dystrophy from MD STARnet 2002–2011. All cases counted as eligible (n = 683) for all medications except testosterone, where only males older than 14 years were counted in the denominator (n = 360). No males younger than 14 years had record of testosterone treatment.
DISCUSSION
We used the multi-site MD STARnet surveillance database to describe the utilization of endocrine and bone health screening and pharmacotherapies in the clinical care of males with DMD. Despite the known complications of DMD and GC use on the skeletal and endocrine systems [21, 22]; we found that only 1/4 of cases had documentation of DXA bone density screening and less than 5% of cases had documentation of an endocrinologist evaluation during the period studied. With the exception of calcium and vitamin D, the documented use of endocrine or bone health pharmacotherapies was very low.
The negative effects of DMD and its treatment with GC on the skeleton including low bone density and increased fracture risk have been widely reported [23–26]. Fractures are an important complication in this population as they adversely affect quality of life, may hasten loss of ambulation [27], and in rare cases can be associated with the life-threatening fat-emboli syndrome [28]. Early consensus statements that recommended the use of DXA to monitor bone health in DMD [5] have been recently updated and now include a recommendation for regular lateral thoracolumbar spine radiographs to assess for vertebral fracture [14]; however there remains uncertainty as to which measure or skeletal site provides the best fracture prediction in this population [29]. There is growing evidence to support the use of bisphosphonates in the DMD population [30, 31], however high quality data from clinical trials is lacking [32].
Our study found evidence of significant variability in DXA and bisphosphonate utilization across surveillance sites. Access to specialists with expertise in the treatment of pediatric osteoporosis may limit availability of DXA in children and there are additional practical limitations that may complicate the use of DXA in the DMD population. DXA interpretation in children is not straightforward, and requires adjustment for short stature and pubertal delay, conditions that are common in GC treated boys with DMD [33]. Additionally, physical limitations that limit the ability to tolerate supine positioning makes obtaining a DXA difficult in many patients with DMD. We also uncovered evidence of racial disparity in DXA utilization; further studies will be needed to identify barriers to DXA use so that adequate access to bone health screening can be provided to all patients. Approximately 22% of GC-exposed cases in our cohort had documentation of bisphosphonate use. This compares to a single center study from Canada which reported that 36% of boys with DMD on GCs had been treated with a bisphosphonate [34]. Other reports from retrospective, single-center analyses confirm the inter-institutional variability in bisphosphonate use in terms of both choice of agent (oral vs intravenous) and indication (primary vs secondary prevention) for patients with DMD [30, 31, 35].
There is limited literature describing the use of endocrine therapies to treat short stature, hypogonadism, or obesity in DMD. Growth [36] and growth hormone secretion [37] have been shown to be impaired in DMD even in the absence of GCs; however concerns about the potential negative effects of rapid growth and/or tall stature on muscle function may limit the use of growth hormone [38]. A single-center retrospective study concluded that growth hormone treatment improved short-term growth velocity without detriment to muscle function [39]. A second small study reported that growth hormone was well tolerated in a trial to assess impact on cardiac function, growth outcomes were not reported [40]. Further data on the use of growth hormone in DMD are not available. Hypogonadism in DMD is most likely attributable to GC exposure [13, 41] and it is likely that many, if not most boys with DMD on GC therapy will have delayed or absent pubertal development [21, 39, 42]. Published data on the use of testosterone to treat hypogonadism in DMD is limited to a single retrospective study of 14 boys that reported increased growth velocity and high patient satisfaction with treatment [43]. While data on pubertal stage was not collected in MD STARnet, the fact that only six boys aged 14 years or greater had been prescribed testosterone suggests the possibility that delayed puberty was not widely recognized or treated during the years studied. Metformin was not widely used in this cohort. Metformin was shown to lower weight and increase insulin sensitivity in a small study in participants with neuromuscular disorders including DMD [44], but is not recommended as standard of care due to insufficient evidence related to safety and efficacy.
The strengths of this study include analyses of a population-based cohort of DMD cases from centers across the US. Data were abstracted from the medical records and therefore expected to represent true clinical practice and be less subject to the recall or participation bias that may result from survey designs. Limitations include retrospective data collection which may have been subject to information and misclassification bias from incomplete or inaccurate abstraction, and bias may have varied across sites. “Endocrinologist” was not a pre-specified provider choice on data collection forms and had to be entered. Bone mineral density and body site were not abstracted from DXA reports and lab data were not reviewed to determine if vitamin D levels were performed. Abstracted data for spine curvature from radiographs were insufficient to make a determination of scoliosis, and radiographs were not specified as “lateral” or “anterior-posterior”, so we could not fully ascertain the utilization of lateral spine radiographs as a means of assessing bone health [45]. The percentage of GC users in our cohort was low; we believe that this may be related to the stringent criteria applied to define GC use (>6 months), the fact that GC therapy is commonly discontinued at time of loss of ambulation, and the slow adoption of GC use into routine clinical practice during the years studied. The results of this study apply to clinical care provided before 2012 and may not reflect current practice, but do represent important baseline data for future comparison.
In summary, this is the first multi-center study to describe the utilization of bone health and endocrine screening tests and treatments in a cohort of males with DMD. We found that a low percentage of cases had bone density assessed by DXA, were evaluated by an endocrinologist, or were treated with an endocrine or bone health pharmacotherapy. We expect that these results will be of interest to providers working to integrate endocrine and bone health care into multi-disciplinary DMD clinics. Ultimately, placebo-controlled clinical trials conducted in DMD participants are needed to determine if the routine use of anti-osteoporosis agents, hormone replacement (growth hormone and/or testosterone), and anti-diabetic medications is indicated to manage or even possibly prevent DMD and GC related complications of the endocrine and skeletal systems.
Supplementary Material
ACKNOWLEDGMENTS
DRW received salary support from the National Institutes of Health, K23DK114477.
FUNDING
This research was supported by the US Centers for Disease Control and Prevention (Cooperative agreements DD000187, DD000189, DD000190, DD000191, DD001126, DD001123, DD001116 and DD001117 for Surveillance and Epidemiologic Research of Duchenne and Becker Muscular Dystrophy).
Footnotes
CONFLICTS OF INTEREST
ST, SWE, DF, JO, SP, YV, CW have nothing to declare. DRW has consulted for Marathon Pharmaceuticals, LLC; EC has consulted for Santhera, Sarepta, Marathon, and Pfizer.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JND-180317.
REFERENCES
- [1].Romitti PA, Zhu Y, Puzhankara S, James KA, Nabukera SK, Zamba GK, et al. Prevalence of Duchenne and Becker muscular dystrophies in the United States. Pediatrics. 2015;135(3):513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Parsons EP, Clarke AJ, Bradley DM. Developmental progress in Duchenne muscular dystrophy: Lessons for earlier detection. European journal of paediatric neurology: EJPN: Official Journal of the European Paediatric Neurology Society. 2004;8(3):145–53. [DOI] [PubMed] [Google Scholar]
- [3].Flanigan KM. Duchenne and Becker muscular dystrophies. Neurologic Clinics. 2014;32(3):671–88. [DOI] [PubMed] [Google Scholar]
- [4].Kohler M, Clarenbach CF, Bahler C, Brack T, Russi EW, Bloch KE. Disability and survival in Duchenne muscular dystrophy. Journal of Neurology, Neurosurgery, and Psychiatry. 2009;80(3):320–5. [DOI] [PubMed] [Google Scholar]
- [5].Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. The Lancet Neurology. 2010;9(1):77–93. [DOI] [PubMed] [Google Scholar]
- [6].Matthews E, Brassington R, Kuntzer T, Jichi F, Manzur AY. Corticosteroids for the treatment of Duchenne muscular dystrophy. The Cochrane Database of Systematic Reviews. 2016;(5):CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Soderpalm AC, Magnusson P, Ahlander AC, Karlsson J, Kroksmark AK, Tulinius M, et al. Bone mass development in patients with Duchenne and Becker muscular dystrophies: A 4-year clinical follow-up. Acta paediatrica (Oslo, Norway: 1992). 2012;101(4):424–32. [DOI] [PubMed] [Google Scholar]
- [8].King WM, Ruttencutter R, Nagaraja HN, Matkovic V, Landoll J, Hoyle C, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68(19):1607–13. [DOI] [PubMed] [Google Scholar]
- [9].Nagel BH, Mortier W, Elmlinger M, Wollmann HA, Schmitt K, Ranke MB. Short stature in Duchenne muscular dystrophy:A study of 34 patients. Acta paediatrica (Oslo, Norway: 1992). 1999;88(1):62–5. [PubMed] [Google Scholar]
- [10].Wood CL, Straub V, Guglieri M, Bushby K, Cheetham T. Short stature and pubertal delay in Duchenne muscular dystrophy. Archives of Disease in Childhood. 2016;101(1): 101–6. [DOI] [PubMed] [Google Scholar]
- [11].Davidson ZE, Ryan MM, Kornberg AJ, Sinclair K, Cairns A, Walker KZ, et al. Observations of body mass index in Duchenne muscular dystrophy: A longitudinal study. European Journal of Clinical Nutrition. 2014;68(8):892–7. [DOI] [PubMed] [Google Scholar]
- [12].Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Implementation of multidisciplinary care. The Lancet Neurology. 2010;9(2):177–89. [DOI] [PubMed] [Google Scholar]
- [13].Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. The Lancet Neurology. 2018;17(3):251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. The Lancet Neurology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Colvin MK, et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. The Lancet Neurology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Andrews JG, Soim A, Pandya S, Westfield CP, Ciafaloni E, Fox DJ, et al. Respiratory Care Received by Individuals With Duchenne Muscular Dystrophy From 2000 to 2011. Respiratory Care. 2016;61(10):1349–59. [DOI] [PubMed] [Google Scholar]
- [17].Miller LA, Romitti PA, Cunniff C, Druschel C, Mathews KD, Meaney FJ, et al. The muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): Surveillance methodology. Birth Defects Research Part A, Clinical and Molecular Teratology. 2006;76(11):793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mathews KD, Cunniff C, Kantamneni JR, Ciafaloni E, Miller T, Matthews D, et al. Muscular Dystrophy Surveillance Tracking and Research Network (MD STARnet): Case definition in surveillance for childhood-onset Duchenne/Becker muscular dystrophy. Journal of Child Neurology. 2010;25(9):1098–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kinane TB, Mayer OH, Duda PW, Lowes LP, Moody SL, Mendell JR. Long-Term Pulmonary Function in Duchenne Muscular Dystrophy: Comparison of Eteplirsen-Treated Patients to Natural History. Journal of Neuromuscular Diseases. 2018;5(1):47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lamb MM, West NA, Ouyang L, Yang M, Weitzenkamp D, James K, et al. Corticosteroid Treatment and Growth Patterns in Ambulatory Males with Duchenne Muscular Dystrophy. The Journal of Pediatrics. 2016;173:207–13.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bianchi ML, Biggar D, Bushby K, Rogol AD, Rutter MM, Tseng B. Endocrine aspects of Duchenne muscular dystrophy. Neuromuscular Disorders:NMD.2011;21(4):298–303. [DOI] [PubMed] [Google Scholar]
- [22].Buckner JL, Bowden SA, Mahan JD. Optimizing Bone Health in Duchenne Muscular Dystrophy. International Journal of Endocrinology. 2015;2015:928385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].McDonald DG, Kinali M, Gallagher AC, Mercuri E, Muntoni F, Roper H, et al. Fracture prevalence in Duchenne muscular dystrophy. Developmental Medicine and Child Neurology. 2002;44(10):695–8. [DOI] [PubMed] [Google Scholar]
- [24].James KA, Cunniff C, Apkon SD, Mathews K, Lu Z, Holtzer C, et al. Risk Factors for First Fractures Among Males With Duchenne or Becker Muscular Dystrophy. Journal of Pediatric Orthopedics. 2015;35(6):640–4. [DOI] [PubMed] [Google Scholar]
- [25].Crabtree NJ, Roper H, McMurchie H, Shaw NJ. Regional changes in bone area and bone mineral content in boys with duchenne muscular dystrophy receiving corticosteroid therapy. The Journal of Pediatrics. 2010;156(3):450–5. [DOI] [PubMed] [Google Scholar]
- [26].King WM, Kissel JT, Visy D, Goel PK, Matkovic V. Skeletal health in Duchenne dystrophy: Bone-size and subcranial dual-energy X-ray absorptiometry analyses. Muscle & Nerve. 2014;49(4):512–9. [DOI] [PubMed] [Google Scholar]
- [27].Larson CM, Henderson RC. Bone mineral density and fractures in boys with Duchenne muscular dystrophy. Journal of Pediatric Orthopedics. 2000;20(1):71–4. [PubMed] [Google Scholar]
- [28].Medeiros MO, Behrend C, King W, Sanders J, Kissel J, Ciafaloni E. Fat embolism syndrome in patients with Duchenne muscular dystrophy. Neurology. 2013;80(14): 1350–2. [DOI] [PubMed] [Google Scholar]
- [29].Tian C, Wong BL, Hornung L, Khoury JC, Miller L, Bange J, et al. Bone health measures in glucocorticoid-treated ambulatory boys with Duchenne muscular dystrophy. Neuromuscular Disorders: NMD. 2016;26(11):760–7. [DOI] [PubMed] [Google Scholar]
- [30].Srinivasan R, Rawlings D, Wood CL, Cheetham T, Jimenez Moreno AC, Mayhew A, et al. Prophylactic oral bisphosphonate therapy in Duchenne muscular dystrophy. Muscle & nerve. 2015. [DOI] [PubMed] [Google Scholar]
- [31].Sbrocchi AM, Rauch F, Jacob P, McCormick A, McMillan HJ, Matzinger MA, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012;23(11):2703–11. [DOI] [PubMed] [Google Scholar]
- [32].Bell JM, Shields MD, Watters J, Hamilton A, Beringer T, Elliott M, et al. Interventions to prevent and treat corticosteroid-induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy. The Cochrane Database of Systematic Reviews. 2017;1: CD010899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, El-Hajj Fuleihan G, Kecskemethy HH, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: The revised 2013 ISCD Pediatric Official Positions. Journal of Clinical Densitometry: The Official Journal of the International Society for Clinical Densitometry. 2014;17(2):225–42. [DOI] [PubMed] [Google Scholar]
- [34].Gordon KE, Dooley JM, Sheppard KM, MacSween J, Esser MJ. Impact of bisphosphonates on survival for patients with Duchenne muscular dystrophy. Pediatrics. 2011;127(2):e353–8. [DOI] [PubMed] [Google Scholar]
- [35].Houston C, Mathews K, Shibli-Rahhal A. Bone density and alendronate effects in Duchenne muscular dystrophy patients. Muscle & Nerve. 2014;49(4):506–11. [DOI] [PubMed] [Google Scholar]
- [36].West NA, Yang ML, Weitzenkamp DA, Andrews J, Meaney FJ, Oleszek J, et al. Patterns of growth in ambulatory males with Duchenne muscular dystrophy. The Journal of Pediatrics. 2013;163(6):1759–63 e1. [DOI] [PubMed] [Google Scholar]
- [37].Merlini L, Granata C, Ballestrazzi A, Cornelio F, Tassoni P, Tugnoli S, et al. Growth hormone evaluation in Duchenne muscular dystrophy. Italian Journal of Neurological Sciences. 1988;9(5):471–5. [DOI] [PubMed] [Google Scholar]
- [38].Bodor M, McDonald CM. Why short stature is beneficial in Duchenne muscular dystrophy. Muscle & Nerve. 2013;48(3):336–42. [DOI] [PubMed] [Google Scholar]
- [39].Rutter MM, Collins J, Rose SR, Woo JG, Sucharew H, Sawnani H, et al. Growth hormone treatment in boys with Duchenne muscular dystrophy and glucocorticoid-induced growth failure. Neuromuscular Disorders: NMD. 2012;22(12):1046–56. [DOI] [PubMed] [Google Scholar]
- [40].Cittadini A, Ines Comi L, Longobardi S, Rocco Petretta V, Casaburi C, Passamano L, et al. A preliminary randomized study of growth hormone administration in Becker and Duchenne muscular dystrophies. European Heart Journal. 2003;24(7):664–72. [DOI] [PubMed] [Google Scholar]
- [41].Rosen H, Jameel ML, Barkan AL. Dexamethasone suppresses gonadotropin-releasing hormone (GnRH) secretion and has direct pituitary effects in male rats: Differential regulation of GnRH receptor and gonadotropin responses to GnRH. Endocrinology. 1988;122(6):2873–80. [DOI] [PubMed] [Google Scholar]
- [42].Dooley JM, Bobbitt SA, Cummings EA. The impact of deflazacort on puberty in Duchenne muscular dystrophy. Pediatric Neurology. 2013;49(4):292–3. [DOI] [PubMed] [Google Scholar]
- [43].Wood CL, Cheetham TD, Guglieri M, Bushby K, Owen C, Johnstone H, et al. Testosterone Treatment of Pubertal Delay in Duchenne Muscular Dystrophy. Neuropediatrics. 2015;46(6):371–6. [DOI] [PubMed] [Google Scholar]
- [44].Casteels K, Fieuws S, van Helvoirt M, Verpoorten C, Goemans N, Coudyzer W, et al. Metformin therapy to reduce weight gain and visceral adiposity in children and adolescents with neurogenic or myogenic motor deficit. Pediatric Diabetes. 2010;11(1):61–9. [DOI] [PubMed] [Google Scholar]
- [45].Ma J, McMillan HJ, Karaguzel G, Goodin C, Wasson J, Matzinger MA, et al. The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporosis international: A journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2017;28(2):597–608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.