Abstract
Proteolytic cleavage of the amyloid precursor protein (APP) into the Aβ peptide has been an extensively researched mechanism for Alzheimer’s disease, but the normal function of the protein is less understood. APP functions to regulate neuronal iron content by stabilizing the surface presentation of ferroportin—the only iron exporter channel of cells. The present study aims to quantify the contribution of APP to brain and peripheral iron by examining the lifetime impact on brain and liver iron levels in APP knockout mice. Consistent with previous reports, we found that wild-type mice exhibited an age-dependent increase in iron and ferritin in the brain, while no age-dependent changes were observed in the liver. APP ablation resulted in an exaggeration of age-dependent iron accumulation in the brain and liver in mice that was assessed at 8, 12, 18, and 22 months of age. Brain ferroportin levels were decreased in APP knockout mice, consistent with a mechanistic role for APP in stabilizing this iron export protein in the brain. Iron elevation in the brain and liver of APP knockout mice correlated with decreased transferrin receptor 1 and increased ferritin protein levels. However, no age-dependent increase in brain ferritin iron saturation was observed in APP-KO mice despite similar protein expression levels potentially explaining the vulnerability of APP-KO mice to parkinsonism and traumatic brain sequelae. Our results support a crucial role of APP in regulating brain and peripheral iron, and show that APP may act to oppose brain iron elevation during aging.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0656-x) contains supplementary material, which is available to authorized users.
Keywords: Amyloid precursor protein, Iron, Ferritin, Brain, Neurodegeneration
Introduction
Alzheimer’s disease (AD) is a slowly progressive neurodegenerative disease characterized by cognitive impairment and profound memory loss and is characterized by 2 pathological hallmarks: the accumulation of extracellular amyloid plaques formed by aggregated amyloid beta peptide (Aβ) and intracellular neurofibrillary tangles formed by phosphorylated microtubule-associated protein tau. Amyloid precursor protein (APP) is a transmembrane protein involved in synapse formation, neuronal plasticity, and ion transport [1]. While most of the research related to APP has focused on its cleavage product Aβ, its function remains poorly understood. We and others have reported that APP acts to promote iron export by stabilizing the iron export protein, ferroportin, on the cell surface in a functional location [2–5]. APP overexpression lowers brain iron levels [6, 7], while APP loss causes brain iron elevation and iron-mediated neuronal death in the substantia nigra and in response to trauma [5, 8, 9].
Iron is essential in metabolic processes, especially in tissues of high oxygen consumption, such as the brain, which constitutively houses high concentrations. Free iron in the cell redox-cycles and so can generate reactive oxygen species. Elaborate cellular machinery at the uptake, storage, and export levels maintains the labile iron pool at levels that supply the needs of the cell, while not causing harmful oxidative stress [10, 11]. Excess iron is effluxed through the ferroportin/APP mechanism, or is caged by intracellular ferritin, which can hold up to 4500 iron atoms [12, 13]. Iron homeostasis in the brain is also controlled at the translational level by iron response proteins (IRPs), which, in response to iron availability, alter the expression of several iron-handling proteins [14]. Brain tissue may rely more on these mechanisms to maintain the stability of the labile iron pool as brain iron homeostasis appears to be largely independent of peripheral iron levels, due to the exclusiveness of the blood–brain barrier [15].
Brain iron elevation is a feature of normal aging [16–18] and could increase the risk for neurodegenerative diseases. Indeed, age-dependent iron elevation is exaggerated in affected tissue in Alzheimer’s, Parkinson’s, and Huntington’s diseases [19–21]. We have recently shown that elevated brain iron levels predict cognitive decline in longitudinal studies of AD [8, 22, 23], which highlights the toxic potential of iron accumulation.
Brain iron levels are relatively independent of peripheral iron levels, and the blood–brain barrier serves to provide the brain with essential iron as a priority, while protecting the brain tissue from peripheral excess [15]. A more complete understanding of the brain iron homeostasis machinery in response to aging is warranted. Since APP physically interacts with ferroportin in human and mouse brain [5], we undertook to survey the impact of APP ablation upon the iron homeostasis machinery in aging mice brain and liver tissue.
Results
Iron Overload in the Liver and Brain of Aging APP Knockout Mice
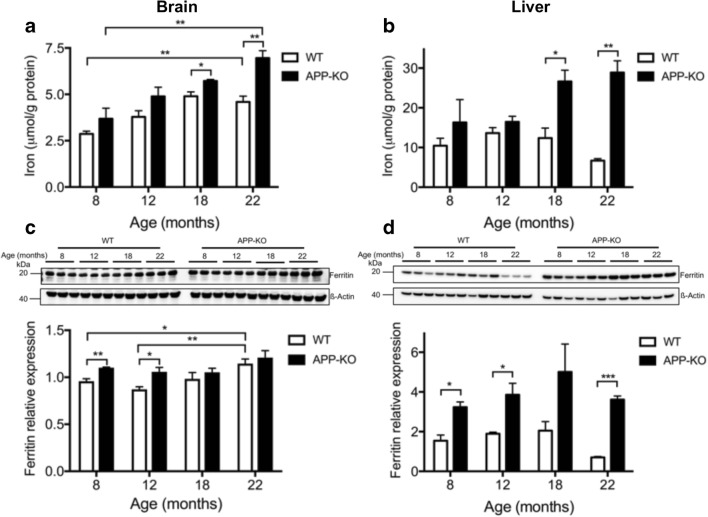
Accumulation of total iron in the brain, liver, and kidney of APP knockout (APP-KO) mice compared with background controls has been previously reported [5]. Here, we examined the developmental timing of iron elevation in the postreproductive era of the animal (reproduction typically ceases at 6 months). Brains and livers from APP-KO mice [24] and background C57BL6/SV129 control mice at 8, 12, 18, and 22 months were analyzed for iron content and iron homeostatic proteins. We observed that iron accumulated progressively in the brains of control mice, plateauing at 18 months (a 70% increase from 8 months of age) (Fig. 1A). APP ablation in mice resulted in an overall increase in brain iron in all age groups, reaching a maximum of ≈ 50% increase over control mice at 22 months of age, and without any apparent plateau (Fig. 1A). APP-KO mice also expressed increased liver iron in comparison to control mice at all age groups, and this increase was exacerbated in older animals, reaching a ≈ 400% increase at 22 months of age (Fig. 1B). In contrast to the brain, liver iron levels in control mice did not increase with age and actually slightly decreased at 22 months of age (Fig. 1B).
Fig. 1.
Ablation of APP exacerbates iron accumulation in the brain and liver of aging mice. (A, B) Total iron levels were measured in perfused (A) brain and (B) liver homogenates of progressively aging (8, 12, 18, and 22 months) APP-KO mice and control littermates. (C, D) The expression of ferritin was analyzed by Western blot in (C) brain and (D) liver homogenates and quantified relative to actin levels. Illustrative blots are shown. For age-dependent changes, statistical analysis was performed for 2 age groups corresponding to the oldest (22 months) and youngest (8 months) animals. Data are means ± SEM, n = 3; 1 asterisk, p < 0.05; 2 asterisks, p < 0.01; 3 asterisks p < 0.001; 2-tailed unpaired Student’s t test
APP Ablation Increases Ferritin Protein Levels
The iron storage protein ferritin has the capacity to store up to 4500 ferric iron atoms in a bioavailable and nontoxic form. Ferritin levels are upregulated at the translational level through the sensing of cytoplasmic labile iron levels by the iron regulatory proteins (IRPs). In APP-KO mice, we found that brain ferritin protein expression was elevated compared with that in normal mice at the youngest age tested (8 months) and remained at these levels even at 22 months of age (Fig. 1C). In control mice, brain ferritin increased slightly with age and eventually reached levels comparable to those of APP-KO mice at 22 months (Fig. 1C). In the liver, aging did not elevate ferritin protein levels in control mice and the ferritin expression profile matched the total iron levels for the same age group (Fig. 1D). Liver ferritin was markedly elevated in APP-KO as compared with that in control mice in all age groups (Fig. 1D).
Ferritin Is the Major Iron-Binding Protein in the Brain and Liver and Its Iron Saturation Is Not Increased in APP-KO Mice
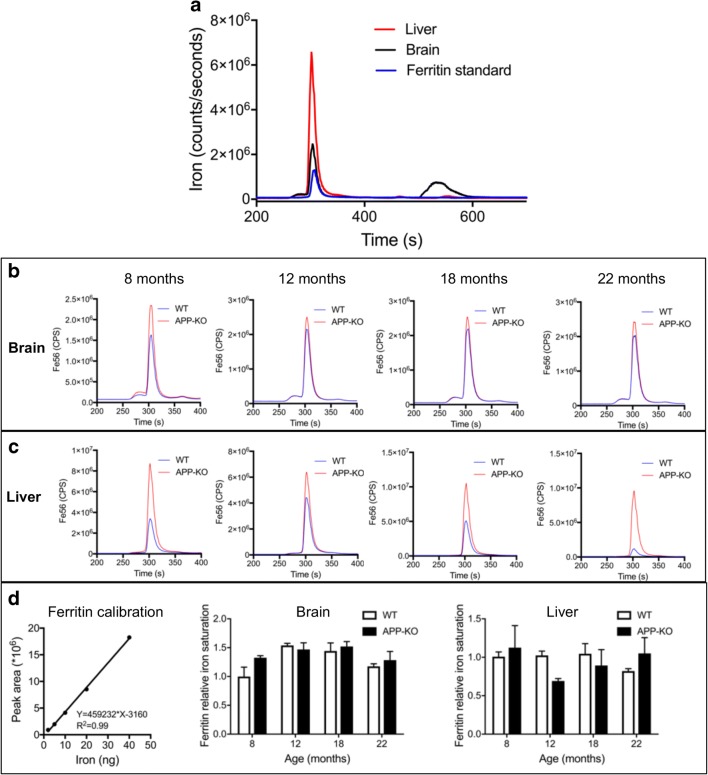
Ferritin stores iron and serves to protect the cell from excess cytosolic iron elevation, which can induce oxidative stress. To study how APP ablation perturbs iron partitioning, we analyzed ferritin iron stores and other iron-binding proteins in brain and liver protein extracts by size-exclusion chromatography–inductively coupled plasma-mass spectrometry (SEC–ICP-MS). This method allows separation of proteins in their native state (according to molecular weight) on a size-exclusion chromatography column coupled to an ICP-MS detector that allows quantification of protein-associated metals in real time [25]. This revealed, as expected, that ferritin is a major iron-binding fraction in both the brain and liver (Fig. 2A). Brain tissue also depicted nonferritin iron species, which eluted as a low molecular weight (MW) peak (below the separation capacity of the column) (Fig. 2A). This low MW peak was below the resolving ability of the column, and so we were not able to reliably quantify it in chromatographic separation. Thus, we focused on ferritin and ferritin-associated iron, as it could be reliably chromatographically measured. Iron levels associated with the ferritin peak were elevated in the APP-KO brain compared with those in control mice at all ages (Fig. 2B), and markedly more so in the liver, especially in the oldest (22 months old) animals (Fig. 2C).
Fig. 2.
Ferritin iron saturation in aging APP-KO compared with wild-type mice. (A) Illustrative chromatograph showing the SEC–ICP-MS profile of brain and liver homogenates and a pure ferritin standard. (B, C) SEC–ICP-MS profiles of (B) brain and (C) liver homogenates derived from the progressively aging (8, 12, 18, and 22 months) APP-KO mice and wild-type control littermates. Each chromatogram represents the average profile derived from 3 mice per group. (D) Iron saturation of ferritin in brain and liver homogenates was quantified using calibration with an external purified ferritin standard (left panel) and normalized to ferritin expression levels previously determined by Western blot. Data are means ± SEM, n = 3. CPS = counts per second
In the liver, ferritin protein levels were markedly elevated in APP-KO mice as compared with those in the control (Fig. 1D) and, hence, ferritin levels may be sufficient to trap excess iron without any net increase in ferritin iron saturation. Indeed, when we normalized ferritin-associated iron levels (measured in Fig. 2B, C) to ferritin expression levels (measured in Fig. 1C, D), no increase in ferritin iron saturation was observed in the brains of APP-KO mice compared with those of wild-type mice (Fig. 2D). Similarly, we did not observe any increase in ferritin iron saturation in livers of APP-KO mice compared with those of wild-type mice (Fig. 2D). In contrast to the liver, brain ferritin protein levels did not increase in APP-KO mice at older age (over 12 months, Fig. 1C) and, therefore, an increase in ferritin iron saturation could not explain the age-dependent increase in brain iron (Fig. 1A).
APP Ablation Impacts on Iron Export Capacity in the Brain and Liver of Aging Mice
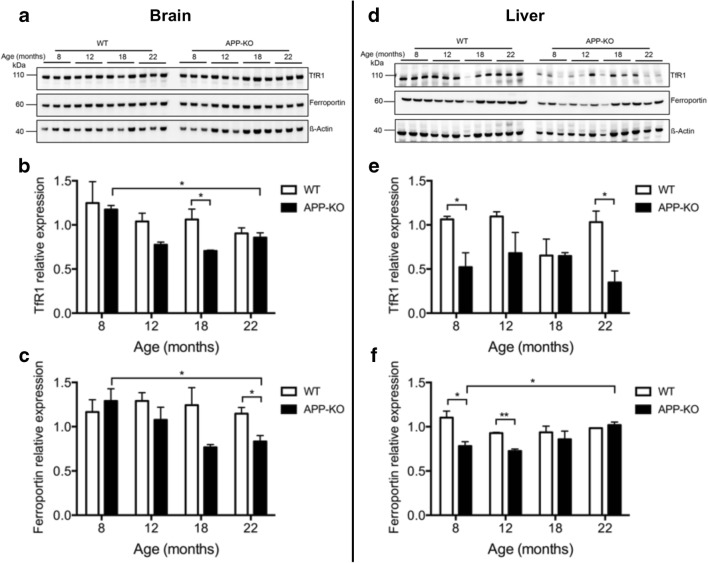
Iron is transported in interstitial fluids by transferrin, which is taken into cells by transferrin receptor 1 (TfR1). Normally, TfR1 is translationally downregulated in response to increased cellular iron to prevent further import of iron-loaded transferrin. TfR1 protein levels are therefore usually inversely correlated with ferritin and iron loading in cells. Consistent with the accumulation of iron in the tissue, brain TfR1 expression in APP-KO mice significantly decreased as they aged and a similar trend was observed in control mice (Fig. 3A, B). In the liver, TfR1 expression was significantly decreased at an early age in APP-KO compared with that in wild-type mice and remained suppressed throughout the different age groups (Fig. 3D, E).
Fig. 3.
APP-KO alters the expression of iron transport proteins in the brains and livers of aging mice. Iron transport proteins were quantified in the brain (A–C) and liver (D–F) homogenates from progressively aging (8, 12, 18, and 22 months) APP-KO mice and wild-type control littermates. Levels of transferrin receptor 1 (TfR1) (B, E) and ferroportin (C, F) were normalized to actin. Illustrative blots are shown. For age-dependent changes, statistical analysis was performed for 2 age groups corresponding to the oldest (22 months) and youngest (8 months) animals. Data are means ± SEM, n = 3; 1 asterisk, p < 0.05; 2 asterisks, p < 0.01; 3 asterisks, p < 0.001; 2-tailed unpaired Student’s t test
Ferroportin is the only known cellular iron export protein, and APP plays a role in its stabilization in neurons [3–5]. We found a progressive decrease in ferroportin levels in the brains of APP-KO mice as they aged, while wild-type mice did not show any change in ferroportin levels over age (Fig. 3A, C). In the liver, ferroportin levels were decreased in APP-KO mice at 8 months and gradually returned to levels similar to those of the wild-type mice at 22 months (Fig. 3D, F).
Discussion
We investigated the effect of APP ablation in aging mice to evaluate its role in maintaining iron homeostasis in the brain and liver during aging. APP loss in mice resulted in a persistent age-dependent increase in brain iron without plateau, in contrast to control mice where age-dependent increases plateaued at 18 months. Normally, ferritin expression is increased in response to a rise in cellular iron levels to sequester excess iron, and indeed, ferritin expression was markedly increased in the liver of APP-KO mice and this increase was sufficient to trap the increased iron, so that there was no increase in apparent ferritin iron content. However, brain ferritin expression levels in APP-KO mice plateaued at 8 months of age and we did not observe any increase in ferritin iron content despite total brain iron levels continuing to increase with advancing age. We hypothesize that brain ferritin expression is less responsive to iron burden compared with the liver and that iron overload in APP-KO mice results in an age-dependent increase in the nonferritin iron pool, which may account for the oxidative stress and cell death observed in APP-KO mice [5, 8]. There are several pools of nonferritin iron in cells, such as heme, which can be found as a low molecular weight species but also as a protein prosthetic group [26]. In the aging Caenorhabditiselegans, iron accumulates in nonferritin low molecular weight species as well as a macromolecular species as the animal ages [27]. We are currently exploring whether an analogous change occurs in mammalian tissue.
Iron export through ferroportin is facilitated by the ferroxidase activity of extracellular ceruloplasmin, which is primarily expressed in astrocytes. Neurons do not express ceruloplasmin; instead, APP is proposed to facilitate neuronal iron efflux through ferroportin stabilization at the cell surface [3, 4]. Our findings are consistent with such a role for APP, as brain iron increase in APP-KO mice at advanced age was associated with a decrease in ferroportin levels. Our previous in vitro data showed that APP impacts on ferroportin membrane stability in cultured neurons [4]. Our current results indicate that despite the increase in brain iron levels with age, ferroportin expression did not appropriately compensate in APP-KO mice, consistent with ferroportin stability substantially depending upon APP expression in vivo. Impaired APP/ferroportin association cannot explain the age-dependent increase in brain iron of wild-type mice since no age-dependent changes in ferroportin or APP (not shown) levels were observed in wild-type mice. The contribution of other factors such as changes in the extracellular ferroxidases ceruloplasmin and hephaestin, or changes in hepcidin, cannot be excluded. Indeed, recent studies showed that hepcidin is widely expressed in the murine brain and that hepcidin mRNA levels increased with aging in areas such as the cerebral cortex, hippocampus, and striatum [28]. Little is known about regulation of hepcidin expression in the brain, although peripheral circulation was reported as contributing to brain hepcidin levels [29].
Similar to the brain, APP ablation resulted in iron overload in the liver with 2 major differences. First, iron increase in the liver was also accompanied by a substantial increase in ferritin levels attesting for the higher iron storage capacity of the liver in comparison to the brain. Second, ferroportin decrease is unlikely to account for the iron increase in the liver since ferroportin levels were only decreased at 8 and 12 months of age and normalized with advancing age, which could reflect greater input from ceruloplasmin or hephaestin in stabilizing ferroportin in the liver than in the brain.
Iron accumulates in several brain regions with normal aging [16, 19, 30], and this is exaggerated in particular regions in several neurodegenerative diseases including AD, where it may contribute to oxidative burden and possibly even pathogenesis [31–34]. A recent in vitro study implicated a major role for hephaestin rather than APP in regulating ferroportin-mediated iron efflux [35]. In our current study, we provide evidence that APP plays a major role in maintaining normal iron levels in the brain and liver during aging and that its deletion is sufficient to induce iron increase in the brain and liver in vivo. Further studies would be required to evaluate the contribution of hephaestin, ceruloplasmin, and hepcidin in brain iron homeostasis during aging. These results may have implications in understanding the pathophysiology of AD, where loss of APP function leading to iron elevation might exacerbate the disease process. Future studies are also required to investigate the impact of APP mutations on its iron function and assess iron homeostasis in AD patients with familial APP mutations, which will consolidate our findings and provide evidence for iron chelation therapies as a therapeutic target for AD.
Methods
Animal Studies
All mouse studies were performed with the approval of the IACUC and in accordance with statutory regulations. Only male mice were used in this study because they were more readily available in our colony at numbers that could permit a long-term aging study. Levels of brain iron increase with age in both sexes [7], although there is some sexual dimorphism in the extent. APP-KO mice and control littermates aged 8, 12, 18, and 22 months were used. After cardiac perfusion with ice-cold phosphate-buffered saline (PBS) under deep anesthesia, the brain and liver were extracted and stored at − 80 °C until required.
Tissue Homogenization and Protein Quantitation
The brain and liver were lysed in cold homogenization buffer containing (250 mM sucrose, 1 mM EDTA, 0.1% SDS, 10 mM Tris HCl buffer, pH 7.2 plus protease inhibitor), and proteins were extracted using sonication and subsequent centrifugation. The supernatant containing the extracted proteins was used for protein quantification with BCA assay (Thermo Fisher Scientific Australia Pty Ltd, Scoresby, Victoria), and aliquots of equal protein amounts were stored at − 80 °C for further analysis.
Western Blot Analysis
Twenty micrograms of total protein crude extracts was separated by SDS-PAGE on 4 to 20% PAGE (Bis-Tris, Thermo Fisher Scientific Australia Pty Ltd, Scoresby, Victoria) and transferred to polyvinylidene difluoride (PVDF) membrane using iBlot (Thermo Fisher Scientific Australia Pty Ltd, Scoresby, Victoria). Primary antibodies used were rabbit anti-ferritin (1:1000, #ab75973, Abcam, Melbourne, Victoria, Australia), mouse anti-transferrin receptor 1 (TfR1) (1:2000, Alpha Diagnostic Intl. Inc. San Antonio, Texas, USA), and rabbit anti-ferroportin (1:1000, MAP23; α/α165–181 gifted by Prof. Tracey Rouault; NIH). The load control was mouse anti-β-actin (1:10,000, Sigma-Aldrich Pty Ltd, Castle Hill, New South Wales, Australia). Membranes were probed with horseradish peroxidase-conjugated secondary antibodies, and signal was detected with Pierce ECL (Thermo Fisher Scientific Australia Pty Ltd, Scoresby, Victoria) and a LAS-3000 Imaging suite. Densitometry analyses were carried out using Image J (NIH), and quantitation was standardized against β-actin levels.
Metal Analysis
Samples of lyophilized homogenates from tissue were dissolved in concentrated HNO3 and H2O2 (Aristar, BDH, VWR International, Pty Ltd, Tingalpa, Queensland, Australia). Metal levels were measured by inductively coupled plasma-mass spectrometry (ICP-MS) with an Ultramass 700 (Varian Australia Pty Ltd, Mulgrave, Victoria) as described [7].
Size-Exclusion Chromatography–Inductively Coupled Plasma-Mass Spectrometry
Brain and liver protein extracts from the aging study were chromatographically separated using a Bio-SEC-3 column (3 μm, 4.6 mm × 300 mm; cat. # 5190-2508, Agilent Technologies Australia, Mulgrave, Victoria) connected to an Agilent 1200 series HPLC. The system was operated at 25 °C and a constant flow rate of 0.4 mL/min ammonium nitrate (200 mM, pH 7.5) as the mobile phase. Antimony chloride and cesium chloride (50 ppb each) were added to the mobile phase as internal standards during ICP-MS acquisition. PEEK tubing (0.12 mm I.D.) exiting the HPLC system variable-wavelength detector was connected to a MicroMist nebulizer (AR35-1-FM04EX, Glass Expansion, Melbourne, Victoria, Australia) attached to an Agilent 7700 series ICP-MS to monitor counts of 56Fe, 63Cu, 66Zn, 121Sb, and 133Cs. The ICP-MS was operated in He mode (3.0 mL/min flow rate) under routine multi-elemental operating conditions [36]. Each sample (100 μg protein) was injected and element counts monitored over a 15.5-min period (6.2 mL, 1.35 column volumes). Protein standards superoxide dismutase 1 (#S8160, Sigma-Aldrich Pty Ltd, Castle Hill, New South Wales, Australia) and ferritin (#28403842B, GE Healthcare Australia, Silverwater New South Wales) were used to calibrate the system by monitoring UV absorbance (280 nm) and counts of 56Fe for ferritin. Data collected were normalized for protein loading by integration of total UV280nm signals and comparing to the average signal across runs. The final ferritin iron saturation depicted in Fig. 2D was determined by normalizing ferritin-associated iron determined by SEC–ICP-MS to ferritin relative expression measured by Western blot in Fig. 1.
Statistical Analysis
Statistical analyses were performed with Graphpad Prism 6 software (Graphpad, La Jolla, California, USA) using 2-tailed unpaired Student’s t test for comparison of less than 3 groups. All statistically significant values are reported in each panel with the corresponding values: *p < 0.05, **p < 0.01, ***p < 0.001. Statistically not significant changes are not highlighted. For age-dependent changes, statistical analysis was performed for 2 age groups corresponding to the oldest (22 months) and youngest (8 months) animals.
Electronic Supplementary Material
(PDF 1225 kb)
Acknowledgments
Support by the National Health and Medical Council of Australia (NHMRC), the Australian Research Council, and the German Research Foundation (DFG: BE 5471/1-1 to AAB) is gratefully acknowledged.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Compliance with Ethical Standards
All mouse studies were performed with the approval of the IACUC and in accordance with statutory regulations.
Conflict of Interest
BR receives research support for Agilent. AIB is a shareholder in Prana Biotechnology Ltd., Cogstate Ltd., Brighton Biotech LLC, Grunbiotics Pty Ltd., Eucalyptus Pty Ltd., and Mesoblast Ltd. He is a paid consultant for, and has a profit share interest in, Collaborative Medicinal Development Pty Ltd.
References
- 1.Caldwell JH, Klevanski M, Saar M, Muller UC. Roles of the amyloid precursor protein family in the peripheral nervous system. Mech Dev. 2013;130:433–446. doi: 10.1016/j.mod.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Needham BE, Ciccotosto GD, Cappai R. Combined deletions of amyloid precursor protein and amyloid precursor-like protein 2 reveal different effects on mouse brain metal homeostasis. Metallomics. 2014;6:598–603. doi: 10.1039/c3mt00358b. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy RC, Park YH, Kosman DJ. sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin. EMBO Rep. 2014;15:809–815. doi: 10.15252/embr.201338064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong BX, Tsatsanis A, Lim LQ, Adlard PA, Bush AI, Duce JA. beta-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS One. 2014;9:e114174. doi: 10.1371/journal.pone.0114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, Cho HH, Galatis D, Moir RD, Masters CL, McLean C, Tanzi RE, Cappai R, Barnham KJ, Ciccotosto GD, Rogers JT, Bush AI. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX. Overexpression of Alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem. 2002;277:44670–44676. doi: 10.1074/jbc.M204379200. [DOI] [PubMed] [Google Scholar]
- 7.Maynard CJ, Cappai R, Volitakis I, Cherny RA, Masters CL, Li QX, Bush AI. Gender and genetic background effects on brain metal levels in APP transgenic and normal mice: implications for Alzheimer beta-amyloid pathology. J Inorg Biochem. 2006;100:952–962. doi: 10.1016/j.jinorgbio.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Ayton S, Lei P, Hare DJ, Duce JA, George JL, Adlard PA, McLean C, Rogers JT, Cherny RA, Finkelstein DI, Bush AI. Parkinson’s disease iron deposition caused by nitric oxide-induced loss of beta-amyloid precursor protein. J Neurosci. 2015;35:3591–3597. doi: 10.1523/JNEUROSCI.3439-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayton S, Zhang M, Roberts BR, Lam LQ, Lind M, McLean C, Bush AI, Frugier T, Crack PJ, Duce JA. Ceruloplasmin and beta-amyloid precursor protein confer neuroprotection in traumatic brain injury and lower neuronal iron. Free Radic Biol Med. 2014;69:331–337. doi: 10.1016/j.freeradbiomed.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 11.Hare D, Ayton S, Bush A, Lei P. A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci. 2013;5:34. doi: 10.3389/fnagi.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arosio P, Ingrassia R, Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta. 2009;1790:589–599. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Arosio P, Levi S. Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage. Biochim Biophys Acta. 2010;1800:783–792. doi: 10.1016/j.bbagen.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Meyron-Holtz EG, Ghosh MC, Iwai K, LaVaute T, Brazzolotto X, Berger UV, Land W, Ollivierre-Wilson H, Grinberg A, Love P, Rouault TA. Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. EMBO J. 2004;23:386–395. doi: 10.1038/sj.emboj.7600041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer's disease and Parkinson's disease: targets for therapeutics. J Neurochem 139 Suppl 1, 179–197. [DOI] [PubMed]
- 16.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramos P, Santos A, Pinto NR, Mendes R, Magalhaes T, Almeida A. Iron levels in the human brain: a post-mortem study of anatomical region differences and age-related changes. J Trace Elem Med Biol. 2014;28:13–17. doi: 10.1016/j.jtemb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. MRI estimates of brain iron concentration in normal aging: comparison of field-dependent (FDRI) and phase (SWI) methods. Neuroimage. 2009;47:493–500. doi: 10.1016/j.neuroimage.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor JR, Snyder BS, Beard JL, Fine RE, Mufson EJ. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer’s disease. J Neurosci Res. 1992;31:327–335. doi: 10.1002/jnr.490310214. [DOI] [PubMed] [Google Scholar]
- 20.Jack CR, Jr, Wengenack TM, Reyes DA, Garwood M, Curran GL, Borowski BJ, Lin J, Preboske GM, Holasek SS, Adriany G, Poduslo JF. In vivo magnetic resonance microimaging of individual amyloid plaques in Alzheimer’s transgenic mice. J Neurosci. 2005;25:10041–10048. doi: 10.1523/JNEUROSCI.2588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeVine SM. Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res. 1997;760:298–303. doi: 10.1016/S0006-8993(97)00470-8. [DOI] [PubMed] [Google Scholar]
- 22.Ayton S, Faux NG, Bush AI, Alzheimer's Disease Neuroimaging I. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun. 2015;6:6760. doi: 10.1038/ncomms7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayton S, Fazlollahi A, Bourgeat P, Raniga P, Ng A, Lim YY, Diouf I, Farquharson S, Fripp J, Ames D, Doecke J, Desmond P, Ordidge R, Masters CL, Rowe CC, Maruff P, Villemagne VL, Australian Imaging B, Lifestyle Research G, Salvado O, Bush AI. Cerebral quantitative susceptibility mapping predicts amyloid-beta-related cognitive decline. Brain. 2017;140:2112–2119. doi: 10.1093/brain/awx137. [DOI] [PubMed] [Google Scholar]
- 24.Zheng H, Jiang M, Trumbauer ME, Sirinathsinghji DJ, Hopkins R, Smith DW, Heavens RP, Dawson GR, Boyce S, Conner MW, Stevens KA, Slunt HH, Sisoda SS, Chen HY, Van der Ploeg LH. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-X. [DOI] [PubMed] [Google Scholar]
- 25.Hare DJ, Grubman A, Ryan TM, Lothian A, Liddell JR, Grimm R, Matsuda T, Doble PA, Cherny RA, Bush AI, White AR, Masters CL, Roberts BR. Profiling the iron, copper and zinc content in primary neuron and astrocyte cultures by rapid online quantitative size exclusion chromatography-inductively coupled plasma-mass spectrometry. Metallomics. 2013;5:1656–1662. doi: 10.1039/c3mt00227f. [DOI] [PubMed] [Google Scholar]
- 26.Gozzelino R. The pathophysiology of heme in the brain. Curr Alzheimer Res. 2016;13:174–184. doi: 10.2174/1567205012666150921103304. [DOI] [PubMed] [Google Scholar]
- 27.James SA, Roberts BR, Hare DJ, de Jonge MD, Birchall IE, Jenkins NL, Cherny RA, Bush AI, McColl G. Direct in vivo imaging of ferrous iron dyshomeostasis in ageing Caenorhabditis elegans. Chem Sci. 2015;6:2952–2962. doi: 10.1039/C5SC00233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SM, Fu LJ, Duan XL, Crooks DR, Yu P, Qian ZM, Di XJ, Li J, Rouault TA, Chang YZ. Role of hepcidin in murine brain iron metabolism. Cell Mol Life Sci. 2010;67:123–133. doi: 10.1007/s00018-009-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vela D. Hepcidin, an emerging and important player in brain iron homeostasis. J Transl Med. 2018;16:25. doi: 10.1186/s12967-018-1399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zecca L, Gallorini M, Schunemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D. Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J Neurochem. 2001;76:1766–1773. doi: 10.1046/j.1471-4159.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- 31.Connor JR, Menzies SL, St Martin SM, Mufson EJ. A histochemical study of iron, transferrin, and ferritin in Alzheimer’s diseased brains. J Neurosci Res. 1992;31:75–83. doi: 10.1002/jnr.490310111. [DOI] [PubMed] [Google Scholar]
- 32.Zhu WZ, Zhong WD, Wang W, Zhan CJ, Wang CY, Qi JP, Wang JZ, Lei T. Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease. Radiology. 2009;253:497–504. doi: 10.1148/radiol.2532082324. [DOI] [PubMed] [Google Scholar]
- 33.Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW, Jr, Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T, Nakamura M, Nunomura A, Perry G. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis. 2010;19:363–372. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin Y, Zhu W, Zhan C, Zhao L, Wang J, Tian Q, Wang W. Investigation on positive correlation of increased brain iron deposition with cognitive impairment in Alzheimer disease by using quantitative MR R2' mapping. J Huazhong Univ Sci Technolog Med Sci. 2011;31:578–585. doi: 10.1007/s11596-011-0493-1. [DOI] [PubMed] [Google Scholar]
- 35.Ji C, Steimle BL, Bailey DK, Kosman DJ. The ferroxidase hephaestin but not amyloid precursor protein is required for ferroportin-supported iron efflux in primary hippocampal neurons. Cell Mol Neurobiol. 2018;38:941–954. doi: 10.1007/s10571-017-0568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunn AP, Roberts BR. Analysis of trace elements and metalloproteins in fractionated human brain samples using size exclusion inductively coupled mass spectrometry. Metals in the Brain: Measurement and Imaging. 2017;124:119–125. doi: 10.1007/978-1-4939-6918-0_7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)