Abstract
The current review highlights the evidence supporting the use of ketogenic diet therapies in the management of a growing number of neurological disorders in adults. An overview of the scientific literature supporting posited mechanisms of therapeutic efficacy is presented including effects on neurotransmission, oxidative stress, and neuro-inflammation. The clinical evidence supporting ketogenic diet use in the management of adult epilepsy, malignant glioma, Alzheimer’s disease, migraine headache, motor neuron disease, and other neurologic disorders is highlighted and reviewed. Lastly, common adverse effects of ketogenic therapy in adults, including gastrointestinal symptoms, weight loss, and transient dyslipidemia are discussed.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-0666-8) contains supplementary material, which is available to authorized users.
Key Words: Modified Atkins diet, epilepsy, malignant glioma, Alzheimer’s disease, migraine headache.
Introduction
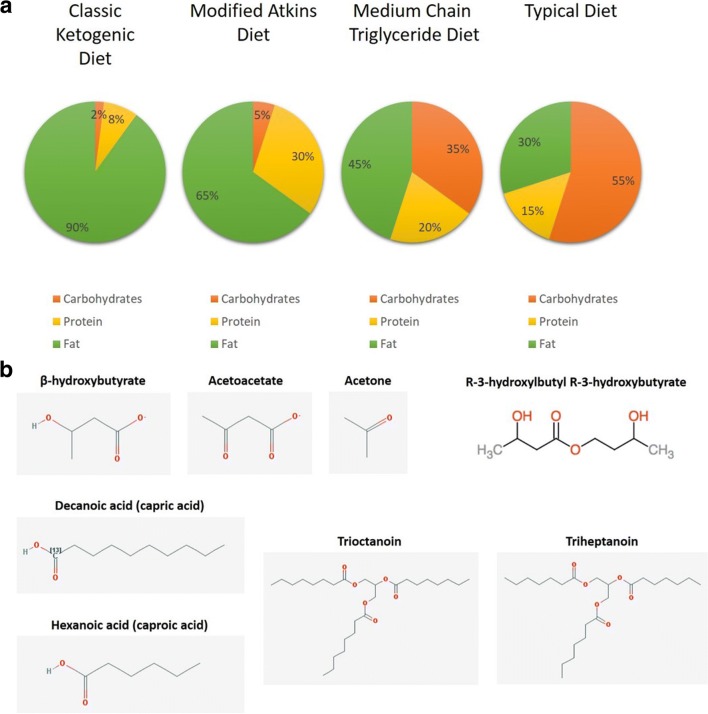
The ketogenic diet (KD) is a high-fat, low-carbohydrate diet that induces ketone body production in the liver through fat metabolism. The goal is to mimic a fasting or starvation state without depriving the body of necessary calories to sustain growth and development [1, 2]. The classic ketogenic diet is composed of a macronutrient ratio of 4:1 (4 grams (g) of fat to every 1 g of protein plus carbohydrates combined), thus shifting the predominant caloric source from carbohydrate to fat. Fatty acids metabolized by the liver produce the ketone bodies acetoacetate (which spontaneously converts to acetone) and β-hydroxybutyrate (Fig. 1) which enter the bloodstream and are utilized by organs including the brain where they are further metabolized in mitochondria to generate energy. With ketogenic diet use, organs utilize ketone bodies as the major fuel source for the central nervous system to replace glucose.
Fig. 1.
Ketogenic diets, ketones, medium-chain triglycerides, and ketogenic supplements. a Macronutrient composition of the classic (4:1 ratio) ketogenic diet and ketogenic diet variants compared to the traditional American diet. b Two-dimensional structures of the endogeneous ketone bodies (β-hydroxybutyrate, acetoacetate, and acetone) produced by hepatic metabolism, examples of the saturated 10- and 6-carbon backbone medium-chain triglycerides found in oils and animal fats (capric and caproic acid), and specific ketogenic chemicals (trioctanoin, triheptanoin, and R-3-hydroxylbutyl R-3-hydroxybutyrate) used in preclinical and clinical studies of neurodegenerative disease
To increase flexibility and palatability, less strict KD variants have been developed (Fig. 1), including lower ratio ketogenic diets, the modified Atkins diet (MAD), and combining these diets with medium-chain triglyceride oil (MCT). Ketogenic diets with lower ratios of 3:1, 2:1, or 1:1 (referred to in some studies as modified ketogenic diets) are chosen based on age, individual tolerability, goal level of ketosis, and protein requirements [3]. The MAD, introduced into practice in 2003, typically employs a 10 to 20-g/day net carbohydrate limit (most often 20 g in adults) which is roughly equivalent to a ratio of 1 to 2:1 of fat to protein plus carbohydrates [4, 5]. The MCT variant ketogenic diet incorporates medium-chain triglycerides, like capric and caproic acid (Fig. 1), provided in coconut and/or palm kernel oil as a diet supplement and allows for greater carbohydrate and protein intake than even a lower ratio KD [6], which can improve compliance. In addition, there is growing interest in the therapeutic use of ketogenic dietary supplements like ketone esters as a potential substitute for ketogenic diets. Although numerous studies have examined the use of ketogenic therapies for epilepsy and weight loss [7, 8], these diets and supplements have more recently been adopted as potential treatments of other potentially diet-sensitive neurological disorders. The current review explores the proposed mechanisms by which ketogenic therapies are thought to achieve therapeutic benefit in a variety of neurologic diseases and to describe the accumulating, predominantly clinical, evidence supporting ketogenic diet use in specific adult neurological disorders including epilepsy, malignant glioma, Alzheimer’s disease, migraine headache, and motor neuron disease.
Mechanisms of Action of the Ketogenic Diet
Synaptic Transmission
An increasing number of experimental studies demonstrate pleiotropic actions of ketone bodies and ketogenic diets [9]. Ketones can induce changes in neurotransmitter concentrations and release, as well as neural membrane polarity which may inhibit increased neuronal excitability. For example, in vitro studies demonstrate that ketones can open adenosine tri-phosphate (ATP)-sensitive potassium channels and slow spontaneous neuronal firing in cultured mouse hippocampal neurons [10, 11]. Medium-chain fatty acids (Fig. 1), such as decanoic acid, may selectively inhibit AMPA receptors, as demonstrated in in vitro and in vivo models of seizure activity [12–14]. Furthermore, ketone bodies acetoacetate and β-hydroxybutyrate increase the accumulation of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) in presynaptic vesicles in rodent models [15]. Rodents in a state of metabolic ketosis have been shown to exhibit lower levels of glutamate in neurons but stable amounts of GABA, which creates a shift towards inhibition [16]. Glutamate recycling via glutamine becomes more efficient when ketone bodies are available which can improve GABA resynthesis for inhibitory neurotransmission [17]. In human studies, patients treated with a ketogenic diet showed increased GABA levels in the cerebrospinal fluid and in brain tissue using magnetic resonance spectroscopy [18, 19]. Some studies have also suggested direct inhibition of vesicular glutamate loading by ketone bodies [20] resulting in reduced glutamatergic synaptic transmission and potential interference with glutamate-mediated toxicity implicated in neuronal injury.
Antioxidant Effects
Ketogenic therapies may also provide additional neuroprotective benefit by improving mitochondrial function. Metabolic ketosis can increase mitochondrial energy reserves while decreasing reactive oxygen species (ROS) production [21]. Animals treated with KD exhibit stimulation of mitochondrial biogenesis, increased cerebral ATP concentrations, and lower ROS production [22, 23]. Another potential mechanism of action of the ketogenic diet is through modulation of intracellular signaling pathways including mammalian target of rapamycin (mTOR) and nuclear factor erythroid 2-related factor 2 (Nrf2). Rodents maintained on a KD showed reduced insulin levels and reduced phosphorylation of Akt and S6, providing evidence for increased AMP-activated protein kinase signaling and decreased mTOR activation [24, 25]. Similarly, rodents consuming a KD exhibit time-dependent changes in mitochondrial hydrogen peroxide production and elevations in lipid peroxidation product 4-hydrocy-2-nonenal (4-HNE) levels, both of which result in enhanced activation of Nrf2—the primary transcription factor responsible for initiating the response to oxidative stress [26, 27]. Administration of ketone bodies can inhibit opening of the membrane permeability transition (mPT) pore, which regulates the homeostatic status of mitochondria, by raising the threshold of calcium-induced mPT [28]. The reduction in oxidative stress resulting from exposure to ketone bodies may also occur via genomic effects. In vitro models demonstrate that the direct application of ketones inhibits histone deactylases (HDACs) resulting in increased transcriptional activity of peroxisome proliferator-activated receptor (PPAR) γ and upregulation of antioxidant genes [29, 30].
Anti-inflammatory Effects
There is emerging evidence that ketone bodies exhibit systemic and neuroprotective anti-inflammatory effects [31]. In rodent models of neurodegenerative and neuro-inflammatory disorders, KD treatment reduces expression of pro-inflammatory cytokines, microglial activation, and pain and inflammation after thermal nociception [32–34]. One potential explanation for this observation is that fatty acids activate peroxisome proliferator-activated receptor α which then inhibits the pro-inflammatory transcription factor nuclear factor kappa B (NF-κB) signal transduction pathway to downregulate expression of two key genes involved in the inflammatory response—COX2 and inducible nitric oxide synthase [35]. Recent studies also show that β-hydroxybutyrate may directly bind to hydroxy-carboxylic acid receptor 2 (HCA2) and/or inhibit the innate immune sensor NOD-like receptor 3 (NLRP3) inflammasome which controls the activation of caspase-1 and the release of pro-inflammatory cytokines IL-1β and IL-18 [9, 34, 36].
Disease-Specific Effects: Evidence from In Vivo Models
Several preclinical studies have explored KDs and/or exogenous supplementation of ketone salts in the treatment of specific neurologic disorders including gliomas, Alzheimer’s disease, and seizure disorders. In rodent glioma models, KDs decrease serum glucose, serum insulin-like growth factor and tumor weight when administered as a stand-alone therapy. When administered in combination with glycolytic inhibitor 2-deoxy-d-glucose (2-DG), radiation therapy, or temozolomide, the diet also reduced peritumoral edema and tumor microvasculature and increased median survival time [37–42]. Thus, KDs favor a metabolic shift in malignant cells towards an anti-angiogenic, anti-invasive, pro-apoptotic, and anti-inflammatory state that contributes to suppressed tumor growth in vivo [43]. Potential mechanisms include enhanced cytotoxic T cell anti-tumor immunity [44], attenuated insulin-activated Akt/mTOR and Ras/MAPK signaling pathways [45, 46], and reduced inflammation as previously described. Other proposed mechanisms include the induction of genes involved in oxidative stress protection, angiogenesis, and vascular remodeling [29, 39, 42].
In vivo studies also suggest that KDs block or improve histological and biochemical changes seen in Alzheimer’s disease pathology. Rodents treated with the ketogenic diet, exogenous β-hydroxybutyrate, and MCT display reduced brain amyloid-β levels, protection from amyloid-β toxicity, and improved mitochondrial function [47–49]. Similarly, in a symptomatic mouse model of AD and in aged rats, exogenous ketone and ketogenic diet administration improved learning and memory [50, 51]. These observations provide insight into potential mechanisms by which AD risk and pathology may be impacted by ketogenic therapies.
There has been controversy over whether the anti-seizure effect of the KD is due to ketosis as blood ketone (i.e., β-hydroxybutyrate) concentrations inconsistently correlate with seizure control amongst clinical studies [52–56], although findings may relate to diet heterogeneity and methodological differences between studies. Rodent studies suggest that modulation of gut microbiota, by feeding or fecal transplant, may confer the anti-seizure effect of KDs. The mechanism involves microbial interactions that reduce bacterial gamma-glutamylation activity, decrease peripheral gamma-glutamylated-amino acids, and elevate bulk hippocampal GABA/glutamate ratios [57]. Whether similar changes in microbiome profile occur in adults with drug-resistant epilepsy and other neurologic disorders in response to KD therapy and whether particular taxonomic changes in gut microbiota correlate with treatment response remains unknown.
In summary, preclinical studies support the use of KDs and/or ketone bodies to thwart or ameliorate the histologic, biochemical, and electrophysiologic changes that lead to neurologic dysfunction and disease and lay the foundation for clinical studies investigating management of these disorders in adults.
Ketogenic Diets in the Management of Adult Neurological Disorders
Epilepsy and Refractory Seizures
Although first recognized as an effective treatment for epilepsy nearly one century ago, the introduction of anti-epileptic drugs (AEDs) supplanted interest in diet therapy until the 1990s when studies re-emerged demonstrating its efficacy in patients with drug-resistant epilepsy and particular pediatric epilepsy syndromes [58–60]. Initial clinical studies and additional evidence since the turn of the century support ketogenic diet use in adult epilepsy management, with modern research reporting efficacy defined by the proportion of patients achieving ≥ 50% seizure reduction (defined as responders). A 2015 meta-analysis reviewed 12 studies using the classic KD, the MAD, and the classic KD in combination with MCT in adults with drug-resistant epilepsy. They found a combined efficacy rate of 52% for the classic KD and 34% for the MAD [61]. A similar 3-month responder rate of 39% was observed in an observational study of 101 adult patients naïve to diet therapy who subsequently began MAD [62]. Overall, intention-to-treat (ITT) data from adult observational studies demonstrate responder rates of 22 to 70% for the classic KD and 12 to 67% for the MAD [61, 63, 64], with some suggestion of increased efficacy in adults with generalized rather than focal epilepsy [65, 66]. Such disparate effects are supported by two recent randomized controlled trials (RCTs) evaluating MAD efficacy in adults with drug-resistant epilepsy. An RCT in Iran compared the proportion of patients with focal or generalized epilepsy achieving ≥ 50% seizure reduction between 34 patients randomized to 2 months of MAD (of whom 22 completed the study) compared to 32 patients randomized to standard medical management and found 35.5% (12/34) efficacy in the MAD group (ITT analysis) at 2 months compared to 0% in the control group, a difference that was statistically significant [67]. In contrast, an RCT from Norway compared the change in seizure frequency in patients with drug-resistant focal or multifocal epilepsy between 37 patients randomized to 12 weeks of MAD (of whom 28 received the intervention and 24 completed the study) and 38 adults randomized to their habitual diet (of whom 34 received the intervention and 32 completed the study) [68]. They found a significant moderate benefit (25-50% seizure reduction) in the diet group compared to controls, but no significant difference in ≥ 50% seizure reduction between groups (no difference in the proportion of responders to diet versus standard medical management). Thus, additional RCTs of larger sample size are warranted to investigate MAD efficacy in different subpopulations of adult epilepsy patients.
Ketogenic diets also show promise for the management of status epilepticus in adults [69–73]. For example, of 10 adults with super-refractory status epilepticus (SRSE; status epilepticus that continues or recurs 24 h or more after the initiation of treatment with anesthetic agents to induce burst or seizure suppression) of median duration 21.5 days treated with a 4:1 or 3:1 KD, successful resolution of status epilepticus over a median of 3 days (range 1-31 days) was observed in 100% of patients who achieved ketosis (9 out of 10) [74, 75]. Similarly, of 15 adult patients with SRSE treated with a 4:1 ratio KD (14 of whom completed therapy) for a median of 10 days, 11 (79% of patients who completed KD therapy, 73% of all patients enrolled) achieved resolution of SRSE in a median of 5 days (range 0-10 days) [76]. As status epilepticus carries a high risk of morbidity and mortality [77], KDs offer a needed adjunctive strategy for management with the advantages of potentially synergizing with concurrent treatments and reducing the need for prolonged use of anesthetic drugs.
Malignant Gliomas
Malignant gliomas, the most frequently diagnosed primary brain tumors, are highly heterogeneous tumors with characteristically poor response to treatment. Glioblastoma multiforme (GBM) is the most aggressive type of glioma in adults and carries an extremely poor prognosis with a median overall survival between 12 and 15 months from the time of diagnosis. The 5-year survival rate is less than 5% [78, 79]. Currently, GBM treatment includes maximal safe tumor resection, followed by radiotherapy and concurrent temozolomide treatment [79]. Other treatment strategies include managing peritumoral edema with glucocorticoids and using bevacicumab for anti-angiogenic effect. However, overall survival remains poor despite these interventions [43]. As tumors exhibit high rates of aerobic glycolysis followed by predominant fermentation of pyruvate to lactate despite sufficient oxygen availability [80, 81], malignant glioma cells critically depend on glucose as the main energy source to survive and sustain their aggressive proliferative properties [82]. Hyperglycemia, moreover, is a negative predictor of overall survival in GBM patients [83–86]. These findings have led researchers to investigate strategies to target glycemic modulation using ketogenic diets, caloric restriction, carbohydrate restriction, intermittent fasting, and combined diet protocols broadly classified as ketogenic metabolic therapies with mixed results.
In 2010, a case report was published of an adult woman with newly diagnosed GBM who was successfully treated with a calorie-restricted ketogenic diet combined with chemotherapy and radiation after partial tumor resection. There was no evidence of residual tumor using fluorodeoxyglucose positron emission tomography (FDG-PET) and magnetic resonance imaging (MRI) after 2 months of treatment. However, tumor recurrence was detected 10 weeks after stopping diet therapy [87]. In 2015, a study was published describing 6 adults with newly diagnosed GBM who were treated with a KD and demonstrated reduced mean serum glucose compared to patients on a regular diet. However, 5 out of 6 showed evidence of disease by 12 months [88]. Similarly, 2 adults with recurrent GBM treated with a 3:1 calorie-restricted KD noted evidence of tumor progression by 12 weeks in another case series [89]. In the largest pilot trial to date, 20 adult patients with recurrent GBM were treated with a low (≤ 60 g/day) carbohydrate diet. Three participants discontinued the diet because of poor tolerability, 3 had stable disease after 6 weeks that lasted for 11 to 13 weeks and 1 had a minor response. Overall, patients with stable ketosis showed a trend towards an increase in survival without tumor progression [90]. The most durable response was observed in an adult with newly diagnosed GBM who experienced significant tumor regression 24 months following subtotal resection, calorie-restricted ketogenic diet, hyperbaric oxygen, and other targeted metabolic therapies combined [91]. These preliminary studies demonstrate feasibility and short-term safety of ketogenic diet therapies in the treatment of GBM. However, given the heterogeneity in study design (differences in diet protocols, combined therapies, endpoints, and outcomes measured) as well as absence of control groups, no conclusive statements can be made regarding overall efficacy compared to standard therapies. Consequently, randomized controlled trials assessing ketogenic diet efficacy on overall survival or progression-free survival in adults with GBM are needed.
Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common form of progressive dementia, affecting approximately 10% of individuals age 65 years and older. Histological hallmarks include hippocampal neuronal death, extracellular amyloid-β peptide deposition, and intracellular tau protein neurofibrillary tangles. Defects in mitochondrial and respiratory chain function may alter amyloid precursor protein (APP) processing, resulting in pathogenic amyloid-β fragment production [92]. Progressive cognitive degeneration in patients with AD has been linked to reduced uptake and metabolism of glucose [93]. Studies demonstrate that asymptomatic individuals with genetic risk factors for AD or a positive family history show reduced glucose uptake in prefrontal cortex, posterior cingulate, entorhinal cortex, and hippocampal tissue compared to normal-risk individuals using FDG-PET. Furthermore, reduction in glucose uptake in these regions correlates with glucose transporter GLUT1 downregulation in the brain of individuals with AD [31, 94]. High-glycemic diets are associated with greater cerebral amyloid burden [95] and increased insulin resistance can contribute to the development of sporadic AD [96, 97]. Therefore, clinical investigators hypothesize that reducing dietary intake of high-glycemic foods may prevent cerebral amyloid accumulation and reduce AD risk.
In an early RCT published in 2004, the investigators randomized 20 patients with mild cognitive impairment (MCI) or AD to receive a single oral dose of either a medium-chain triglyceride supplement or placebo on different days. Serum ketone levels increased following MCT ingestion, but only patients without the apolipoprotein E (APOE) ε4 allele showed enhanced short-term cognitive performance on a screening tool of attention, memory, language, and praxis, whereas patients with the allele did not [98]. These findings were replicated in a study of 19 elderly patients without dementia who received an MCT supplement and exhibited similar improvements in working memory, visual attention, and task switching [99]. A third RCT comparing response to a very restricted (5-10%) or liberal (50%) carbohydrate intake diet over 6 weeks in adults with MCI showed an improvement in verbal memory performance. This improvement correlated with serum ketone concentrations in the carbohydrate restricted group [100]. A 2015 case report demonstrated that a patient with AD using ketone monoester (R)-3-hydroxybutyl (R)-3-hydroxybutyrate (Fig. 1) supplementation over 20 months without altering their diet displayed improved cognitive and daily activity performance as well as diurnal elevations in circulating serum β-hydroxybutyrate levels [101]. In a single-arm pilot study of 15 patients with mild-moderate AD, 9 out of 10 compliant patients that completed 3 months of treatment with a ≥ 1:1 ratio KD combined with MCT demonstrated improved Alzheimer’s disease Assessment Scale—cognitive subscale scores [102]. Interestingly, two subsequent randomized studies of MCT or a ketogenic product compared to placebo for 3 to 6 months as well as an observational study administering a ketogenic meal over 3 months reported that the cognitive benefit of ketogenic therapies was greatest in patients who did not have the APOE ε4 allele [103, 104]. In the observational study, cognitive improvement was limited to APOE ε4 negative patients with mild AD only [105]. A recent study of patients with mild-moderate AD treated with 1 month of MCT supplements demonstrated an increase in brain 11C-acetoacetate PET uptake, suggesting ketones from MCT can compensate for the brain glucose deficit observed in AD [106]. In sum, the preliminary clinical evidence suggests that ketogenic therapies including ketogenic diets and/or supplements designed to induce metabolic ketosis may improve cognitive outcomes in patients with AD. However, APOE ε4 genotype and the degree of disease progression may impact response to metabolic ketosis.
Migraine Headache
Migraine headache is a primary disorder of brain excitatory-inhibitory imbalance leading to the periodic activation and sensitization of the trigemino-vascular pain pathway. Experimental evidence suggests that the ketogenic diet may act at different stages of migraine pathophysiology to restore brain metabolism and excitability and counteract inflammatory and oxidative mechanisms [107]. For example, a MCT KD administered to rodents reduced the propagation of cortical spreading depression, the neurophysiological event underpinning migraine aura [108]. Although a clinical case series of KD use in migraine dates back to 1928 with improvement observed in 39% of cases [109], more recent studies including 2 case reports and 2 prospective studies demonstrate KD effectiveness for migraine prophylaxis in adults with benefit ranging from reduction in attack frequency and intensity to migraine cessation [110–113]. Notably, all but 8 of the 117 patients in these collective studies were overweight and treated with a hypocaloric (≤ 800 kcal) ketogenic diet. However, in the larger prospective study comparing 96 patients treated with either a hypocaloric ketogenic diet (≤ 30 g/day carbohydrates) or a nonketogenic standard weight-loss diet during the 1st month of a 6-month dietary treatment, overweight migraineurs treated with the KD showed a significant reduction in attack frequency and analgesic use during the 1st month of the study followed by a modest worsening of migraine control when patients were gradually transitioned to a standard diet over the remaining 5 months of the study. As weight loss was observed in both the KD treatment arm and the control arm and the change in weight persisted for the full 6 month of the study, the early headache benefit achieved with the ketogenic diet appears to be independent of weight-loss [111]. The smaller prospective study included 8 normal weight patients with migraine treated with a normocaloric KD (≤ 15 g/day carbohydrates) who appeared to experience the same reduction in migraine frequency and duration after 1 month of treatment exhibited by their 10 overweight peers treated with a hypocaloric KD, but small sample size did not allow for subgroup analyses [112]. A recent single arm clinical trial evaluating the effect of 12 weeks of MAD (10 g/day carbohydrate limit the 1st month and 20 to 30 g/day carbohydrate limit thereafter) in 18 patients with chronic cluster headache demonstrated either full resolution of headache or at least 50% reduction in attack frequency in 83% (15/18) [114], suggesting the potential benefit of KDs for prophylaxis of other headache phenotypes that share similar mechanisms with migraine headache.
Motor Neuron Disease
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by the degeneration of motor neurons in the spinal cord and brain which leads to progressive motor weakness and ultimately death 2 to 5 years from symptom onset due to respiratory failure [115]. Posited mechanisms of motor neuron degeneration include glutamate excitotoxicity, abnormal protein aggregation, impaired axonal transport, inflammation, oxidative stress, and abnormalities in energy metabolism [116]. Altered glucose uptake has been observed in the brain and spinal cord of ALS patients as well as disturbances in energy metabolism [117–120]. Approaches targeting the metabolic impairments found in ALS through the use of alternative fuels include the Deanna Protocol (a collection of arginine α-ketoglutarate, medium-chain triglycerides, vitamins, and antioxidants), specific medium-chain triglycerides (trioctanoin and triheptanoin) (Fig. 1), and pyruvate dehydrogenase kinase inhibitors [116, 121]. Although these have yet to be studied clinically for ALS, preclinical work in rodent ALS models suggests that these approaches improve mitochondrial metabolism, reduce motor neuron toxicity, improve or delay motor symptoms/disease progression, and in some cases extend survival [122–126]. Controlled clinical trials investigating these approaches are warranted.
Other Neurologic Disorders
Recent studies have begun to explore the use of ketogenic therapies in other adult neurological and psychiatric disorders including Parkinson’s disease, traumatic and ischemic brain and spinal cord injury, mood disorders, and multiple sclerosis. Preclinical work has demonstrated regeneration in motor or cognitive performance with ketogenic therapies in rodent models of neurologic disease. In one study, KD administration improved forelimb motor function in rodents after spinal cord injury with maintenance of the functional benefits when returning to a standard diet after 12 weeks [127]. KD administration for 7 days following traumatic brain injury in adolescent rats improved recovery of memory function with several studies suggesting a possible age-dependent effect and an underlying mechanism of reduced alterations in cell metabolism and cell death [128–130]. KD and ketone body administration have also been shown to protect dopaminergic neurons from degeneration and improve motor function in rodent models of Parkinson’s disease via mechanisms that include improved mitochondrial function and ATP production [131–134]. A pilot study of the use of a KD for 4 weeks in patients with Parkinson’s disease showed a global enhancement in the Unified Parkinson’s Disease Rating Scale, which included motor function, in the 5 patients who completed the study [135].
Ketogenic diets have also been shown to significantly dampen motor disability and memory dysfunction by preventing inflammation and enhancing neuroprotection in a murine model of multiple sclerosis (MS) [136]. A single randomized pilot trial has been reported assessing the safety and feasibility of 6 months of ketogenic diet (< 50 g of carbohydrates/day) therapy compared to standard diet therapy in patients with relapsing-remitting multiple sclerosis [137]. The authors concluded that the KD is safe, feasible with 90% compliance, and potentially effective as the KD cohort displayed clinically meaningful improvements in health-related quality of life (HRQOL) summary scale scores as well as a mild reduction in expanded disability scale status [137]. Recent clinical evidence suggests that the impaired colonic microbiome function observed in patients with multiple sclerosis is normalized by 6 months of ketogenic diet therapy; however, as no specific microbiome pattern was apparent and shifts in bacterial populations were multidirectional, the precise role for microbiome changes in MS pathology remains unclear [138]. Further clinical studies including imaging outcomes, blinded clinical trials, and immune assays are warranted to better define KD efficacy in these conditions.
Interestingly, several studies in adults receiving diet therapy for epilepsy have reported benefits of KD treatment beyond seizure control. These include improvements in arousal, mood, alertness, energy, and concentration [139–141]. Furthermore, quality of life scores tend to increase rather than decrease with diet therapy [142]. Thus, the potential benefits of KDs may extend further than the primary neurologic disorder and also impact comorbid psychiatric and other medical conditions.
Ketogenic Diet Adverse Effects and Contraindications in Adults
As ketogenic diets are being utilized with increasing frequency for adult neurologic disorders, recognition and management of potential adverse effects and contraindications must be considered. The adverse effects most often reported by adults on ketogenic diets for the treatment of epilepsy are weight loss, gastrointestinal effects, and a transient increase in lipids. In clinical studies of KD use in other neurological disorders in adults, similar side effects have been reported although a true estimate of prevalence in these populations is difficult due to the small number of participants, often with lack of control groups for comparison, short duration of follow up, reliance on self-report of adverse effects in some studies, and heterogeneity in KD therapy applied [43, 143]. Gastrointestinal side effects can include constipation, diarrhea (typically with MCT supplementation), nausea, vomiting, and rarely pancreatitis (KD therapies are therefore contraindicated in acute pancreatitis). The side effects can improve with continued diet use and with minor adjustments, rarely necessitating pharmaceutical intervention or diet discontinuation. Strategies utilized by dietitians and nutritionists include recommending multiple small meals throughout the day; increasing fiber, sodium, and fluid intake; and daily exercise. When used, gradual introduction of MCT may reduce gastrointestinal side effects. Given that many adults with neurologic disorders are overweight or obese, weight loss may be a welcomed effect, and for those who are normal or underweight and wish to maintain or gain weight, regulation and monitoring of caloric intake is recommended. Anorexia is a contraindication to using KD therapies.
Fasting serum total cholesterol and low-density lipoprotein (LDL) cholesterol have been shown to increase in adults with epilepsy after initiation of a ketogenic diet and then normalize with continued treatment (after approximately 1 year) or after cessation of treatment [66, 144, 145]. Although the influence of ketogenic diets on cholesterol levels and other markers of cardiovascular disease in adults with neurologic disorders is limited, some reports note improvements in weight, body mass index (BMI) and triglyceride level but a shift in predominant LDL particle size in adults with epilepsy on long-term (at least 12 months) MAD compared to controls [146], whereas other reports note no difference in blood lipid parameters, BMI, blood pressure, or carotid artery thickness after 10 years of KD use [147]. More extensive evidence of ketogenic diet impact on cardiovascular health in adults has been garnered from studies of weight loss comparing low-carbohydrate to low-fat diets. For example, meta-analysis and systemic reviews of randomized controlled trials exploring the effect of a ketogenic or low-carbohydrate diet on cardiovascular risk in overweight or obese participants have generally noted positive effects on cardiovascular health measures with noted reductions in weight, BMI, blood pressure, and triglycerides, no significant change in LDL, and in some instances increased high-density lipoprotein (HDL) cholesterol [148–150]. However, it is worth noting that the macronutrient composition of these low-carbohydrate diets ranged from the traditional Atkins diet (which typically involves a ≤ 20 g/day carbohydrate restriction at induction) to diets permissive of carbohydrate intake of 50 g/day or 30 to 40% of caloric intake. Thus, the effect of more restrictive ketogenic diets on cardiovascular health measures should be further explored in adults of normal weight and with neurological disorders [151]. Elevated fasting lipids prior to KD therapy are not an absolute contraindication to treatment although they should be closely monitored.
Vitamin and mineral deficiencies can also occur when restricting carbohydrates and supplementing with a recommended daily allowance of a multivitamin and minerals can prevent these. This is of particular importance in postmenopausal women and adults that are not ambulatory as prolonged ketonemia can cause osteopenia and osteoporosis through mechanisms that are poorly understood and require further investigation [2, 152, 153]. Lastly, as with any therapy, potential interactions with other treatments are important to consider. Basic management principles (e.g., minimizing carbohydrates and sugar additives in medications, parental and intravenous fluids) are necessary in order to avoid inadvertently bringing the patient out of ketosis. Still, clinical data supporting significant pharmacodynamic or pharmacokinetic interactions between KDs and pharmacological drugs is limited. A recent RCT in adults showed that serum concentrations of AEDs decreased by 16% in the diet group compared to controls (p < 0.0001), including one participant in whom serum concentration of zonisamide decreased 41% and carbamazepine decreased 21%. This patient had an increase in seizures following MAD initiation by greater than 200% [68]. From the pediatric epilepsy literature, serum levels of the most commonly used AEDs, with the exception of valproic acid, do not appear to be significantly altered with concomitant KD use [154–156]. Valproic acid use in rare cases has also interfered with the development of ketosis and contributed to the worsening of secondary carnitine deficiency (which can occur with either KD or valproic acid use), so close monitoring of ketosis and carnitine level is advised and carnitine supplementation is recommended if a deficiency is observed [157, 158]. As the ketogenic diet can also lead to an asymptomatic metabolic acidosis, particularly early after initiation, caution has been advised for concomitant use of carbonic anhydrase inhibitors (acetazolamide, topiramate, and zonisamide) which may worsen acidosis. There is a theoretical higher risk of kidney stones when KDs are combined with carbonic anhydrate inhibitors, as both have been independently associated with nephrolithiasis [159, 160], and increased risk in combination-therapy populations has been demonstrated for zonisamide in some studies of pediatric patients [161], whereas not others [162]. Although dose adjustments for the majority of AEDs are not typically merited, if clinical concern for a medication side effect arises, plasma AED levels should be monitored and adjustments in KD ratio considered [163]. A similar strategy is warranted for any medication used in combination with KD therapy.
Conclusions
Although the neurological conditions described in this review have distinct underlying pathologies, many are caused by a disruption in energy metabolism and exhibit evidence of increased oxidative stress and neuro-inflammation. Diet manipulation could alter the course and outcomes of these and other neurologic disorders that share common pathways. Extensive preclinical work supports the use of metabolic ketosis induced by ketogenic diets and/or exogenous ketone ingestion to prevent or slow progression of physiologic, histological, and biochemical changes leading to neurodegeneration. The scientific evidence presented here from clinical studies of adults with a wide variety of neurological disorders supports KD therapy (as highlighted in Table 1), with the strongest evidence supporting use in epilepsy, Alzheimer’s disease, migraine headache, and glioma, although further clinical investigation using randomized, blinded KD trials in large populations, and in patient subpopulations where feasible, is warranted.
Table 1.
Highlighted clinical studies investigating KD use in comparison to a control group in adults with epilepsy, mild cognitive impairment, migraine, and multiple sclerosis
| Disease | Reference | Study design | Pts (n) | KD type | Duration (months) | Control diet | Results | KD adherence |
|---|---|---|---|---|---|---|---|---|
| Epilepsy | Zare [67] | RCT | 66 | MAD (15 g/day) | 2 | Habitual diet | 35% (12/34) responder rate (≥ 50% seizure reduction) in MAD treatment arm (significant difference in response rate compared to control arm) | 65% (22/34) |
| Kverneland [68] | RCT | 75 | MAD (15-20 g/day) | 3 | Habitual diet | No significant difference in seizure frequency after intervention between groups | 65% (24/37) | |
| MCI | Krikorian [100] | RCT | 23 | KD (5-10% Carbs) | 1.5 | High Carbs (> 50%) | Improved verbal memory performance, weight, fasting glucose, and insulin | 100% (12/12) |
| Migraine | Di Lorenzo [111] | Obs | 96 | Hypocaloric KD (30 g/day, ≤ 800 kcal) | 6 (1 KD) | Hypocaloric standard diet | Improved attack frequency, headache days, and analgesic use during the 1st month | 91% (41/45) |
| Multiple sclerosis | Choi [137] | RCT | 40 | KD (< 50 g/day) | 6 | Habitual diet | Improved health-related quality of life and expanded disability status scale scores | 90% (18/20) |
Carbs = carbohydrates, g = grams, KD = ketogenic diet, MAD = modified Atkins diet, MCI = mild cognitive impairment, Obs = observational study, RCT = randomized controlled trial
Electronic Supplementary Material
(PDF 514 kb)
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.McNally MA, Hartman AL. Ketone bodies in epilepsy. J Neurochem. 2012;121(1):28–35. doi: 10.1111/j.1471-4159.2012.07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cervenka MC, Kossoff EH. Dietary treatment of intractable epilepsy. Continuum (Minneap Minn) [Internet]. 2013;19(June):756–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23739109 [DOI] [PMC free article] [PubMed]
- 3.Zupec-Kania BA, Spellman E. An overview of the ketogenic diet for pediatric epilepsy. Nutr Clin Pract. 1998;23(6):589–96. doi: 10.1177/0884533608326138. [DOI] [PubMed] [Google Scholar]
- 4.Kossoff EH, Rowley H, Sinha SR, Vining EPG. A prospective study of the modified Atkins diet for intractable epilepsy in adults. Epilepsia. 2008;49(2):316–9. doi: 10.1111/j.1528-1167.2007.01256.x. [DOI] [PubMed] [Google Scholar]
- 5.Cervenka MC, Terao NN, Bosarge JL, Henry BJ, Klees AA, Morrison PF, et al. E-mail management of the modified Atkins diet for adults with epilepsy is feasible and effective. Epilepsia. 2012;53(4):728–32. doi: 10.1111/j.1528-1167.2012.03406.x. [DOI] [PubMed] [Google Scholar]
- 6.Neal EG, Cross JH. Efficacy of dietary treatments for epilepsy. J Hum Nutr Diet. 2010;23(2):113–9. doi: 10.1111/j.1365-277X.2010.01043.x. [DOI] [PubMed] [Google Scholar]
- 7.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr [Internet]. Nature Publishing Group; 2013;67(8):789–96. Available from: 10.1038/ejcn.2013.116 [DOI] [PMC free article] [PubMed]
- 8.McDonald TJW, Cervenka MC. Ketogenic diets for adults with highly refractory epilepsy. Epilepsy Curr. 2017;17(6). [DOI] [PMC free article] [PubMed]
- 9.Simeone TA, Simeone KA, Stafstrom CE, Rho JM. Do ketone bodies mediate the anti-seizure effects of the ketogenic diet? Neuropharmacology [Internet]. Elsevier Ltd; 2018;133:233–41. Available from: 10.1016/j.neuropharm.2018.01.011 [DOI] [PMC free article] [PubMed]
- 10.Tanner GR, Lutas A, Martinez-Francois JR, Yellen G. Single KATP Channel Opening in Response to Action Potential Firing in Mouse Dentate Granule Neurons. J Neurosci [Internet]. 2011;31(23):8689–96. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.5951-10.2011 [DOI] [PMC free article] [PubMed]
- 11.Ma W, Berg J, Yellen G. Ketogenic Diet Metabolites Reduce Firing in Central Neurons by Opening KATP Channels. J Neurosci [Internet]. 2007;27(14):3618–25. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0132-07.2007 [DOI] [PMC free article] [PubMed]
- 12.Tan KN, Carrasco-Pozo C, McDonald TS, Puchowicz M, Borges K. Tridecanoin is anticonvulsant, antioxidant, and improves mitochondrial function. J Cereb Blood Flow Metab. 2017;37(6):2035–48. doi: 10.1177/0271678X16659498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wlaź P, Socała K, Nieoczym D, Zarnowski T, Zarnowska I, Czuczwar SJ, et al. Acute anticonvulsant effects of capric acid in seizure tests in mice. Prog Neuro-Psychopharmacology Biol Psychiatry. 2015;57:110–6. doi: 10.1016/j.pnpbp.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. 2016;139(2):431–43. doi: 10.1093/brain/awv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erecińska M, Nelson D, Daikhin Y, Yudkoff M. Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium, and ketone bodies. J Neurochem [Internet]. 1996;67(6):2325–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8931464 [DOI] [PubMed]
- 16.Melø TM, Nehlig A, Sonnewald U. Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48(6–7):498–507. doi: 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Yudkoff M, Daikhin Y, Melø TM, Nissim I, Sonnewald U, Nissim I. The Ketogenic Diet and Brain Metabolism of Amino Acids: Relationship to the Anticonvulsant Effect. Annu Rev Nutr [Internet]. 2007;27(1):415–30. Available from: http://www.annualreviews.org/doi/10.1146/annurev.nutr.27.061406.093722 [DOI] [PMC free article] [PubMed]
- 18.Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, et al. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy—Exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49(4):615–9. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- 19.Dahlin M, Elfving Å, Ungerstedt U, Åmark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64(3):115–25. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci [Internet]. Elsevier Ltd; 2013;36(1):32–40. 10.1016/j.tins.2012.11.005 [DOI] [PMC free article] [PubMed]
- 21.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev [Internet]. Elsevier B.V.; 2009;59(2):293–315. 10.1016/j.brainresrev.2008.09.002 [DOI] [PMC free article] [PubMed]
- 22.Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The Ketogenic Diet Increases Mitochondrial Uncoupling Protein Levels and Activity. 2004;576–80. [DOI] [PubMed]
- 23.Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial Biogenesis in the Anticonvulsant Mechanism of the Ketogenic Diet. 2006;5:223–35. [DOI] [PubMed]
- 24.Yamada KA. Calorie restriction and glucose regulation. Epilepsia. 2008;49(SUPPL. 8):94–6. doi: 10.1111/j.1528-1167.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- 25.McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52(3):7–11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis [Internet]. Elsevier Inc.; 2010;40(1):238–44. 10.1016/j.nbd.2010.05.030 [DOI] [PMC free article] [PubMed]
- 27.Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res [Internet]. 2014;55(11):2211–28. Available from: http://www.jlr.org/lookup/doi/10.1194/jlr.R048975 [DOI] [PMC free article] [PubMed]
- 28.Kim DY, Simeone KA, Simeone TA, Pandya JD, Wilke JC, Ahn Y, et al. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann Neurol. 2015;78(1):77–87. doi: 10.1002/ana.24424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimazu T, Hirschey M, Newman J, He W, Shirakawa K, Moan N Le, et al. Suppression of Oxidative Stress by. 2013;(January):211–4. [DOI] [PMC free article] [PubMed]
- 30.Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, et al. Ketogenic diet-induced peroxisome proliferator-activated receptor-γ activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp Neurol. 2011;232(2):195–202. doi: 10.1016/j.expneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Koppel SJ, Swerdlow RH. Neurochemistry International Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem Int [Internet]. Elsevier Ltd; 2017; 10.1016/j.neuint.2017.05.019 [DOI] [PMC free article] [PubMed]
- 32.Ruskin DN, Kawamura M, Masino SA. Reduced Pain and Inflammation in Juvenile and Adult Rats Fed a Ketogenic Diet. 2009;4(12):1–6. [DOI] [PMC free article] [PubMed]
- 33.Yang X, Cheng B. Neuroprotective and Anti-inflammatory Activities of Ketogenic Diet on MPTP-induced Neurotoxicity. 2010;145–53. [DOI] [PubMed]
- 34.Youm Y, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med [Internet]. Nature Publishing Group; 2015;21(3):263–9. 10.1038/nm.3804 [DOI] [PMC free article] [PubMed]
- 35.Cullingford TE. The ketogenic diet; fatty acids, fatty acid-activated receptors and neurological disorders. Prostaglandins Leukot Essent Fat Acids. 2004;70(3):253–64. doi: 10.1016/j.plefa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Müller-Fielitz H, et al. The b-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5(May):1–11. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 37.Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer. 2003;89(7):1375–82. doi: 10.1038/sj.bjc.6601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marsh J, Mukherjee P, Seyfried TN. Drug/diet synergy for managing malignant astrocytoma in mice: 2-deoxy-D-glucose and the restricted ketogenic diet. Nutr Metab. 2008;5(1):1–5. doi: 10.1186/1743-7075-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poff A, Koutnik AP, Egan KM, Sahebjam S, D’Agostino D, Kumar NB. Targeting the Warburg effect for cancer treatment: Ketogenic diets for management of glioma. Semin Cancer Biol. 2018;(June 2017). [DOI] [PMC free article] [PubMed]
- 40.Woolf EC, Syed N, Scheck AC. Tumor Metabolism, the Ketogenic Diet and β-Hydroxybutyrate: Novel Approaches to Adjuvant Brain Tumor Therapy. Front Mol Neurosci [Internet]. 2016;9(November):1–11. Available from: http://journal.frontiersin.org/article/10.3389/fnmol.2016.00122/full [DOI] [PMC free article] [PubMed]
- 41.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7(5):1–7. doi: 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woolf EC, Curley KL, Liu Q, Turner GH, Charlton JA, Preul MC, et al. The ketogenic diet alters the hypoxic response and affects expression of proteins associated with angiogenesis, invasive potential and vascular permeability in a mouse glioma model. PLoS One. 2015;10(6):1–18. doi: 10.1371/journal.pone.0130357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winter SF, Loebel F, Dietrich J. Role of ketogenic metabolic therapy in malignant glioma: A systematic review. Crit Rev Oncol Hematol [Internet]. Elsevier Ireland Ltd; 2017;112:41–58. 10.1016/j.critrevonc.2017.02.016 [DOI] [PubMed]
- 44.Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer [Internet]. BMC Cancer; 2016;16(1):1–10. 10.1186/s12885-016-2337-7 [DOI] [PMC free article] [PubMed]
- 45.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer [Internet]. Nature Publishing Group; 2011;11(2):85–95. 10.1038/nrc2981 [DOI] [PubMed]
- 46.Bowers LW, Rossi EL, O’Flanagan CH, De Graffenried LA, Hursting SD. The role of the insulin/IGF system in cancer: Lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol (Lausanne) 2015;6(MAY):1–16. doi: 10.3389/fendo.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging [Internet]. Elsevier Inc; 2013;34(6):1530–9. 10.1016/j.neurobiolaging.2012.11.023 [DOI] [PMC free article] [PubMed]
- 48.Studzinski CM, MacKay WA, Beckett TL, Henderson ST, Murphy MP, Sullivan PG, et al. Induction of ketosis may improve mitochondrial function and decrease steady-state amyloid-β precursor protein (APP) levels in the aged dog. Brain Res. 2008;1226:209–17. doi: 10.1016/j.brainres.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Van Der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab. 2005;2:1–8. doi: 10.1186/1743-7075-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin JX, Maalouf M, Han P, Zhao M, Gao M, Dharshaun T, et al. Ketones block amyloid entry and improve cognition in an Alzheimer’s model. Neurobiol Aging. 2016;39:25–37. doi: 10.1016/j.neurobiolaging.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 51.Xu K, Sun X, Eroku BO, Tsipis CP, Puchowicz MA, Lamanna JC. Oxygen Transport to Tissue XXXI. 2010;662:71–5. Available from: http://link.springer.com/10.1007/978-1-4419-1241-1 [DOI] [PMC free article] [PubMed]
- 52.Gilbert DL, Pyzik PL, Freeman JM. The ketogenic diet: seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. J Child Neurol. 2000;15(12):787–90. doi: 10.1177/088307380001501203. [DOI] [PubMed] [Google Scholar]
- 53.van Delft R, Lambrechts D, Verschuure P, Hulsman J, Majoie M. Blood beta-hydroxybutyrate correlates better with seizure reduction due to ketogenic diet than do ketones in the urine. Seizure. 2010;19(1):36–9. doi: 10.1016/j.seizure.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving the ketogenic diet: Recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50(2):304–17. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- 55.Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics [Internet]. 2009;6(2):406–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19332337%0A, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4071763 [DOI] [PMC free article] [PubMed]
- 56.Buchhalter JR, D’Alfonso S, Connolly M, Fung E, Michoulas A, Sinasac D, et al. The relationship between D-beta-hydroxybutyrate blood concentrations and seizure control in children treated with the ketogenic diet for medically intractable epilepsy. Epilepsia Open [Internet]. 2017;2(3):317–21. Available from: http://doi.wiley.com/10.1002/epi4.12058 [DOI] [PMC free article] [PubMed]
- 57.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell [Internet]. Elsevier; 2018;173(7):1728–1741.e13. 10.1016/j.cell.2018.04.027 [DOI] [PMC free article] [PubMed]
- 58.Martin K, Jackson CF, Levy RG, Cooper PN. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2016;(2). [DOI] [PubMed]
- 59.Barborka CJ. Epilepsy in adults: results of treatment by ketogenic diet in one hundred cases. Arch Neurol Psych. 1930;23:904–14. doi: 10.1001/archneurpsyc.1930.02220110066004. [DOI] [Google Scholar]
- 60.Barborka CJ. Ketogenic diet treatment of epilepsy in adults. JAMA. 1928;9(2):73–8. doi: 10.1001/jama.1928.02700020007003. [DOI] [Google Scholar]
- 61.Ye F, Li XJ, Jiang WL, Sun HB, Liu J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: A meta-analysis. J Clin Neurol. 2015;11(1):26–31. doi: 10.3988/jcn.2015.11.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cervenka MC, Henry BJ, Felton EA, Patton K, Kossoff EH. Establishing an Adult Epilepsy Diet Center: Experience, efficacy and challenges. Epilepsy Behav. 2016;58:61–8. doi: 10.1016/j.yebeh.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 63.Williams T, Cervenka MC. The role for ketogenic diets in epilepsy and status epilepticus in adults. Clin Neurophysiol Pract [Internet]. 2017;2:154–60. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2467981X1730015X [DOI] [PMC free article] [PubMed]
- 64.Liu H, Yang Y, Wang Y, Tang H, Zhang F, Zhang Y, et al. Ketogenic diet for treatment of intractable epilepsy in adults: A meta-analysis of observational studies. Epilepsia Open [Internet]. 2018;3(1):9–17. Available from: http://doi.wiley.com/10.1002/epi4.12098 [DOI] [PMC free article] [PubMed]
- 65.Kverneland M, Selmer KK, Nakken KO, Iversen PO, Taubøll E. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav [Internet]. Elsevier Inc.; 2015;53(2010):197–201. 10.1016/j.yebeh.2015.10.021 [DOI] [PubMed]
- 66.Klein P, Janousek J, Barber A, Weissberger R. Ketogenic diet treatment in adults with refractory epilepsy. Epilepsy Behav [Internet]. Elsevier Inc.; 2010;19(4):575–9. 10.1016/j.yebeh.2010.09.016 [DOI] [PubMed]
- 67.Zare M, Okhovat AA, Esmaillzadeh A, Mehvari J, Najafi MR, Saadatnia M. Modified atkins diet in adult patients with refractory epilepsy: A controlled randomized clinical trial. Iran J Neurol [Internet]. 2017;16(2):72–7. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed17&NEWS=N&AN=72155838 [PMC free article] [PubMed]
- 68.Kverneland M, Molteberg E, Iversen PO, Veierød MB, Taubøll E, Selmer KK, et al. Effect of modified Atkins diet in adults with drug-resistant focal epilepsy: A randomized clinical trial. 2018;(May):1–10. [DOI] [PubMed]
- 69.Bodenant M, Moreau C, Sejourné C, Auvin S, Delval A, Cuisset J-M, et al. [Interest of the ketogenic diet in a refractory status epilepticus in adults]. Rev Neurol (Paris) [Internet]. 2008;164(2):194–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18358881 [DOI] [PubMed]
- 70.Wusthoff CJ, Kranick SM, Morley JF, Bergqvist AGC. The ketogenic diet in treatment of two adults with prolonged nonconvulsive status epilepticus. Epilepsia. 2010;51(6):1083–5. doi: 10.1111/j.1528-1167.2009.02388.x. [DOI] [PubMed] [Google Scholar]
- 71.Martikainen MH, Paivarinta M, Jaaskelainen S, Majamaa K., Martikainen M.H. PM. JS. MK. Successful treatment of POLG-related mitochondrial epilepsy. Epileptic Disord. 2012;14(4):438–41. [DOI] [PubMed]
- 72.Nam SH, Lee BL, Lee CG, Yu HJ, Joo EY, Lee J, et al. The role of ketogenic diet in the treatment of refractory status epilepticus. Epilepsia. 2011;52(11):e181–4. doi: 10.1111/j.1528-1167.2011.03289.x. [DOI] [PubMed] [Google Scholar]
- 73.Strzelczyk A, Reif PS, Bauer S, Belke M, Oertel WH, Knake S, et al. Intravenous initiation and maintenance of ketogenic diet: Proof of concept in super-refractory status epilepticus. Seizure. 2013;22(7):581–3. doi: 10.1016/j.seizure.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 74.Thakur KT, Probasco JC, Hocker SE, Roehl K, Henry B, Kossoff EH, et al. Ketogenic diet for adults in super-refractory status epilepticus. Neurology. 2014;82(8):665–70. doi: 10.1212/WNL.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hocker SE, Britton JW, Mandrekar JN, Wijdicks EFM, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA Neurol [Internet]. 2013;70(1):72–7. Available from: http://archneur.jamanetwork.com/article.aspx?articleid=1557592 [DOI] [PubMed]
- 76.Cervenka MC, Hocker SE, Koenig M, Bar B, Henry-Barron B, Kossoff EH, et al. A phase I/II multicenter ketogenic diet study for adult super-refractory status epilepticus. Neurology. 2017;In Press:938–43. [DOI] [PMC free article] [PubMed]
- 77.Shorvon S, Ferlisi M. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain. 2012;135(8):2314–28. doi: 10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 78.Wen P, Kesari S. Malignant Gliomas in Adults—NEJM. Malig gliomas adults [Internet]. 2008;492–507. Available from: http://www.nejm.org.lib-proxy.fullerton.edu/doi/full/10.1056/NEJMra0708126 [DOI] [PubMed]
- 79.Carlsson SK, Brothers SP, Wahlestedt C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med [Internet]. 2014;6(11):1359–70. Available from: http://embomolmed.embopress.org/cgi/doi/10.15252/emmm.201302627 [DOI] [PMC free article] [PubMed]
- 80.Warburg O. On the origin of cancer cells. Science (80- ) 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 81.Seyfried TN, Flores RE, Poff AM, D’Agostino DP. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis. 2014;35(3):515–27. doi: 10.1093/carcin/bgt480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jelluma N. Glucose Withdrawal Induces Oxidative Stress followed by Apoptosis in Glioblastoma Cells but not in Normal Human Astrocytes. Mol Cancer Res [Internet]. 2006;4(5):319–30. Available from: http://mcr.aacrjournals.org/cgi/doi/10.1158/1541-7786.MCR-05-0061 [DOI] [PubMed]
- 83.Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27(7):1082–6. doi: 10.1200/JCO.2008.19.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Ruiz AJ, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63(2):286–91. doi: 10.1227/01.NEU.0000315282.61035.48. [DOI] [PubMed] [Google Scholar]
- 85.Mayer A, Vaupel P, Struss HG, Giese A, Stockinger M, Schmidberger H. Ausgeprägt negativer prognostischer Einfluss von hyperglykämischen Episoden während der adjuvanten Radiochemotherapie des Glioblastoma multiforme. Strahlentherapie und Onkol. 2014;190(10):933–8. doi: 10.1007/s00066-014-0696-z. [DOI] [PubMed] [Google Scholar]
- 86.Adeberg S, Bernhardt D, Foerster R, Bostel T, Koerber SA, Mohr A, et al. The influence of hyperglycemia during radiotherapy on survival in patients with primary glioblastoma. Acta Oncol (Madr). 2016;55(2):201–7. doi: 10.3109/0284186X.2015.1043397. [DOI] [PubMed] [Google Scholar]
- 87.Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case Report. Nutr Metab. 2010;7:1–7. doi: 10.1186/1743-7075-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, Evans JJ, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117(1):125–31. doi: 10.1007/s11060-014-1362-0. [DOI] [PubMed] [Google Scholar]
- 89.Schwartz K, Chang HT, Nikolai M, Pernicone J, Rhee S, Olson K, et al. Treatment of glioma patients with ketogenic diets: report of two cases treated with an IRB-approved energy-restricted ketogenic diet protocol and review of the literature. Cancer Metab [Internet]. 2015;3(1):3. Available from: http://www.cancerandmetabolism.com/content/3/1/3 [DOI] [PMC free article] [PubMed]
- 90.Rieger J, Bähr O, Maurer GD, Hattingen E, Franz K, Brucker D, et al. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;45(6):1843–52. doi: 10.3892/ijo.2014.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Elsakka AMA, Bary MA, Abdelzaher E, Elnaggar M, Kalamian M, Mukherjee P, et al. Management of Glioblastoma Multiforme in a Patient Treated With Ketogenic Metabolic Therapy and Modified Standard of Care: A 24-Month Follow-Up. Front Nutr [Internet]. 2018;5(March):1–11. Available from: http://journal.frontiersin.org/article/10.3389/fnut.2018.00020/full [DOI] [PMC free article] [PubMed]
- 92.Wilkins HM, Swerdlow RH. Amyloid precursor protein processing and bioenergetics. Brain Res Bull. Elsevier Inc.; 2017;133:71–9. [DOI] [PMC free article] [PubMed]
- 93.Castellano C-A, Nugent S, Paquet N, Tremblay S, Bocti C, Lacombe G, et al. Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer’s disease dementia. J Alzheimer’s Dis. 2015;43(4):1343–53. doi: 10.3233/JAD-141074. [DOI] [PubMed] [Google Scholar]
- 94.Winkler EA, Nishida Y, Sagare AP, Rege S V., Bell RD, Perlmutter D, et al. GLUT1 reductions exacerbate Alzheimer’s disease vasculo-neuronal dysfunction and degeneration. Nat Neurosci. Nature Publishing Group; 2015;18(4):521–30. [DOI] [PMC free article] [PubMed]
- 95.Taylor MK, Sullivan DK, Swerdlow RH, Vidoni ED, Morris JK, Mahnken JD, et al. A high-glycemic diet is associated with cerebral amyloid burden in cognitively normal older adults. Am J Clin Nutr. 2017;106(6):1463–70. doi: 10.3945/ajcn.117.162263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de la Monte SM. Insulin Resistance and Neurodegeneration: Progress Towards the Development of New Therapeutics for Alzheimer’s Disease. Drugs. Springer International Publishing; 2017;77(1):47–65. [DOI] [PMC free article] [PubMed]
- 97.Gaspar JM, Baptista FI, MacEdo MP, Ambrósio AF. Inside the Diabetic Brain: Role of Different Players Involved in Cognitive Decline. ACS Chem Neurosci. 2016;7(2):131–42. doi: 10.1021/acschemneuro.5b00240. [DOI] [PubMed] [Google Scholar]
- 98.Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, et al. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25(3):311–4. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 99.Ota M, Matsuo J, Ishida I, Hattori K, Teraishi T, Tonouchi H, et al. Effect of a ketogenic meal on cognitive function in elderly adults: potential for cognitive enhancement. Psychopharmacology (Berl) [Internet]. Psychopharmacology; 2016;233(21–22):3797–802. 10.1007/s00213-016-4414-7 [DOI] [PubMed]
- 100.Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. Elsevier Inc.; 2012;33(2):425.e19–425.e27. [DOI] [PMC free article] [PubMed]
- 101.Newport MT, Vanitallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: Use of ketone ester in a case of Alzheimer’s disease. Alzheimer’s Dement. 2015;11(1):99–103. doi: 10.1016/j.jalz.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Taylor MK, Sullivan DK, Mahnken JD, Burns JM, Swerdlow RH. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement Transl Res Clin Interv. 2018;4:28–36. doi: 10.1016/j.trci.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab (Lond) [Internet]. 2009;6(1):31. Available from: http://nutritionandmetabolism.biomedcentral.com/articles/10.1186/1743-7075-6-31 [DOI] [PMC free article] [PubMed]
- 104.Rebello CJ, Keller JN, Liu AG, Johnson WD, Greenway FL. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: A randomized controlled trial. BBA Clin. 2015;3:123–5. doi: 10.1016/j.bbacli.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohnuma T, Toda A, Kimoto A, Takebayashi Y, Higashiyama R, Tagata Y, et al. Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: A prospective, open-label pilot study. Clin Interv Aging. 2016;11:29–36. doi: 10.2147/CIA.S95362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Croteau E, Castellano C-A, Richard MA, Fortier M, Nugent S, Lepage M, et al. Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer’s Disease. J Alzheimers Dis. 2018;1–2. [DOI] [PubMed]
- 107.Barbanti P, Fofi L, Aurilia C, Egeo G, Caprio M. Ketogenic diet in migraine: rationale, findings and perspectives. Neurol Sci. 2017;38:111–5. doi: 10.1007/s10072-017-2889-6. [DOI] [PubMed] [Google Scholar]
- 108.de Almeida Rabello Oliveira M, da Rocha AT, de Oliveira SL, de Melo Lucena AL, de Lira CEPR, Soares AA, et al. Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats. Neurosci Lett. 2008;434(1):66–70. doi: 10.1016/j.neulet.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 109.Schnabel T. An experience with a ketogenic dietary in migraine. Ann Intern Med. 1928;2:341–7. doi: 10.7326/0003-4819-2-4-341. [DOI] [Google Scholar]
- 110.Strahlman R. Can Ketosis Help Migraine Sufferers? A Case Report. Headache. 2006;46:182. doi: 10.1111/j.1526-4610.2006.00321_5.x. [DOI] [PubMed] [Google Scholar]
- 111.Di Lorenzo C, Coppola G, Sirianni G, Di Lorenzo G, Bracaglia M, Di Lenola D, et al. Migraine improvement during short lasting ketogenesis: A proof-of-concept study. Eur J Neurol. 2015;22(1):170–7. doi: 10.1111/ene.12550. [DOI] [PubMed] [Google Scholar]
- 112.Di Lorenzo C, Coppola G, Bracaglia M, Di Lenola D, Evangelista M, Sirianni G, et al. Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J Headache Pain. 2016;17(1):2377. doi: 10.1186/s10194-016-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Di Lorenzo C, Curra A, Sirianni G, Coppola G, Bracaglia M, Cardillo A, et al. Diet transiently improves migraine in twin sisters: possible role of ketogenesis? Funct Neurol. 2013;28(4):305–8. [PMC free article] [PubMed] [Google Scholar]
- 114.Di Lorenzo C, Coppola G, Di Lenola D, Evangelista M, Sirianni G, Rossi P, et al. Efficacy of modified atkins ketogenic diet in chronic cluster headache: An open-label, single-arm, clinical trial. Front Neurol. 2018;9(FEB). [DOI] [PMC free article] [PubMed]
- 115.Körner S, Kollewe K, Ilsemann J, Müller-Heine A, Dengler R, Krampfl K, et al. Prevalence and prognostic impact of comorbidities in amyotrophic lateral sclerosis. Eur J Neurol. 2013;20(4):647–54. doi: 10.1111/ene.12015. [DOI] [PubMed] [Google Scholar]
- 116.Tefera TW, Tan KN, McDonald TS, Borges K. Alternative Fuels in Epilepsy and Amyotrophic Lateral Sclerosis. Neurochem Res. 2017;42(6):1610–20. doi: 10.1007/s11064-016-2106-7. [DOI] [PubMed] [Google Scholar]
- 117.Cistaro A, Valentini MC, Chiò A, Nobili F, Calvo A, Moglia C, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: A FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging. 2012;39(2):251–9. doi: 10.1007/s00259-011-1979-6. [DOI] [PubMed] [Google Scholar]
- 118.Ludolph AC, Langen KJ, Regard M, Herzog H, Kemper B, Kuwert T, et al. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand [Internet]. 1992;85(2):81–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1574993 [DOI] [PubMed]
- 119.Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10(1):75–82. doi: 10.1016/S1474-4422(10)70224-6. [DOI] [PubMed] [Google Scholar]
- 120.Körner S, Hendricks M, Kollewe K, Zapf A, Dengler R, Silani V, et al. Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BioMed Cent. 2013;13(84):83. doi: 10.1186/1471-2377-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fournier C, Bedlack R, Hardiman O, Heiman-Patterson T, Gutmann L, Bromberg M, et al. ALS untangled no. 20: The Deanna Protocol. Amyotroph Lateral Scler Front Degener. 2013;14(4):319–23. doi: 10.3109/21678421.2013.788405. [DOI] [PubMed] [Google Scholar]
- 122.Miquel E, Cassina A, Martínez-Palma L, Bolatto C, Trías E, Gandelman M, et al. Modulation of astrocytic mitochondrial function by dichloroacetate improves survival and motor performance in inherited amyotrophic lateral sclerosis. PLoS One. 2012;7(4):1–9. doi: 10.1371/journal.pone.0034776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao W, Varghese M, Vempati P, Dzhun A, Cheng A, Wang J, et al. Caprylic Triglyceride as a Novel Therapeutic Approach to Effectively Improve the Performance and Attenuate the Symptoms Due to the Motor Neuron Loss in ALS Disease. PLoS One. 2012;7(11):1–8. doi: 10.1371/journal.pone.0049191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ari C, Poff AM, Held HE, Landon CS, Goldhagen CR, Mavromates N, et al. Metabolic therapy with Deanna Protocol supplementation delays disease progression and extends survival in amyotrophic lateral sclerosis (ALS) mouse model. PLoS One. 2014;9(7). [DOI] [PMC free article] [PubMed]
- 125.Tefera TW, Wong Y, Barkl-Luke ME, Ngo ST, Thomas NK, McDonald TS, et al. Triheptanoin protects motor neurons and delays the onset of motor symptoms in a mouse model of amyotrophic lateral sclerosis. PLoS One. 2016;11(8):1–24. doi: 10.1371/journal.pone.0161816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, et al. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:1–10. doi: 10.1186/1471-2202-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Streijger F, Plunet WT, Lee JHT, Liu J, Lam CK, Park S, et al. Ketogenic Diet Improves Forelimb Motor Function after Spinal Cord Injury in Rodents. PLoS One [Internet]. 2013;8(11):e78765. 10.1371/journal.pone.0078765 [DOI] [PMC free article] [PubMed]
- 128.Appelberg KS, Hovda DA, Prins ML. The Effects of a Ketogenic Diet on Behavioral Outcome after Controlled Cortical Impact Injury in the Juvenile and Adult Rat. J Neurotrauma [Internet]. 2009;26(4):497–506. Available from: http://www.liebertonline.com/doi/abs/10.1089/neu.2008.0664 [DOI] [PMC free article] [PubMed]
- 129.Prins ML, Fujima LS, Hovda DA. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res. 2005;82(3):413–20. doi: 10.1002/jnr.20633. [DOI] [PubMed] [Google Scholar]
- 130.Hu ZG, Wang HD, Qiao L, Yan W, Tan QF, Yin HX. The protective effect of the ketogenic diet on traumatic brain injury-induced cell death in juvenile rats. Brain Inj. 2009;23(5):459–65. doi: 10.1080/02699050902788469. [DOI] [PubMed] [Google Scholar]
- 131.Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL. D-b-Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Pnas. 2000;97(10):5440–4. doi: 10.1073/pnas.97.10.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheng B, Yang X, An L, Gao B, Liu X, Liu S. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res [Internet]. Elsevier B.V.; 2009;1286:25–31. 10.1016/j.brainres.2009.06.060 [DOI] [PubMed]
- 133.Shaafi S, Najmi S, Aliasgharpour H, Mahmoudi J, Sadigh-Etemad S, Farhoudi M, et al. The efficacy of the ketogenic diet on motor functions in Parkinson’s disease: A rat model. Iran J Neurol [Internet]. 2016;15(2):63–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27326359%0A, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4912670 [PMC free article] [PubMed]
- 134.Tieu K, Ramasamy R, Przedborski S, Tieu K, Perier C, Caspersen C, et al. D-b-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease Find the latest version : D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. 2003;112(6):892–901. [DOI] [PMC free article] [PubMed]
- 135.VanItallie T, Nonas C, Di Rocco A, Boyar N, Hymans K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: A. Neurology. 2005;64:728–30. doi: 10.1212/01.WNL.0000152046.11390.45. [DOI] [PubMed] [Google Scholar]
- 136.Kim DY, Hao J, Liu R, Turner G, Shi F, Rho JM. Inflammation-Mediated Memory Dysfunction and Effects of a Ketogenic Diet in a Murine Model of Multiple Sclerosis. 2012;7(5). [DOI] [PMC free article] [PubMed]
- 137.Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, et al. A Diet Mimicking Fasting Promotes Regeneration and Reduces Autoimmunity and Multiple Sclerosis Symptoms. Cell Rep [Internet]. The Author(s); 2016;15(10):2136–46. 10.1016/j.celrep.2016.05.009 [DOI] [PMC free article] [PubMed]
- 138.Swidsinski A, Dörffel Y, Loening-Baucke V, Gille C, Göktas Ö, Reißhauer A, et al. Reduced mass and diversity of the colonic microbiome in patients with multiple sclerosis and their improvement with ketogenic diet. Front Microbiol. 2017;8(JUN):1–9. doi: 10.3389/fmicb.2017.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sirven J, Whedon B, Caplan D, Liporace J, Glosser D, O’Dwyer J, et al. The ketogenic diet for intractable epilepsy in adults: preliminary results. Epilepsia [Internet]. 1999;40(12):1721–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10612335 [DOI] [PubMed]
- 140.Schoeler NE, Wood S, Aldridge V, Sander JW, Cross JH, Sisodiya SM. Ketogenic dietary therapies for adults with epilepsy: Feasibility and classification of response. Epilepsy Behav [Internet]. Elsevier Inc.; 2014;37:77–81. 10.1016/j.yebeh.2014.06.007 [DOI] [PubMed]
- 141.Carrette E, Vonck K, de Herdt V, Dewaele I, Raedt R, Goossens L, et al. A pilot trial with modified Atkins’ diet in adult patients with refractory epilepsy. Clin Neurol Neurosurg. 2008;110(8):797–803. doi: 10.1016/j.clineuro.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 142.Lambrechts DAJE, Wielders LHP, Aldenkamp AP, Kessels FGH, de Kinderen RJA, Majoie MJM. The ketogenic diet as a treatment option in adults with chronic refractory epilepsy: Efficacy and tolerability in clinical practice. Epilepsy Behav [Internet]. Elsevier Inc.; 2012;23(3):310–4. 10.1016/j.yebeh.2012.01.002 [DOI] [PubMed]
- 143.Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-Oxidant and Anti-Inflammatory Activity of Ketogenic Diet: New Perspectives for Neuroprotection in Alzheimer’s Disease. Antioxidants [Internet]. 2018;7(5):63. Available from: http://www.mdpi.com/2076-3921/7/5/63 [DOI] [PMC free article] [PubMed]
- 144.Mosek A, Natour H, Neufeld MY, Shiff Y, Vaisman N. Ketogenic diet treatment in adults with refractory epilepsy: A prospective pilot study. Seizure. 2009;18(1):30–3. doi: 10.1016/j.seizure.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 145.Cervenka MC, Patton K, Eloyan A, Henry B, Kossoff EH. The impact of the modified Atkins diet on lipid profiles in adults with epilepsy. Nutr Neurosci [Internet]. 2016;19(3):131–7. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L609460799%5Cn, 10.1179/1476830514Y.0000000162 [DOI] [PubMed]
- 146.McDonald TJW, Ratchford EV, Henry-Barron BJ, Kossoff EH, Cervenka MC. Impact of the modified Atkins diet on cardiovascular health in adults with epilepsy. Epilepsy Behav. 2018;79. [DOI] [PubMed]
- 147.Heussinger N, Della Marina A, Beyerlein A, Leiendecker B, Hermann-Alves S, Dalla Pozza R, et al. 10 patients, 10 years—Long term follow-up of cardiovascular risk factors in Glut1 deficiency treated with ketogenic diet therapies: A prospective, multicenter case series. Clin Nutr. 2017;1–6. [DOI] [PubMed]
- 148.Santos FL, Esteves SS, da Costa Pereira A, Yancy WS, Nunes JPL. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors. Obes Rev. 2012;13(11):1048–66. doi: 10.1111/j.1467-789X.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 149.Bueno NB, De Melo ISV, De Oliveira SL, Da Rocha Ataide T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of Randomised controlled trials. Br J Nutr. 2013;110(7):1178–87. doi: 10.1017/S0007114513000548. [DOI] [PubMed] [Google Scholar]
- 150.Naude CE, Schoonees A, Senekal M, Young T, Garner P, Volmink J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: A systematic review and meta-analysis. PLoS One. 2014;9(7). [DOI] [PMC free article] [PubMed]
- 151.Iacovides S, Meiring RM. The effect of a ketogenic diet versus a high-carbohydrate, low-fat diet on sleep, cognition, thyroid function, and cardiovascular health independent of weight loss: Study protocol for a randomized controlled trial. Trials. Trials. 2018;19(1):1–9. doi: 10.1186/s13063-018-2462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mackay MT, Bicknell-Royle J, Nation J, Humphrey M, Harvey AS. The ketogenic diet in refractory childhood epilepsy. J Paediatr Child Health. 2005;41(7):353–7. doi: 10.1111/j.1440-1754.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 153.Bergqvist AGC, Schall JI, Stallings VA, Zemel BS. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am J Clin Nutr. 2008;88(6):1678–84. doi: 10.3945/ajcn.2008.26099. [DOI] [PubMed] [Google Scholar]
- 154.Heo G, Kim SH, Chang MJ. Effect of ketogenic diet and other dietary therapies on anti-epileptic drug concentrations in patients with epilepsy. J Clin Pharm Ther. 2017;42(6):758–64. doi: 10.1111/jcpt.12578. [DOI] [PubMed] [Google Scholar]
- 155.Coppola G, Verrotti A, D’Aniello A, Arcieri S, Operto FF, Della Corte R, et al. Valproic acid and phenobarbital blood levels during the first month of treatment with the ketogenic diet. Acta Neurol Scand. 2010;122(4):303–7. doi: 10.1111/j.1600-0404.2010.01359.x. [DOI] [PubMed] [Google Scholar]
- 156.Dahlin MG, Beck OML, Åmark PE. Plasma Levels of Antiepileptic Drugs in Children on the Ketogenic Diet. Pediatr Neurol. 2006;35(1):6–10. doi: 10.1016/j.pediatrneurol.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 157.Coppola G, Epifanio G, Auricchio G, Federico RR, Resicato G, Pascotto A. Plasma free carnitine in epilepsy children, adolescents and young adults treated with old and new antiepileptic drugs with or without ketogenic diet. Brain Dev. 2006;28(6):358–65. doi: 10.1016/j.braindev.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 158.Spilioti M, Pavlou E, Gogou M, Katsanika I, Papadopoulou-Alataki E, Grafakou O, et al. Valproate effect on ketosis in children under ketogenic diet. Eur J Paediatr Neurol [Internet]. Elsevier Ltd; 2016;20(4):555–9. 10.1016/j.ejpn.2016.04.003 [DOI] [PubMed]
- 159.Furth SL, Casey JC, Pyzik PL, Neu AM, Docimo SG, Vining EP, et al. Risk factors for urolithiasis in children on the ketogenic diet. Pediatr Nephrol. 2000;15(1–2):125–8. doi: 10.1007/s004670000443. [DOI] [PubMed] [Google Scholar]
- 160.Maalouf NM, Langston JP, Van Ness PC, Moe OW, Sakhaee K. Nephrolithiasis in topiramate users. Urol Res. 2011;39(4):303–7. doi: 10.1007/s00240-010-0347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kossoff EH, Pyzik PL, Furth SL, Hladky HD, Freeman JM, Vining EPG. Kidney stones, carbonic anhydrase inhibitors, and the ketogenic diet. Epilepsia. 2002;43(10):1168–71. doi: 10.1046/j.1528-1157.2002.11302.x. [DOI] [PubMed] [Google Scholar]
- 162.Paul E, Conant KD, Dunne IE, Pfeifer HH, Lyczkowski DA, Linshaw MA, et al. Urolithiasis on the ketogenic diet with concurrent topiramate or zonisamide therapy. Epilepsy Res [Internet]. Elsevier B.V.; 2010;90(1–2):151–6. 10.1016/j.eplepsyres.2010.04.005 [DOI] [PMC free article] [PubMed]
- 163.Kossoff EH, Zupec-Kania BA, Auvin S, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open [Internet]. 2018;3(2):175–92. Available from: http://doi.wiley.com/10.1002/epi4.12225 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 514 kb)