Abstract
Ryanodine receptor type 1-related myopathies (RYR1-RM) are the most common class of congenital myopathies. Historically, RYR1-RM classification and diagnosis have been guided by histopathologic findings on muscle biopsy. Main histological subtypes of RYR1-RM include central core disease, multiminicore disease, core–rod myopathy, centronuclear myopathy, and congenital fiber-type disproportion. A range of RYR1-RM clinical phenotypes has also emerged more recently and includes King Denborough syndrome, RYR1 rhabdomyolysis-myalgia syndrome, atypical periodic paralysis, congenital neuromuscular disease with uniform type 1 fibers, and late-onset axial myopathy. This expansion of the RYR1-RM disease spectrum is due, in part, to implementation of next-generation sequencing methods, which include the entire RYR1 coding sequence rather than being restricted to hotspot regions. These methods enhance diagnostic capabilities, especially given historic limitations of histopathologic and clinical overlap across RYR1-RM. Both dominant and recessive modes of inheritance have been documented, with the latter typically associated with a more severe clinical phenotype. As with all congenital myopathies, no FDA-approved treatments exist to date. Here, we review histopathologic, clinical, imaging, and genetic diagnostic features of the main RYR1-RM subtypes. We also discuss the current state of treatments and focus on disease-modulating (nongenetic) therapeutic strategies under development for RYR1-RM. Finally, perspectives for future approaches to treatment development are broached.
Keywords: RYR1, Myopathies, Therapeutics, Central core disease, N-acetylcysteine, Rycal, 4PBA, Salbutamol
Introduction
Congenital myopathies (CM) are an expanding group of genetic disorders typically characterized by childhood onset of skeletal muscle hypotonia and slowly or nonprogressive skeletal muscle weakness [1]. The collective true prevalence of CM is not well understood. Regional estimates range from 1:26,000 (pediatric) in the USA to 1:135,000 in the UK (pediatric and adult) [2–5]. Of all CM, ryanodine receptor 1-related myopathies (RYR1-RM), with an estimated US pediatric point prevalence of at least 1:90,000, are recognized as the most frequently diagnosed. This is in part owing to advances in genetic testing [6, 7].
The RYR1 gene (19q 13.2) encodes a calcium ion (Ca2+) channel (RyR1), which is embedded within the sarcoplasmic reticulum (SR) membrane in skeletal muscle. This large gene spans > 159 kb and features an open reading frame encoded in 106 exons (2 alternatively spliced) [8]. RyR1 is a homotetrameric protein structure comprised of 4 identical ~ 560-kDa subunits, each of which houses an FK506-binding protein (FKBP) subunit. The RyR1-FKBP interaction is crucial for channel stability, as are accessory proteins such as calmodulin, triadin, junctin, and calsequestrin [9]. RYR1-RM encompass a heterogeneous spectrum of histopathological and clinical subtypes accompanied by varying disease severity.
Histopathological RYR1-RM subtypes include central core disease (CCD) [10, 11], multiminicore disease (MmD) [12], centronuclear myopathy (CNM) [13], core–rod myopathy (CRM) [14], and congenital fiber-type disproportion (CFTD) [15]. Clinical phenotypes resulting from RYR1 variants are diverse and include interrelated conditions such as malignant hyperthermia (MH) susceptibility, exertional heat stroke, rhabdomyolysis-myalgia syndrome, King Denborough syndrome, and atypical periodic paralysis [5, 16, 17].
This review focuses on disease-modulating (nongenetic) therapeutic strategies under development for RYR1-RM. An overview of RYR1-RM diagnostic features [18] and potential therapeutic approaches are discussed.
Genetic Etiology
Historically, dominant RYR1 variants have been enriched in 3 hotspot regions (N-terminal: MH/CCD region 1, amino acids 35–614; central: MH/CCD region 2, amino acids 2163–2458; and C-terminal: MH/CCD region 3, amino acids 4550–4940), with region 1 and 2 variants predominantly associated with the MH susceptibility phenotype and region 3 variants with the classic CCD phenotype. RYR1 variants attributed to a recessive mode of inheritance have been reported as being evenly distributed throughout the RYR1 sequence [19–21]. Dominantly inherited RYR1 variants have been most frequently linked to CCD and MH susceptibility, whereas recessively inherited variants are prevalent in individuals with MmD [19], CNM [22], and CFTD histopathology [15].
MH (MIM# 145600) is a potentially lethal hypermetabolic condition triggered by exposure of susceptible individuals to certain volatile anesthetics and muscle relaxants. MH-like events triggered by nonpharmacologic factors (high environmental and body temperature, stress) have also been reported [23, 24]. With incomplete penetrance of MH susceptibility (~ 50%) and no common RYR1 causative variant, the diagnostic test for MH susceptibility is the in vitro caffeine–halothane contracture test (IVCT), which determines contracture thresholds. The IVCT requires an open biopsy to obtain sufficient fresh skeletal muscle tissue and may yield false-negative results [25]. Approximately 50% of MH-susceptible individuals exhibit cores on biopsy whereas others, without a history of myopathy prior to an MH episode (MH trait only), do not [8, 26, 27]. It is therefore challenging to rule out MH susceptibility in individuals with pathogenic RYR1 missense variants, and this is compounded by the fact that susceptible individuals do not always exhibit symptoms following initial exposure(s) to triggering agents. Furthermore, although MH susceptibility is typically associated with dominant RYR1-RM cases, MH episodes have been reported in recessive cases [28]. As such, all RYR1-RM-affected individuals are considered potentially MH susceptible [29].
Once a clinical phenotype of CM is suspected, histopathological findings such as cores, rods, centralized nuclei, and type 1 fiber predominance on skeletal muscle biopsy provide evidence for classification of RYR1-RM subtypes. Muscle imaging (magnetic resonance imaging and ultrasound) has become utilized more recently as part of the differential diagnosis. Muscle imaging has proven particularly useful for RYR1-RM owing to a specific pattern of muscle involvement that is often visible on MRI and ultrasound, namely relative sparing of the rectus femoris compared with the vastus lateralis [30].
Advancements in next-generation sequencing (NGS) technologies have made it possible to screen for variants in large genes such as RYR1 and assess genotype–phenotype correlations [31, 32]. RYR1 variants have been implicated in sarcoplasmic reticulum (SR) calcium Ca2+ release dysfunction (RyR1 channel hyper/hyposensitivity and chronic Ca2+ leak) [33]. Recessive variants have also been associated with hypomorphic RyR1 protein expression and RyR1-dihydropyridine (DHPR) misalignment [33, 34]. However, further research is required in order to fully elucidate the complex pathomechanism(s) elicited by specific RYR1 variants with regard to channel and calcium regulation.
As is the case with other congenital myopathies, such as SEPN1-related myopathy (SEPN1-RM), another core myopathy, no FDA-approved treatments are available to date. Nevertheless, there have been reports of off-label drug responses to manage related symptoms, which we discuss below under “RYR1-RM Therapeutic Approaches” [7, 9, 35–37].
Ryanodine Receptor Isoform 1
Ryanodine receptor isoform 1 (RyR1) is the major Ca2+ channel expressed in skeletal muscle and is a critical component of the triad system that is required for effective excitation–contraction (EC) coupling. RyR1 are embedded in the membranes of sarco/endoplasmic reticula (SR/ER) and regulate the rapid intracellular release of Ca2+ following transverse tubule depolarization. RyR isoforms also contribute to maintaining cellular Ca2+ homeostasis under resting conditions [38]. With a total molecular weight of ~ 2200 kDa, these homotetrameric membrane proteins are the largest known ion channels, capable of raising cytosolic Ca2+ by 2 orders of magnitude in < 0.5 ms [38, 39]. There are 2 additional mammalian RyR isoforms (RyR2 and RyR3) for which expression has been detected in numerous tissues and cell types, including cardiac muscle (where RyR2 is the predominant isoform), brain, smooth muscle, exocrine cells, epithelial cells, dendritic cells, and B-lymphocytes [9, 40, 41]. There is approximately 65% homology between isoforms [42]; however, RyR1 was the first to be cloned [43] and extensively described using both cryo-electron microscopy (cryo-EM) [44] and X-ray crystallography of individual domains [45]. RyR1 serves as a connection between the SR and the transverse tubule (invaginations of the sarcolemma) in skeletal muscle, allowing for direct protein–protein coupling to the L-type Ca2+ channel dihydropyridine (DHPR) receptors that are localized to the transverse tubule membrane. Depolarization across the plasma membrane causes conformational changes in DHPR that trigger RyR1 opening independently of extracellular Ca2+ concentration [46].
Aberrant SR Ca2+ release leading to disruption of cytosolic Ca2+ homeostasis is the direct consequence of RyR1 dysfunction [33, 47]. Consequently, EC uncoupling between RyR1 and DHPR, RyR1 channel destabilization causing SR Ca2+ leak which is ineffectively compensated by the sarco-endoplasmic reticulum Ca2+ ATPase (SERCA) pump (decompensated leak), decreased RyR1 expression, and elevated oxidative and nitrative stress resulting from mitochondrial Ca2+ uptake dysregulation could explain the phenotypes associated with RYR1-RM [48–51].
RYR1-RM Diagnostic Approaches
A summary of diagnostic clues suggestive of RYR1-RM is presented in Table 1.
Table 1.
Diagnostic clues suggestive of RYR1-RM
| Central core disease | Multiminicore disease | Core–rod myopathy | Centronuclear myopathy | Congenital fiber type disproportion | |
|---|---|---|---|---|---|
| Histopathology | Single or multiple cores spanning the longitudinal fiber axis | Multiple cores; limited on longitudinal sections | Presence of both central cores and nemaline bodies upon muscle biopsy | Centrally located nuclei | Fiber size disproportion (type 1 fibers consistently at least 35–40% smaller than type 2 fibers diameter) |
| Type 1 fiber predominance and uniformity | Type 1 fiber predominance and uniformity | Type 1 fiber predominance and uniformity | Type 1 fiber predominance | ||
| Increased internal nuclei | Type 1 hypotrophy | ||||
| Central aggregation or reduction of oxidative stains | |||||
| Cores on oxidative stains | |||||
| Clinical features | Hypotonia and motor development delay | Hypotonia | Axial hypotonia | Static or slowly progressive generalized muscle weakness | |
| Respiratory, bulbar, and cardiac involvement are uncommon | Early respiratory impairment with or without cardiac complications | Respiratory impairment | Mild respiratory involvement | Respiratory weakness | |
| Proximal weakness pronounced in hip girdle | Distal weakness | Diffuse muscle weakness | Diffuse and progressive muscle weakness | Proximal axial weakness | |
| Extraocular muscle involvement and ophthalmoplegia in severe cases | Ptosis/extraocular involvement | Ophthalmoplegia | |||
| Mild facial involvement | Facial dysmorphism | Facial muscle weakness | |||
| Orthopedic deformities (scoliosis) and ligamentous laxity | Spinal rigidity and scoliosis | Multiple joint contractures and scoliosis | Joint contractures | ||
| Moderate bulbar involvement | Bulbar weakness | Dysphagia | |||
| Myalgia and/or exertional weakness with or without rhabdomyolysis | Exercise-induced myalgia | ||||
| High malignant hyperthermia susceptibility | Malignant hyperthermia rarely reported |
Genetic Testing
Although genotype–phenotype correlations remain challenging to establish across subtypes of this disease, the significant overlap and common occurrence of histologic features in the RYR1-RM spectrum make it increasingly likely that associated disorders will ultimately be defined by genetics and pathophysiology, and less by histopathology [1, 52]. RYR1-RM genetic heterogeneity and the increasing utility of NGS approaches to variant identification, coupled with reduction in sequencing cost, should prompt clinicians and researchers to screen patients for pathogenic variants with congenital myopathy sequencing panels over single gene analysis [53, 54]. Indeed, genetic sequencing (by gene, targeted variant, exome, and/or genomic testing) is becoming an increasingly crucial diagnostic tool for RYR1-RM and sequencing the entire RYR1 gene rather than only the 3 hotspots is now best practice.
Muscle imaging, including both magnetic resonance imaging and ultrasound, has also become utilized as an adjunct diagnostic tool. A specific pattern of muscle involvement in RYR1-RM is typically evident on MRI and ultrasound [30]. The combination of neuromuscular panel genetic testing and muscle imaging facilitates diagnosis in many cases, avoiding an invasive biopsy and the use of anesthesia in this malignant hyperthermia-susceptible population. If genetic testing and muscle imaging are inconclusive, biopsy may then be performed to further direct a diagnosis. Muscle imaging is discussed further below.
Clinical Phenotypes Indicative of RYR1-RM
Clinical features associated with RYR1-RM have been extensively described [1, 16, 20]. Across all ages, RYR1-RM clinical features include symmetric proximal muscle weakness, often with pronounced facial weakness with or without dysmorphism and ophthalmoparesis/ophthalmoplegia with ptosis, bulbar weakness, significant respiratory involvement, severe neonatal hypotonia, scoliosis, orthopedic deformities including arthrogryposis, hip dislocation, club feet, and King Denborough syndrome (pectus carinatum or excavatum, short stature, joint contractures, facial and skeletal deformities) [16], MH susceptibility, anesthesia-induced rhabdomyolysis [55], fatigue, exercise-induced hyperthermia/exertional heat stroke, and exertional myalgia [56].
Histopathology, Clinical Features, and Genetic Considerations
Central Core Disease
Histopathologic features: On oxidative histological staining, CCD presents with clearly demarcated central cores (regions of pale staining) that can span the length of the muscle fiber. The amorphous central cores predominate within type 1 fibers and result from the absence or depletion of mitochondria [8]. These patterns show variability ranging from more than 1 core per muscle fiber, variable core diameters, to typical or eccentric cores and centrally or peripherally located cores [57]. Sarcomeric filamentous disorganization (Z line streaming) and proliferation of sarcotubular membranes are typical on electron microscopy [58].
Clinical features: As one of the most frequent CM [59], the CCD phenotype of dominant RYR1 causative variations is usually mild and nonprogressive. Clinical features include hypotonia in infancy, delayed motor milestones, axial muscle involvement, proximal weakness, myalgia, muscle stiffness, hip dislocation, spinal deformities, and exertional weakness with or without rhabdomyolysis. Extraocular muscle weakness and bulbar and respiratory involvement are not typical [33, 60]; however, strabismus has been reported in dominant cases [31, 61]. Recessively inherited or de novo dominant RYR1-related CCD present with more severe features such as fetal akinesia syndrome (severe hypotonia, multiple arthrogryposis, respiratory failure) [8, 61, 62]. Serum creatinine kinase (CK) may be mildly elevated or normal, and muscle MRI imaging shows selective involvement and sparing of certain muscle groups in dominant RYR1-related cases [30]. MRI findings in recessively inherited cases are not as consistent. There is an overlap between CCD and MH susceptibility, with RYR1-related CCD patients with causative variants in the N-terminal region having a higher probability of MH susceptibility than CCD patients with variations in the C-terminal region [8].
Genetic considerations: Approximately 60% of RYR1-related CCD causative variants are located in the CCD hotspots including the C-terminal domain. CCD is usually inherited in an autosomal dominant pattern with few recessively inherited cases [61]; therefore, parental genetic testing should be performed when possible. To increase the probability of detecting a causative variant, individuals suspected of RYR1-RM should have full RYR1 gene sequencing performed [8, 63]. A CCD patient, with an asymptomatic parent who tests positive for the same variant, should be investigated for additional RYR1 variants by sequence analysis of the entire RYR1 gene to investigate the possibility of recessive modes of inheritance. More than half of the variants associated with RYR1-RM are private, and only 10% or so of RYR1 variants have been functionally characterized. Therefore, additional variants identified through genetic testing may have unknown or unreported functions (variants of unknown clinical significance) [29]. Variations in the MYH7 gene are increasingly associated with CCD histopathology, making up an estimated 10% of CCD cases [64]. Other genes implicated in CCD histopathology include SEPN1, ACTA1, TTN, KBTBD13, and CCDC78, CACNA1S [7, 18, 65].
Multiminicore Disease
Histopathologic features: MmD is a recessive RYR1-RM subtype that presents with numerous amorphous cores on oxidative muscle biopsy stains, resulting in a characteristic “moth-eaten” appearance. The cores often vary in size, are observed in most fibers, and typically do not span the entire length of the muscle fiber, unlike those in CCD. Multiple internally placed nuclei and type 1 fiber predominance are also noted in affected muscles. Minimal disruption of myofibrillar structure may be present on electron microscopy. Abnormal expression of desmin and other sarcomeric and intermediate filament proteins may be observed within or around the core area in immunohistochemical studies.
Clinical features: As with most RYR1-RM subtypes, MmD shows clinical diversity and genetic heterogeneity. Clinical features in MmD patients include delayed development, proximal and axial weakness, hip girdle involvement, marked distal weakness and wasting, feeding difficulty during infancy, prominent facial weakness, axial hypotonia, scoliosis, ophthalmoplegia, and severe respiratory impairment [66]. Homozygosity or compound heterozygosity for either 2 missense variants or 1 nonsense and 1 missense variant is the most common mode of inheritance in recessive RYR1-related MmD [66, 67]. The clinical phenotype of recessively inherited RYR1-associated MmD may be partly explained by decreased RyR1 protein expression in affected individuals [33].
Genetic considerations: RYR1 should be considered the second most likely causative gene to SEPN1 for MmD [18]. SEPN1 encodes selenoprotein-N, which is involved in cellular redox-regulated calcium homeostasis. SEPN1-related MmD is characterized by early spinal rigidity, high-pitched voice, myopathic facies, respiratory involvement, and scoliosis, and does not typically present with extraocular muscle involvement and ophthalmoplegia [18, 60]. If cardiomyopathy is present, variants in TTN and MYH7 genes should be considered [68]. Variations in MEGF10 and CACNA1S have also been associated with MmD [65, 69]. Screening of the entire RYR1 coding sequence as part of a congenital myopathy sequencing panel is recommended to effectively elucidate the genetic etiology of MmD histopathology.
Core–Rod Myopathy
Histopathologic features: CRM can be identified by the presence of both central cores and nemaline bodies (rods), characteristic of nemaline myopathy (MIM# 256030), upon biopsy. RYR1 variants are the most common cause of CRM, although causative variants in other genes have been reported [70, 71]. Rods are largely composed of actin and α-actinin with the same electron density similar to the Z lines of adjacent sarcomeres on EM. Rods are arranged in clusters or diffusely distributed within the fibers. Structured cores spanning almost the entire fiber length with absent mitochondria are seen in longitudinal sections. CRM myopathic features include centralized nuclei, variability in fiber size diameter, type 1 fiber predominance, and mild fatty infiltration. Single and multiple cores, centrally and peripherally located, coexist with rods [14].
Clinical features: Patients present with generalized hypotonia, mild facial weakness, diffuse muscle weakness with axial predominance, multiple joint contractures, scoliosis, and respiratory insufficiency. Serum CK levels are normal and cardiac complications are typically absent.
Genetic considerations: Variants in KTBTBD13 and CFL2 genes have been associated with CRM. KTBTBD13 encodes a muscle-specific ubiquitin ligase, and variants in this gene can cause both CRM and nemaline myopathy. KTBTBD13 variants should be suspected in CRM patients with proximal weakness and “slowness” in muscle movement [18]. Compound heterozygous cases with NEB variants have also been associated with CRM [71, 72]. Additionally, variations in genes associated with nemaline myopathy (NEM1, ACTA1, TPM3) should be considered and screened as part of a NGS congenital myopathy sequencing panel to identify causative variants.
Centronuclear Myopathy
Histopathologic features: CNM is characterized by numerous centrally located nuclei in approximately ≥ 25% of muscle fibers [73]. The number of centralized nuclei may increase with age, confounding the minimum number required for diagnosis [74]. Central and multiple internalized nuclei are the main pathological features noted when muscle biopsy is performed in early life, but other histopathological features associated with recessive RYR1-RM appear over time [75]. Histopathological features include a central zone with either accumulation or absence of oxidative enzyme activity on NADH staining, radial strands (myofibrils) surrounding the central zone in many fibers, variability in fiber diameter, and type 1 fiber predominance and hypotrophy [33].
Clinical features: CNM is part of the recessive RYR1-related continuum, and therefore, patients present with substantial clinical similarities as noted with MmD. Clinical presentations include facial weakness, external ophthalmoplegia, and predominantly proximal muscle involvement [22]. Variants in multiple genes encoding proteins linked to various aspects of phosphoinositide metabolism and membrane trafficking, T-tubule formation, triad assembly, and EC coupling (DNM2, TTN, BIN1, MTMR14, CCDC78) [76] have been associated with CNM, including the most severe fatal X-linked form (myotubular myopathy) attributed to mutations in the MTM1 gene [77]. Most frequent clinical features of autosomal CNM include delayed motor milestones, distal muscle weakness, ptosis, ophthalmoparesis, or ophthalmoplegia [78]. More recently, late-onset distal myopathy has also been associated with RYR1-CNM [79].
Genetic considerations: Caution should be taken to exclude recessive inheritance if only a single variant is identified, especially as RYR1 is a large gene and has many variants of unknown significance. Screening of the entire RYR1 coding sequence as part of a congenital myopathy sequencing panel is recommended in identification of CNM causative variants. In severely affected males, the investigative focus should begin with variants in the MTM1 gene, including deep intronic regions to capture rare variants, for the X-linked form of CNM. Female carriers of MTM1 variants with moderate to severe symptoms may have skewed X-inactivation [75]. Variations in DNM2 gene should be investigated if family history and clinical features are consistent with autosomal dominant inheritance and predominant distal weakness [74, 78, 79].
Congenital Fiber-Type Disproportion
Histopathological features: CFTD histopathological diagnosis requires observation of type 1 fibers that are 35–40% consistently smaller than type 2 fibers in the absence of other structural abnormalities [80]. This disproportion has to be the main diagnostic abnormality after ruling out other histopathological findings for the diagnosis of CFTD to hold. When fiber size disproportion appears in conjunction with rods, cores, or central nuclei, it is superseded by those histopathological findings. RYR1 variants may account for up to 20% of CFTD cases, while other well-established genetic etiologies include variations in ACTA1, TPM3, TPM2, SEPN1, and MYH7 genes [15, 81]. No single clinical or histologic feature is specific to CFTD; therefore, clinical features should be consistent with congenital myopathies to make a CFTD diagnosis.
Congenital neuromuscular disease with uniform type 1 fiber (CNMDU1) is associated with variations in the C-terminal domain of RYR1, a hotspot region for CCD causative variants. CNMDU1 is a rare form of CM that is pathologically diagnosed by a muscle fiber composition of > 99% type 1 fibers with no other specific structural changes [82]. The few type 2 fibers present are predominantly immature type 2C.
Clinical features: Overlapping with other recessive RYR1-RM, RYR1-related CFTD clinical presentations include prominent hypotonia and weakness of axial muscles, myopathic facies, respiratory failure, feeding difficulties, and ophthalmoparesis. Joint contractures, scoliosis, and cardiac involvement are relatively uncommon. Muscle imaging of the leg typically shows sparing of the rectus femoris compared with other quadriceps muscles [83]. Cases of RYR1-CNMDU1 present with clinical features similar to other CM such as infantile hypotonia, mild proximal muscle weakness, respiratory distress, high arched palate, craniofacial dysmorphism, normal CK levels, and no mental retardation.
Genetic considerations: Known genes account for 50 to 70% of causative variations in CFTD; therefore, other genetic causes remain unidentified [84]. Variants in TPM3 and ACTA1 genes should be investigated if an autosomal dominant inheritance pattern is suspected. Identification of causative variations in particular genes can determine which disease surveillance is recommended, for example, cardiac surveillance in MYH7 and TMP2 pathogenic variations [80]. Heterozygous RYR1 sequence variations (2 missense, 1 substitution of 2 consecutive nucleotides, and 1 de novo frame-shift deletion) have been reported in CNMDU1 patients [82]. However, the genetic etiology and pathomechanism of the CNMDU1 remain uncertain. Exome sequencing or NGS congenital myopathy sequencing panel is recommended for detection of possible causative variants in individuals affected by CFTD and CNMDU1.
Magnetic Resonance and Ultrasound Imaging
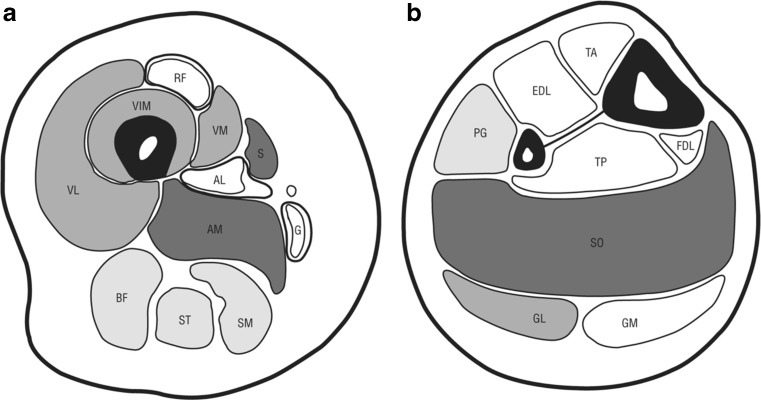
Muscle magnetic resonance imaging (MRI) and muscle ultrasound imaging can reveal patterns of involvement or sparing of specific muscles in RYR1-RM. It is important to recognize, however, that there is overlap among subtypes of congenital myopathies, for example, relative sparing of vastus lateralis in CM resulting from variants in RYR1, SEPN1, MYH7, and BIN1 [85]. In the upper leg of CCD-dominant cases (Fig. 1), there is fatty infiltration and muscle wasting of the vasti, adductor magnus, and sartorius, while the adductor longus, gracilis, and rectus femoris muscles are relatively spared. In the lower leg, the most affected muscle is the soleus, followed by the lateral head of the gastrocnemius, with selective sparing of the medial head of the gastrocnemius. In the anterior compartment muscles of the calf, the peroneal group is more affected than the tibialis anterior. There is selective involvement in bicep brachii, subscapularis, lumbar paravertebral, and glutei muscles [18, 86]. Recessively inherited RYR1-RM subtypes, other than CCD, typically show more diffuse muscle involvement rather than the classic pattern above [30].
Fig. 1.
Schematic diagram of the typical pattern in RYR1-related myopathies. (A) In the thighs, the rectus femoris (RF), adductor longus (AL), and gracilis (G) are spared and in some patients hypertrophied; the adductor magnus (AM), sartorius (S), vastus lateralis (VL), vastus intermedius (VIM), and vastus medialis (VM) are affected; the hamstrings are less affected; and the involvement of semimembranosus (SM) and semitendinosus (ST) is nonspecific. BF = biceps femoris. (B) In the calf, the most affected muscle is the soleus (SO), followed by the gastrocnemius lateralis (GL) and to a lesser effect the gastrocnemius medialis (GM). In the anterior compartment, which is less affected than the posterior, the peroneal group (PG) is more affected than the tibialis anterior (TA). EDL = extensor digitorum longus; FDL = flexor digitorum longus; TP = tibialis posterior. (From: Klein et al., 2011, JAMA Neurology, with permission)
RYR1-RM Therapeutic Approaches
Preclinical Models
In the preclinical setting, investigation of potential therapeutic candidates for RYR1-RM is undertaken by utilizing models of the disease (in vitro, ex vivo, and in vivo). Patient biopsy-derived, cultured mature skeletal muscle cells (myotubes) are an ideal in vitro model of the disease through which to test potential therapies that target a specific variant or a shared disease pathomechanism. Patient-derived fibroblasts (obtained through skin biopsy or skeletal muscle primary culture) should be retained as such cells can undergo MyoD transfection to achieve a myogenic phenotype [87]. Yet, when skeletal muscle tissue is not available from a patient, unaffected immortalized cells (e.g., lymphoblastoid cells, HEK-293 cells which do not have endogenous RyR) represent a stable model through which to recapitulate the disease phenotype by transiently transfecting mutant RyR1 cDNA [88].
Two of the 4 RYR1 mouse models have been primarily used to test therapeutic candidates for RYR1-RM in vivo. The I4895T knock-in mouse model exhibits a progressively severe clinical phenotype associated with decreased RyR1-mediated Ca2+ permeation and may therefore not be directly comparable to the human disease process resulting from the equivalent variant (I4898T) [47]. In contrast, the Y524S knock-in mouse (corresponding to Y522S in humans) exhibits a mild phenotype with elevated RyR1-mediated Ca2+ leak and is comparable to rhabdomyolysis and MH susceptibility clinical phenotypes in humans [51]. A homozygous and RyR1-null, dyspedic mouse model has also been established [89]. Although dyspedic mice die at birth owing to asphyxia resulting from skeletal muscle paralysis, this model has been successfully used to investigate components of EC coupling [90]. An MH mouse model had also been developed with the p.Arg163Cys RYR1 variant. In the homozygous state, the variant is embryonically lethal whereas the heterozygous form survives and exhibits halothane hypersensitivity [91]. However, there is no histopathology associated with this model [92], suggesting it is a valuable tool for researching MH but perhaps less so for RYR1-RM.
Alternatively, a severe homozygous zebrafish RYR1-RM model (relatively relaxed) has been established which resembles RYR1-related MmD [93]. This has proven useful for conducting high-throughput drug screening, and studies, using this model, provided rationale for the first RYR1-RM clinical trial [50]. High-throughput drug screening, especially using the relatively relaxed zebrafish model but also other techniques, such as assessing calcium ER/SR stress using genetically encoded biosensors in vitro [94, 95], has recently enabled identification of several candidate therapeutic compounds, signifying that the era of therapeutics for RYR1-RM is advancing at a more rapid pace than before.
Nevertheless, collectively, there is no ideal in vivo RYR1-RM animal model, but work is underway to develop a model that better resembles this spectrum of diseases as a whole. Caution must also be taken when comparing results across different model systems [26].
Therapies
Despite an unmet medical need, there remain no approved therapies for RYR1-RM and those discussed in this section are, at best, in investigational stages. Therapies currently under development can be broadly assigned into 2 categories: 1) drugs with a mechanism of action that may negate the downstream deleterious effects of oxidative and nitrosative stress on the cellular milieu and RyR1 posttranslational modifications or 2) drugs that act either directly on RyR1 itself or modulate interacting proteins that determine RyR1 functionality.
RYR1 exceeds 159 kb and thus far supersedes the current packaging capacity for adenovirus-mediated therapy (∼ 5 kb) [96]. As such, gene therapy approaches, such as those that have shown great promise for spinal muscular atrophy [97], are not currently feasible for RYR1-RM. An additional factor complicating this approach is that more than 700 variants have been located throughout the RYR1 coding region and canonical splice sites. Although not suitable for all cases, exon-skipping has shown promise, in vitro, as a gene-directed therapeutic strategy for compound heterozygous RYR1-RM-affected individuals with pseudo-exon inclusion [98]. Despite the abovementioned limitations, at least 3 nongenetic medications have been used, off label, to manage RYR1-RM-related symptoms. These include dantrolene, salbutamol/albuterol, and N-acetylcysteine (NAC) (Table 2). The current state of nongenetic medications and therapies under development for RYR1-RM are detailed herein with an overview of proposed mechanism(s) of action provided in Fig. 2.
Table 2.
Compounds under development as potential therapeutics for RYR1-RM
| Compound/drug | Mechanism of action | Stage of clinical development |
|---|---|---|
| N-Acetylcysteine (NAC) | Reduction of aberrant oxidative stress | Phase I/II clinical trial data collection complete, data analysis pending, FDA approved for other indications |
| Rycal ® (S48168) | RyR1 closed channel stabilization | Phase I complete, phase IIa pending |
| Sodium 4-phenylbutyrate (4BPA) | Chemical chaperone | Preclinical, FDA approved for other indications |
| 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) | RyR1 channel antagonist | Preclinical |
| Salbutamol/albuterol | Enhancement of SERCA expression | Open-label study complete, FDA approved for other indications |
| Dantrolene | RyR1 channel antagonist | FDA-approved medical antidote for MH crises, but no formal analyses in RYR1-RM |
| Carvedilol | Beta-blocker | Preclinical, FDA approved for other indications |
| Pyridostigmine | Acetylcholinesterase inhibitor | Case report of initial response in myasthenic-like presentation, FDA approved for other indications |
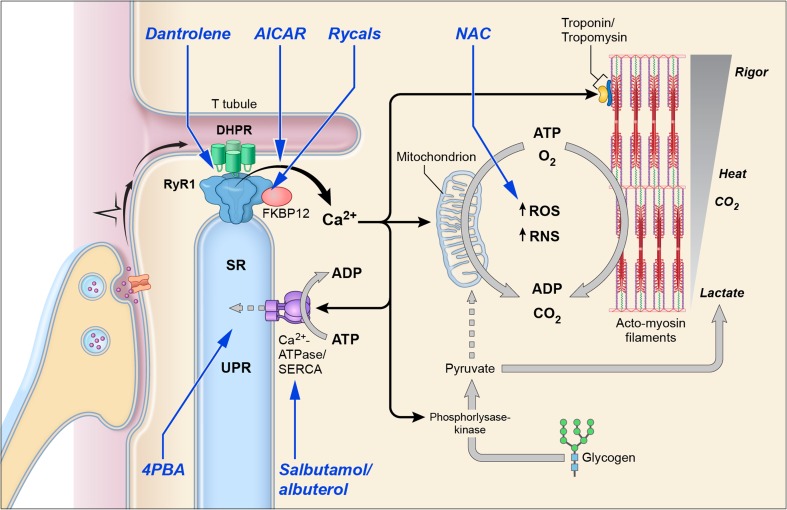
Fig. 2.
RyR1 channel in the open and closed state. Purported mechanisms of action of various therapies: salbutamol increases SERCA expression levels to facilitate reuptake of cytosolic Ca2+ into the SR lumen; NAC works in the sarcoplasm to reduce levels of mitochondrially derived oxidative stress via restoring redox balance; rycals increase FKBP12 binding to RyR1, which in turn maintains the RyR1 channel in the closed state, reducing Ca2+ leak into the sarcoplasm; dantrolene antagonizes the RyR1 channel and thus reduces Ca2+ leak; 4PBA reduces ER stress markers/UPR and cytosolic Ca2+ levels and reduces calpain activation while increasing SR Ca2+ content. (Adapted from Witherspoon and Meilleur, 2017 and https://www.biozentrum.uni-wuerzburg.de/humangenetik/forschung/emeritus/prof-mueller-reible/ with permission from Dr. M. Anetseder and Dr. A. Hoyer, Dept. of Anesthesiology, University of Wuerzburg, Germany)
Therapies Targeting Downstream Pathomechanisms
Elevated oxidative stress is a hallmark pathological feature in RYR1-RM and has been identified in several preclinical models of the disease [47, 50, 51, 99]. In particular, lipid peroxidation of mitochondrial membranes has been implicated as a central pathomechanism that leads to severe mitochondrial dysfunction and/or death in the RYR1Y522S/W mouse model [51]. Owing to elevated mitochondrial Ca2+ uptake in response to RyR1-mediated SR Ca2+ leak, respiratory chain activity is thought to be upregulated. Resultant reactive byproducts, such as reactive oxygen species (ROS) and nitrogen species (RNS), trigger posttranslational modifications of RyR1 resulting in further calcium dysregulation [100–102].
NAC
NAC is a precursor to the ubiquitous antioxidant glutathione and is available in 3 formulations: intravenous (IV), inhaled, and oral. Upon administration, NAC is enzymatically de-acetylated to form cysteine, the rate-limiting component of reduced glutathione resynthesis [103, 104]. The oral formulation is available over the counter, and the intravenous and inhaled formulations are FDA approved for multiple conditions, including acetaminophen overdose and various pulmonary conditions [104–109]. Strong preclinical evidence for a beneficial effect of NAC treatment on skeletal muscle function and histology has been provided by studies conducted in mouse and zebrafish models of the disease. Notably, treatment of the RYR1Y522S/W mice with N-acetylcysteine (NAC) (ad lib via drinking water for 8 weeks) rescued lipid peroxidation and concomitantly prevented previously observed decrements in force generation [51]. These findings have recently been supported by a longer-term study of NAC treatment in the same mouse model in which NAC treatment led to a decreased number of structural cores and improved skeletal muscle function [99]. Further preclinical evidence supporting the therapeutic potential of NAC was provided by Dowling and colleagues (2012) [50]. In an elegant study conducted in relatively relaxed zebrafish and RYR1-RM patient myotubes, NAC was found to improve endurance and decrease biomarkers of mitochondrial oxidative stress, respectively, when compared to wildtype/control. A double-blind, placebo-controlled trial of NAC (NCT02362425) was recently completed at the National Institutes of Health by our team, and results are pending.
Medical considerations: The dose of NAC required to elicit a potential beneficial effect in this population may be greater than that used OTC for other purposes, possibly owing to decreased basal GSH:GSSG ratio in RYR1-RM individuals compared to the general population. Therefore, although available OTC, affected individuals should use NAC under the care of their local neurologist or clinician, and dosing should be weight based.
Salbutamol/Albuterol
Salbutamol is a beta 2-adrenergic agonist, typically used for asthma control, but purported to build muscle volume and increase strength. Salbutamol has been reported to increase the overall expression of SERCA (type 1/fast-twitch isoform), a Ca2+ ATPase pump responsible for SR Ca2+ reuptake although potential mechanisms of action require further investigation [110]. Increased cytosolic Ca2+ is a well-established consequence of certain, gain-of-function RYR1 variants, such as p.Arg4861His and p.Asp4505His, that cause RyR1-mediated Ca2+ leak. Subsequently, increased cytosolic Ca2+ causes a negative feedback loop to the mitochondria resulting in elevated oxidative and nitrosative stress and possibly poor ATP production.
This drug has been tested in RYR1-RM-affected individuals in 2 studies in the past 14 years. The first was an open-label pilot study conducted in London, UK, and included RYR1-RM-affected individuals with CCD or MmD histopathology [111]. RYR1-RM-affected individuals improved in Medical Research Council (MRC) scores, forced vital capacity (FVC), and myometry values [111]. The second report of albuterol treatment involved a single 9-year-old male case with recessive inheritance and purported mitochondrial dysfunction [37]. Treatment led to an improvement in FEV1, motor function scales, and clinical observations (such as ability to raise arms above shoulders, speech, and self-care). In both studies, the participants underwent an exercise regimen while taking this medication as it had been alluded, in previous neuromuscular disease intervention, that a combinational effect of albuterol with exercise may exist [112, 113]. Of note, individuals with several neuromuscular diseases anecdotally report improvements with exercise alone; however, exercise as an intervention has not been tested independently in a blinded fashion. Ideally, salbutamol needs to be tested in a randomized controlled trial.
Medical considerations: Given the effect of beta-adrenergics such as salbutamol on cardiac function, an electrocardiogram should be undertaken prior to dosing, in order to rule out long QT syndrome, and monitoring of cardiac rhythm is recommended during administration. This was performed regularly in the case report above via vital signs and periodic electrocardiograms [37].
Sodium 4-Phenylbutyrate
Sodium 4-phenylbutyrate (4PBA) is a chemical chaperone and is FDA approved for the management of hyperammonemia due to urea cycle disorders and alleviates endoplasmic reticulum (ER)/sarcoplasmic reticulum (SR) stress. Its mechanism of action is to aid in protein folding, decrease protein aggregation, and lower oxidative stress [47].
A recent study by Lee et al. (2017), using the I4895T RYR1-RM mouse model, sought to determine if alleviation of endoplasmic reticulum (ER) stress, uncoupled protein response (UPR), and oxidative stress with 4PBA would reverse skeletal muscle pathology [47]. 4PBA treatment of mice carrying the I4895T RYR1 variant decreased markers of mitochondrial oxidative stress, muscle protein ubiquitination, and ER stress/UPR and improved skeletal muscle function. Yet 4PBA treatment did not restore SR Ca2+ transients in I4895T fibers to wild-type levels, suggesting that decreased SR Ca2+ release may not be the central pathomechanism in RYR1-RM resulting from this specific variant. Overall, these findings indicate that 4PBA has potential to be repurposed as a therapeutic intervention for RYR1-RM patients, at least for those affected by the equivalent human variant (p.Ile4898Thr).
In support of the therapeutic potential of 4PBA, Ma et al. reported decreased muscle wasting in rats with severe burn injuries, following treatment [114]. ER stress markers were elevated in these rats compared with those in the placebo group, especially at postburn days 4 and 7. 4PBA treatment decreased ER swelling, ER stress markers, cytosolic Ca2+ concentration, and calpain activation while increasing SR Ca2+ content. 4PBA treatment also improved muscle structure and decreased skeletal muscle wasting.
Therapies Targeting the RyR1 or Modulating Interacting Proteins
Dantrolene
Dantrolene is a muscle relaxant and is approved for use as the only medical treatment for an active malignant hyperthermia episode. Although the mechanism of action for dantrolene is not completely understood, this postsynaptic muscle relaxant likely interferes with SR Ca2+ release via disruption of communication between RyR1 and Cav1.1 [115]. By inhibiting SR Ca2+ efflux, it decreases excitation–contraction coupling and relaxes cardiac and skeletal muscle [116]. After its implementation for treatment of MH, mortality due to MH decreased from 80 to 10% [117].
Reports of off-label dantrolene use for management of RYR1-RM symptoms have yielded mixed findings. For example, in a single case with mild CCD, motor function improved [118]. Anecdotally, affected individuals who have used dantrolene off-label state it improves symptoms such as myalgia; however, despite the likely overlap between MH, myalgias, and exertional rhabdomyolysis, further studies are needed to identify the efficacy of dantrolene in these latter 2 phenotypes [36]. Another concern with dantrolene use for RYR1-RM is that, given an inhibitory mechanism of action on RyR1, use by affected individuals with variants that already cause decreased sensitivity to RyR1 agonists may result in deleterious sequelae. Although motor function improved in the above case, muscle weakness from dantrolene use has also been reported [36]. RYR1 variants associated with decreased sensitivity to agonists (also termed “blocked channel variants”) include p.Ile4898Thr, which has been recapitulated in the I4895T mouse model and exhibits decreased resting cytosolic Ca2+ and an uncoupled protein response. However, studies of patient-derived myotubes with the corresponding human mutation, p.Ile4898Thr, show a differing phenotype of minimal leak. To this point, Marty and Faure discuss the difficulty of identifying exact pathophysiologic mechanisms while differing preclinical models are used for the same variant, and they consequently surmise that actual insults are likely more complex than our current understanding [26].
Medical considerations: Although it is plausible that affected individuals with variant(s) associated with RyR1 Ca2+ leak may benefit from periodic dantrolene use, confirmation of channel leakiness may be difficult for a given variant. More preclinical research is necessary to confirm the functional impact of specific RYR1 variants, and likely impact of cytosolic free magnesium concentrations on dantrolene action in this population [119, 120], in order to determine the safety and utility of dantrolene administration [36] with the potential for a future personalized health approach in the clinic.
Rycals
Rycals are a class of benzothiazepine-derived Ca2+ channel stabilizers that have been developed by ARMGO Pharma Inc. (Tarrytown, NY) for chronic heart failure, cardiac arrhythmia, and catecholaminergic polymorphic ventricular tachycardia [121]. Rycals target the interaction between RyR and calstabin isoforms (RyR1 to FKBP12 (calstabin1) in skeletal muscle and RyR2 to FKBP12.6 in cardiac muscle), the latter of which stabilize the RyR closed state under normal physiological conditions without perturbing functional isoforms expressed elsewhere [122]. In skeletal muscle, each RyR1 monomer houses a single FKBP12 binding site localized to the cytosolic shell domain. Preclinical work in skeletal muscle using Rycal compounds has focused on muscular dystrophies, which are considered to have a secondary defect in EC coupling. Animal studies [123], particularly those conducted in the mdx mouse model of Duchenne muscular dystrophy [124], have revealed that Rycal compounds restore RyR1-calstabin1 binding, an interaction which is diminished in muscle diseases associated with excessive oxidative and/or nitrosative stress [125]. Under such conditions, Rycal treatment has been demonstrated to restore RyR1-calstabin1 binding thereby stabilizing RyR1 in the closed state [124, 126, 127]. Owing to therapeutic potential, ARMGO Pharma Inc. have expanded their clinical testing pipeline to encompass Rycal treatment for indications related to skeletal muscle diseases, including RYR1-RM.
The Rycal class of drugs emerged from initial work using K201 (JTV-519), a compound first described in the early 1990s [128] that has shown mixed effects on RyR1-mediated Ca2+ release in vitro and may diminish SR Ca2+ re-uptake via SERCA [129]. Owing to this nonspecific mechanism of action, a number of refined JTV-519-derivatives have since been developed including the first Rycal compound to be tested clinically (ARM036; Aladorian), and the current lead Rycal compound (ARM210; S48168). First-in-man phase 1 trials for ARM210 have recently been completed; however, to date, there are no published reports of Rycal treatment in preclinical models of RYR1-RM. To address this, using skeletal muscle obtained from RYR1-RM-affected individuals, Rycal treatment is currently being tested ex vivo through a collaboration between our laboratory and Marks and colleagues at Columbia University. Due to the promising safety profile and therapeutic potential of ARM210, by addressing RyR1-mediated Ca2+ leak, a central pathomechanism associated with RYR1-RM, a phase 1 trial in this population is in planning stages. In addition, in August 2018, the FDA awarded ARM210 Orphan Drug Designation for RYR1-RM.
Carvedilol
Carvedilol, a beta-blocker commonly used to treat and manage heart failure and cardiac arrhythmias, suppresses store overload-induced calcium release (SOICR) in HEK-293 cells expressing MH/CCD-associated RYR1 variants in different regions of the RyR1 channel, including leaky N-terminal variants [130]. SOICR is a depolarization-independent Ca2+ overload-induced SR Ca2+ release that result in spontaneous muscle contractions [131]. Although the molecular mechanisms underlying the suppression of enhanced SOICR remain undetermined, carvedilol is hypothesized to suppress the RyR1 open state-dependent luminal Ca2+ activation and SOICR by stabilizing the closed state of the RyR1 channel [132]. As HEK-293 cells lack certain muscle-specific proteins (L-type Ca2+ channels, calsequestrin, triadin, junction), these findings await demonstration in skeletal muscle cells. Carvedilol may represent a potential long-term therapeutic agent for MH and other RYR1-RM if future studies show similar action in skeletal muscle [130].
5-Aminoimidazole-4-Carboxamide Ribonucleoside
5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) has been shown to improve muscle endurance without exercise by activating AMPK, a kinase and cellular energy sensor. Its activation depends on increases in AMP:ATP. Lanner et al. demonstrated that AICAR significantly decreased Ca2+ leak and in turn, ROS and RNS generation in the Y524S mouse model [133]. Death typically occurs following heat exposure in this mouse model; however, this was prevented with AICAR treatment, thus indicating that AICAR may be beneficial, prophylactically, in those with exercise or heat intolerance due to variants in RYR1. AICAR has also shown benefit in aged myostatin knockout mice, improving running time and peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1alpha expression. To some degree, AICAR also improved mitochondrial function, although it did not increase the number of mitochondria. These benefits were either not observed or not observed to the same degree in aged wild-type mice [134]. Mitochondrial oxidative stress is associated with elevated cytosolic Ca2+ levels and has been implicated as a downstream pathomechanism in preclinical models of RYR1-RM [50, 135].
Pyridostigmine
Pyridostigmine, an acetylcholinesterase inhibitor, has been reported to improve fatigability related to neuromuscular junction (NMJ) signaling in CNM-RYR1-related cases [36]. Structural changes (reduced synaptic vesicles; short, shallow, and unbranched synaptic clefts) and abnormal innervation at the NMJ have been correlated with muscle disuse or motor endplate immaturity in CNM [136]. Additionally, decreased quantal release, reduced postsynaptic response, and acetylcholine receptor deficiency compromise neuromuscular transmission at the NMJ in CNM [137]. Response of RYR1-RM fatigable weakness to NMJ therapy such as pyridostigmine has been beneficial but nonsustained and therefore awaits further systematic confirmation [136].
Future Directions
Drug Development Considerations
The time taken to reach a definitive RYR1-RM diagnosis has been bolstered by NGS advances. However, the lag in bringing therapeutic compounds to clinical trial is recognized as a shortfall that needs addressing for rare diseases as a whole. For example, despite being successfully tested in an open-label study 14 years ago, salbutamol has not been tested in a randomized controlled trial and is still not officially approved for RYR1-RM-affected individuals.
This can present a financial burden for affected individuals, whose insurance may or may not cover off-label drugs in certain countries such as the USA. Indeed, passing of the Orphan Drug Act (ODA) in the USA paved the way for the FDA to establish “Orphan Drug Designation” which provides drug development incentives for sponsors working in the rare disease field. Despite this milestone, the drug development timeline for rare disease indications remains slow, in part owing to a backlog of ODA requests and, in 2017, the FDA unveiled a strategic plan to streamline the process. The resources, expertise, and sites available to perform simultaneous clinical trials remain a critical need and cannot be underestimated. In fact, a NAC trial in the SEPN1-RM population has been put on extended hiatus due to lack of continued resources, a common problem [138].
While advancing novel compound development is important for advancing potential RYR1-RM therapeutics, focus should also be placed on repurposing drugs already approved by regulatory bodies, such as the FDA, for other indications. The latter approach may represent a faster path to RYR1-RM (or congenital myopathy) orphan drug designation because such drugs have an established safety profile, and therefore, phase I trials may not be required.
Combinatorial Therapy
Future clinical trials may need to involve more complex designs with more than 2 arms in order to assess the potential benefits of combinational therapeutic approaches. A traditional parallel-group RCT with 2 arms (treatment and placebo) may need to be supplemented by adding an arm combining 2 treatments to test for their synergistic effect. Factorial RCT or crossover designs (with washout) may be helpful to this effect. As in various other conditions, future approved treatments may ultimately involve more than one compound, or a “cocktail,” to address different angles of the pathomechanism of the disease [127].
Clinical Trial Readiness
As advances in NGS technology improve genetic testing methodology, the likelihood of identifying causative variants in diseases such as RYR1-RM is increasing. The trend towards using molecular genetic testing as the first diagnostic tool in CM, without muscle biopsy, is gaining momentum in the field of neuromuscular diseases. This genetics-led approach is justified based on the abovementioned histopathological overlap and its time- and cost-effective nature. By proxy, detailed genetic characterization of RYR1-RM-affected individuals will aid in optimizing the specificity of inclusion criteria for clinical trials. As such, clinical trial teams will be able to take into account the drug’s mechanism of action in the context of each individual’s genotype, as well as expected structural modifications, and/or functional consequences.
Accordingly, personalized therapeutic approaches may be required to adequately address the deleterious effects of specific variants and alleviate affected individuals’ symptoms. Emphasis should be placed on developing infrastructure that supports such approaches as it is highly unlikely that a “one treatment suits all” approach will be successful for this population.
Conclusion
The heterogeneous and expanding spectrum of RYR1-RM reflects the implication of calcium dysregulation on fundamental processes in the skeletal muscle cell. Presently, drugs approved for alternative indications have been used to manage symptoms in affected individuals, reflecting a palpable need for additional clinical trials in this population. However, securing sites to perform this labor-intensive work remains challenging, especially given the obstacles of performing trials in rare diseases. Current clinical trial efforts, further understanding of the structure and function of RyR1, and the role of specific pathogenic RYR1 variants will provide a path forward to investigating effective therapeutic agents to manage and treat RYR1-RM.
Acknowledgments
This work was supported by funding from the NIH NINR Division of Intramural Research. We would also like to acknowledge Drs. Ronald Cohn, James Dowling, and Joan Austin for their expert review of this manuscript.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Summary Although there have been reports of off-label drug responses to manage RYR1-related myopathy (RYR1-RM) symptoms, no FDA-approved treatments exist to date. We present an overview of RYR1-RM diagnostic approaches, including genetic testing, muscle imaging, and histopathological findings. We also discuss the current state of investigational treatments and focus on disease-modulating (nongenetic) therapeutic strategies for RYR1-RM. Finally, perspectives for future approaches to treatment development are broached.
References
- 1.Dowling JJ, North KN, Goebel HH, Beggs AH. Chapter 28 - Congenital and Other Structural Myopathies A2 - Darras, Basil T. In: Jones HR, Ryan MM, Vivo DCD, editors. Neuromuscular Disorders of Infancy, Childhood, and Adolescence. Second Edition. San Diego: Academic Press; 2015. pp. 499–537. [Google Scholar]
- 2.Darin N, Tulinius M. Neuromuscular disorders in childhood: a descriptive epidemiological study from western Sweden. Neuromuscular disorders : NMD. 2000;10(1):1–9. doi: 10.1016/s0960-8966(99)00055-3. [DOI] [PubMed] [Google Scholar]
- 3.Norwood FL, Harling C, Chinnery PF, Eagle M, Bushby K, Straub V. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain : a journal of neurology. 2009;132(Pt 11):3175–86. doi: 10.1093/brain/awp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amburgey K, McNamara N, Bennett LR, McCormick ME, Acsadi G, Dowling JJ. Prevalence of congenital myopathies in a representative pediatric united states population. Annals of neurology. 2011;70(4):662–5. doi: 10.1002/ana.22510. [DOI] [PubMed] [Google Scholar]
- 5.Witting N, Werlauff U, Duno M, Vissing J. Phenotypes, genotypes, and prevalence of congenital myopathies older than 5 years in Denmark. Neurology: Genetics. 2017;3(2):e140. doi: 10.1212/NXG.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo I, Scoto M, Manzur AY, et al. Congenital myopathies: Natural history of a large pediatric cohort. Neurology. 2015;84(1):28–35. doi: 10.1212/WNL.0000000000001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonorazky HD, Bonnemann CG, Dowling JJ. The genetics of congenital myopathies. Handbook of clinical neurology. 2018;148:549–64. doi: 10.1016/B978-0-444-64076-5.00036-3. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Ibarra MC, Malicdan MC, et al. Central core disease is due to RYR1 mutations in more than 90% of patients. Brain : a journal of neurology. 2006;129(Pt 6):1470–80. doi: 10.1093/brain/awl077. [DOI] [PubMed] [Google Scholar]
- 9.Meissner G. Regulation of mammalian ryanodine receptors. Frontiers in bioscience: a journal and virtual library. 2002;7:d2072–80. doi: 10.2741/A899. [DOI] [PubMed] [Google Scholar]
- 10.Magee KR, Shy GM. A new congenital non-progressive myopathy. Brain : a journal of neurology. 1956;79(4):610–21. doi: 10.1093/brain/79.4.610. [DOI] [PubMed] [Google Scholar]
- 11.Quane KA, Healy JM, Keating KE, et al. Mutations in the ryanodine receptor gene in central core disease and malignant hyperthermia. Nature genetics. 1993;5(1):51–5. doi: 10.1038/ng0993-51. [DOI] [PubMed] [Google Scholar]
- 12.Ferreiro A, Monnier N, Romero NB, et al. A recessive form of central core disease, transiently presenting as multi-minicore disease, is associated with a homozygous mutation in the ryanodine receptor type 1 gene. Annals of neurology. 2002;51(6):750–9. doi: 10.1002/ana.10231. [DOI] [PubMed] [Google Scholar]
- 13.Fattori F, Maggi L, Bruno C, et al. Centronuclear myopathies: genotype-phenotype correlation and frequency of defined genetic forms in an Italian cohort. Journal of neurology. 2015;262(7):1728–40. doi: 10.1007/s00415-015-7757-9. [DOI] [PubMed] [Google Scholar]
- 14.Scacheri PC, Hoffman EP, Fratkin JD, et al. A novel ryanodine receptor gene mutation causing both cores and rods in congenital myopathy. Neurology. 2000;55(11):1689–96. doi: 10.1212/wnl.55.11.1689. [DOI] [PubMed] [Google Scholar]
- 15.Clarke NF, Waddell LB, Cooper ST, et al. Recessive mutations in RYR1 are a common cause of congenital fiber type disproportion. Human mutation. 2010;31(7):E1544–50. doi: 10.1002/humu.21278. [DOI] [PubMed] [Google Scholar]
- 16.Dowling JJ, Lillis S, Amburgey K, et al. King-Denborough syndrome with and without mutations in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscular disorders : NMD. 2011;21(6):420–7. doi: 10.1016/j.nmd.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Matthews E, Neuwirth C, Jaffer F, et al. Atypical periodic paralysis and myalgia: A novel RYR1 phenotype. Neurology. 2018;90(5):e412–e8. doi: 10.1212/WNL.0000000000004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.North KN, Wang CH, Clarke N, et al. Approach to the diagnosis of congenital myopathies. Neuromuscular disorders : NMD. 2014;24(2):97–116. doi: 10.1016/j.nmd.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jungbluth H. Central core disease. Orphanet journal of rare diseases. 2007;2:25. doi: 10.1186/1750-1172-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H, Jungbluth H, Sewry CA, et al. Molecular mechanisms and phenotypic variation in RYR1-related congenital myopathies. Brain : a journal of neurology. 2007;130(8):2024–36. doi: 10.1093/brain/awm096. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan DH, Duff C, Zorzato F, et al. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990;343(6258):559–61. doi: 10.1038/343559a0. [DOI] [PubMed] [Google Scholar]
- 22.Wilmshurst JM, Lillis S, Zhou H, et al. RYR1 mutations are a common cause of congenital myopathies with central nuclei. Annals of neurology. 2010;68(5):717–26. doi: 10.1002/ana.22119. [DOI] [PubMed] [Google Scholar]
- 23.Tobin JR, Jason DR, Challa VR, Nelson TE, Sambuughin N. Malignant hyperthermia and apparent heat stroke. Jama. 2001;286(2):168–9. doi: 10.1001/jama.286.2.168. [DOI] [PubMed] [Google Scholar]
- 24.Groom L, Muldoon SM, Tang ZZ, et al. Identical de novo Mutation in the Type 1 Ryanodine Receptor Gene Associated with Fatal, Stress-induced Malignant Hyperthermia in Two Unrelated Families. Anesthesiology. 2011;115(5):938–45. doi: 10.1097/ALN.0b013e3182320068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iaizzo PA, Wedel DJ, Gallagher WJ. In vitro contracture testing for determination of susceptibility to malignant hyperthermia: a methodologic update. Mayo Clinic proceedings. 1991;66(10):998–1004. doi: 10.1016/s0025-6196(12)61722-4. [DOI] [PubMed] [Google Scholar]
- 26.Marty I, Fauré J. Excitation-Contraction Coupling Alterations in Myopathies. Journal of Neuromuscular Diseases. 2016;3(4):443–53. doi: 10.3233/JND-160172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy TV, Quane KA, Lynch PJ. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Human mutation. 2000;15(5):410–7. doi: 10.1002/(SICI)1098-1004(200005)15:5<410::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 28.Shaaban S, Ramos-Platt L, Gilles FH, et al. Ryr1 mutations as a cause of ophthalmoplegia, facial weakness, and malignant hyperthermia. JAMA Ophthalmology. 2013;131(12):1532–40. doi: 10.1001/jamaophthalmol.2013.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riazi S, Kraeva N, Muldoon SM, et al. Clinical Significance of Ryanodine Receptor 1 Gene (RYR1) Variants: Proceedings of the 2013 MHAUS Scientific Conference. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2014;61(11):1040–9. doi: 10.1007/s12630-014-0227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein A, Jungbluth H, Clement E, et al. Muscle magnetic resonance imaging in congenital myopathies due to ryanodine receptor type 1 gene mutations. Archives of neurology. 2011;68(9):1171–9. doi: 10.1001/archneurol.2011.188. [DOI] [PubMed] [Google Scholar]
- 31.Todd JJ, Razaqyar MS, Witherspoon JW, et al. Novel Variants in Individuals with RYR1-Related Congenital Myopathies: Genetic, Laboratory, and Clinical Findings. Frontiers in neurology. 2018;9:118. doi: 10.3389/fneur.2018.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd JJ, Sagar V, Lawal TA, et al. Correlation of phenotype with genotype and protein structure in RYR1-related disorders. Journal of neurology. 2018. [DOI] [PMC free article] [PubMed]
- 33.Treves S, Jungbluth H, Muntoni F, Zorzato F. Congenital muscle disorders with cores: the ryanodine receptor calcium channel paradigm. Current opinion in pharmacology. 2008;8(3):319–26. doi: 10.1016/j.coph.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H, Yamaguchi N, Xu L, et al. Characterization of recessive RYR1 mutations in core myopathies. Human molecular genetics. 2006;15(18):2791–803. doi: 10.1093/hmg/ddl221. [DOI] [PubMed] [Google Scholar]
- 35.Jungbluth H, Dowling JJ, Ferreiro A, Muntoni F. 182nd ENMC International Workshop: RYR1-related myopathies, 15-17th April 2011, Naarden, The Netherlands. Neuromuscular disorders : NMD. 2012;22(5):453–62. doi: 10.1016/j.nmd.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Dowling JJ, Lawlor MW, Dirksen RT. Triadopathies: An Emerging Class of Skeletal Muscle Diseases. Neurotherapeutics. 2014;11(4):773–85. doi: 10.1007/s13311-014-0300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreuder LTW, MWG N-v d S, de Hair A, et al. Successful use of albuterol in a patient with central core disease and mitochondrial dysfunction. Journal of Inherited Metabolic Disease. 2010;33(Suppl 3):205–9. doi: 10.1007/s10545-010-9085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zahradnikova A, Zahradnik I, Gyorke I, Gyorke S. Rapid activation of the cardiac ryanodine receptor by submillisecond calcium stimuli. The Journal of general physiology. 1999;114(6):787–98. doi: 10.1085/jgp.114.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santulli G, Lewis DR, Marks AR. Physiology and pathophysiology of excitation-contraction coupling: the functional role of ryanodine receptor. Journal of muscle research and cell motility. 2017;38(1):37–45. doi: 10.1007/s10974-017-9470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine Receptors: Structure, Expression, Molecular Details, and Function in Calcium Release. Cold Spring Harbor Perspectives in Biology. 2010;2(11):a003996. doi: 10.1101/cshperspect.a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McPherson PS, Campbell KP. Characterization of the major brain form of the ryanodine receptor/Ca2+ release channel. The Journal of biological chemistry. 1993;268(26):19785–90. [PubMed] [Google Scholar]
- 42.Hakamata Y, Nakai J, Takeshima H, Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS letters. 1992;312(2–3):229–35. doi: 10.1016/0014-5793(92)80941-9. [DOI] [PubMed] [Google Scholar]
- 43.Takeshima H, Nishimura S, Matsumoto T, et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339(6224):439–45. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- 44.des Georges A, Clarke OB, Zalk R, et al. Structural Basis for Gating and Activation of RyR1. Cell. 2016;167(1):145–157.e17. doi: 10.1016/j.cell.2016.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuchi Z, Van Petegem F. Ryanodine receptors under the magnifying lens: Insights and limitations of cryo-electron microscopy and X-ray crystallography studies. Cell calcium. 2016;59(5):209–27. doi: 10.1016/j.ceca.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Van Petegem F. Ryanodine receptors: structure and function. The Journal of biological chemistry. 2012;287(38):31624–32. doi: 10.1074/jbc.R112.349068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee CS, Hanna AD, Wang H, et al. A chemical chaperone improves muscle function in mice with a RyR1 mutation. Nature communications. 2017;8:14659. doi: 10.1038/ncomms14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou H, Rokach O, Feng L, et al. RyR1 deficiency in congenital myopathies disrupts excitation-contraction coupling. Human mutation. 2013;34(7):986–96. doi: 10.1002/humu.22326. [DOI] [PubMed] [Google Scholar]
- 49.Avila G, Dirksen RT. Functional effects of central core disease mutations in the cytoplasmic region of the skeletal muscle ryanodine receptor. The Journal of general physiology. 2001;118(3):277–90. doi: 10.1085/jgp.118.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowling JJ, Arbogast S, Hur J, Nelson DD, McEvoy A, Waugh T, et al. Oxidative stress and successful antioxidant treatment in models of RYR1-related myopathy. Brain : a journal of neurology. 2012;135(Pt 4):1115–27. doi: 10.1093/brain/aws036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Durham WJ, Aracena-Parks P, Long C, et al. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133(1):53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathews KD, Moore SA. Multiminicore myopathy, central core disease, malignant hyperthermia susceptibility, and RYR1 mutations: one disease with many faces? Archives of neurology. 2004;61(1):27–9. doi: 10.1001/archneur.61.1.27. [DOI] [PubMed] [Google Scholar]
- 53.TUoCGSL. Congenital Myopathy Sequencing Panel [Available from: http://dnatesting.uchicago.edu/tests/congenital-myopathy-sequencing-panel.
- 54.PG. Congenital Myopathy Sequencing Panel [Available from: https://www.preventiongenetics.com/testInfo?sel=test&val=Congenital+Myopathy+Sequencing+Panel.
- 55.Gray RM. Anesthesia-induced rhabdomyolysis or malignant hyperthermia: is defining the crisis important? Paediatric anaesthesia. 2017;27(5):490–3. doi: 10.1111/pan.13130. [DOI] [PubMed] [Google Scholar]
- 56.Dlamini N, Voermans NC, Lillis S, et al. Mutations in RYR1 are a common cause of exertional myalgia and rhabdomyolysis. Neuromuscular Disorders. 2013;23(7):540–8. doi: 10.1016/j.nmd.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Sewry CA, Muller C, Davis M, et al. The spectrum of pathology in central core disease. Neuromuscular disorders : NMD. 2002;12(10):930–8. doi: 10.1016/s0960-8966(02)00135-9. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi K, Miller Rodman G, Brownell AKW. Central core disease: Ultrastructure of the sarcoplasmic reticulum and T-tubules. Muscle & Nerve. 2004;12(2):95–102. doi: 10.1002/mus.880120203. [DOI] [PubMed] [Google Scholar]
- 59.Fardeau M TF. Congenital myopathies In: Myology. 2nd ed. New York: McGraw-Hill;; 1994.
- 60.Jungbluth H, Sewry CA, Muntoni F. Core myopathies. Seminars in pediatric neurology. 2011;18(4):239–49. doi: 10.1016/j.spen.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Romero NB, Monnier N, Viollet L, et al. Dominant and recessive central core disease associated with RYR1 mutations and fetal akinesia. Brain : a journal of neurology. 2003;126(Pt 11):2341–9. doi: 10.1093/brain/awg244. [DOI] [PubMed] [Google Scholar]
- 62.Bharucha-Goebel DX, Santi M, Medne L, et al. Severe congenital RYR1-associated myopathy: the expanding clinicopathologic and genetic spectrum. Neurology. 2013;80(17):1584–9. doi: 10.1212/WNL.0b013e3182900380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shepherd S, Ellis F, Halsall J, Hopkins P, Robinson R. RYR1 mutations in UK central core disease patients: more than just the C-terminal transmembrane region of the RYR1 gene. Journal of Medical Genetics. 2004;41(3):e33–e. doi: 10.1136/jmg.2003.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naddaf E, Waclawik AJ. Two families with MYH7 distal myopathy associated with cardiomyopathy and core formations. Journal of clinical neuromuscular disease. 2015;16(3):164–9. doi: 10.1097/CND.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 65.Schartner V, Romero NB, Donkervoort S, Treves S, Munot P, Pierson TM, et al. Dihydropyridine receptor (DHPR, CACNA1S) congenital myopathy. Acta neuropathologica. 2017;133(4):517–33. doi: 10.1007/s00401-016-1656-8. [DOI] [PubMed] [Google Scholar]
- 66.Jungbluth H, Zhou H, Hartley L, et al. Minicore myopathy with ophthalmoplegia caused by mutations in the ryanodine receptor type 1 gene. Neurology. 2005;65(12):1930–5. doi: 10.1212/01.wnl.0000188870.37076.f2. [DOI] [PubMed] [Google Scholar]
- 67.Monnier N, Marty I, Faure J, et al. Null mutations causing depletion of the type 1 ryanodine receptor (RYR1) are commonly associated with recessive structural congenital myopathies with cores. Human mutation. 2008;29(5):670–8. doi: 10.1002/humu.20696. [DOI] [PubMed] [Google Scholar]
- 68.Carmignac V, Salih MA, Quijano-Roy S, et al. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Annals of neurology. 2007;61(4):340–51. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- 69.Boyden SE, Mahoney LJ, Kawahara G, et al. Mutations in the satellite cell gene MEGF10 cause a recessive congenital myopathy with minicores. Neurogenetics. 2012;13(2):115–24. doi: 10.1007/s10048-012-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tosch V, Rohde HM, Tronchere H, et al. A novel PtdIns3P and PtdIns(3,5)P2 phosphatase with an inactivating variant in centronuclear myopathy. Human molecular genetics. 2006;15(21):3098–106. doi: 10.1093/hmg/ddl250. [DOI] [PubMed] [Google Scholar]
- 71.Scoto M, Cullup T, Cirak S, et al. Nebulin (NEB) mutations in a childhood onset distal myopathy with rods and cores uncovered by next generation sequencing. European journal of human genetics : EJHG. 2013;21(11):1249–52. doi: 10.1038/ejhg.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Romero NB, Lehtokari VL, Quijano-Roy S, et al. Core-rod myopathy caused by mutations in the nebulin gene. Neurology. 2009;73(14):1159–61. doi: 10.1212/WNL.0b013e3181bacf45. [DOI] [PubMed] [Google Scholar]
- 73.Zanoteli E, Oliveira AS, Kiyomoto BH, Schmidt B, Gabbai AA. Centronuclear myopathy. Histopathological aspects in ten patients with childhood onset. Arquivos de neuro-psiquiatria. 1998;56(1):1–8. doi: 10.1590/s0004-282x1998000100001. [DOI] [PubMed] [Google Scholar]
- 74.Bitoun M, Bevilacqua JA, Prudhon B, et al. Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Annals of neurology. 2007;62(6):666–70. doi: 10.1002/ana.21235. [DOI] [PubMed] [Google Scholar]
- 75.Bevilacqua JA, Monnier N, Bitoun M, et al. Recessive RYR1 mutations cause unusual congenital myopathy with prominent nuclear internalization and large areas of myofibrillar disorganization. Neuropathology and Applied Neurobiology. 2010;37(3):271–84. doi: 10.1111/j.1365-2990.2010.01149.x. [DOI] [PubMed] [Google Scholar]
- 76.Jungbluth H, Gautel M. Pathogenic Mechanisms in Centronuclear Myopathies. Frontiers in Aging Neuroscience. 2014;6:339. doi: 10.3389/fnagi.2014.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laporte J, Guiraud-Chaumeil C, Vincent M-C, et al. Mutations in the MTM1 Gene Implicated in X-Linked Myotubular Myopathy. Human molecular genetics. 1997;6(9):1505–11. doi: 10.1093/hmg/6.9.1505. [DOI] [PubMed] [Google Scholar]
- 78.Jeannet PY, Bassez G, Eymard B, et al. Clinical and histologic findings in autosomal centronuclear myopathy. Neurology. 2004;62(9):1484–90. doi: 10.1212/01.wnl.0000124388.67003.56. [DOI] [PubMed] [Google Scholar]
- 79.Laughlin RS, Niu Z, Wieben E, Milone M. RYR1 causing distal myopathy. Molecular Genetics & Genomic Medicine. 2017;5(6):800–4. doi: 10.1002/mgg3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Clarke NF. Congenital fiber-type disproportion. Seminars in pediatric neurology. 2011;18(4):264–71. doi: 10.1016/j.spen.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 81.Laing NG, Clarke NF, Dye DE, et al. Actin mutations are one cause of congenital fibre type disproportion. Annals of neurology. 2004;56(5):689–94. doi: 10.1002/ana.20260. [DOI] [PubMed] [Google Scholar]
- 82.Sato I, Wu S, Ibarra MC, et al. Congenital neuromuscular disease with uniform type 1 fiber and RYR1 mutation. Neurology. 2008;70(2):114–22. doi: 10.1212/01.wnl.0000269792.63927.86. [DOI] [PubMed] [Google Scholar]
- 83.Jungbluth H, Davis MR, Muller C, et al. Magnetic resonance imaging of muscle in congenital myopathies associated with RYR1 mutations. Neuromuscular disorders : NMD. 2004;14(12):785–90. doi: 10.1016/j.nmd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 84.Lawlor MW, DeChene ET, Roumm E, Geggel AS, Moghadaszadeh B, Beggs AH. Mutations of tropomyosin 3 (TPM3) are common and associated with type 1 myofiber hypotrophy in congenital fiber type disproportion. Human mutation. 2010;31(2):176–83. doi: 10.1002/humu.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cassandrini D, Trovato R, Rubegni A, et al. Congenital myopathies: clinical phenotypes and new diagnostic tools. Italian journal of pediatrics. 2017;43(1):101. doi: 10.1186/s13052-017-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quijano-Roy S, Avila-Smirnow D, Carlier RY. Whole body muscle MRI protocol: pattern recognition in early onset NM disorders. Neuromuscular disorders : NMD. 2012;22(Suppl 2):S68–84. doi: 10.1016/j.nmd.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Ishibashi J, Perry RL, Asakura A, Rudnicki MA. MyoD induces myogenic differentiation through cooperation of its NH(2)- and COOH-terminal regions. The Journal of Cell Biology. 2005;171(3):471–82. doi: 10.1083/jcb.200502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Brini M, Manni S, Pierobon N, et al. Ca2+ signaling in HEK-293 and skeletal muscle cells expressing recombinant ryanodine receptors harboring malignant hyperthermia and central core disease mutations. The Journal of biological chemistry. 2005;280(15):15380–9. doi: 10.1074/jbc.M410421200. [DOI] [PubMed] [Google Scholar]
- 89.Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature. 1996;380:72. doi: 10.1038/380072a0. [DOI] [PubMed] [Google Scholar]
- 90.Filipova D, Walter AM, Gaspar JA, et al. Gene profiling of embryonic skeletal muscle lacking type I ryanodine receptor Ca(2+) release channel. Scientific reports. 2016;6:20050. doi: 10.1038/srep20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kushnir A, Betzenhauser MJ, Marks AR. Ryanodine receptor studies using genetically engineered mice. FEBS letters. 2010;584(10):1956–65. doi: 10.1016/j.febslet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang MDPDT, Riehl DVMPDJ, Esteve PDE, et al. Pharmacologic and Functional Characterization of Malignant Hyperthermia in the R163C RyR1 Knock-in Mouse. Anesthesiology. 2006;105(6):1164–75. doi: 10.1097/00000542-200612000-00016. [DOI] [PubMed] [Google Scholar]
- 93.Hirata H, Watanabe T, Hatakeyama J, et al. Zebrafish relatively relaxed mutants have a ryanodine receptor defect, show slow swimming and provide a model of multi-minicore disease. Development (Cambridge, England) 2007;134(15):2771–81. doi: 10.1242/dev.004531. [DOI] [PubMed] [Google Scholar]
- 94.Murayama T, Kurebayashi N, Ikegami-Yuasa M, et al. Efficient high-throughput screening by ER Ca<sup>2+</sup> measurement to identify inhibitors of ryanodine receptor Ca2+−release channels. Molecular Pharmacology. 2018. [DOI] [PubMed]
- 95.Henderson MJ, Wires ES, Trychta KA, Richie CT, Harvey BK. SERCaMP: a carboxy-terminal protein modification that enables monitoring of ER calcium homeostasis. Molecular biology of the cell. 2014;25(18):2828–39. doi: 10.1091/mbc.E14-06-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chamberlain K, Riyad JM, Weber T. Expressing Transgenes That Exceed the Packaging Capacity of Adeno-Associated Virus Capsids. Human Gene Therapy Methods. 2016;27(1):1–12. doi: 10.1089/hgtb.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mendell JR, Al-Zaidy S, Shell R, et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. New England Journal of Medicine. 2017;377(18):1713–22. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 98.Rendu J, Brocard J, Denarier E, et al. Exon Skipping as a Therapeutic Strategy Applied to an RYR1 Mutation with Pseudo-Exon Inclusion Causing a Severe Core Myopathy. Human Gene Therapy. 2013;24(7):702–13. doi: 10.1089/hum.2013.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Michelucci A, De Marco A, Guarnier FA, Protasi F, Boncompagni S. Antioxidant Treatment Reduces Formation of Structural Cores and Improves Muscle Function in RYR1Y522S/WT Mice. Oxidative Medicine and Cellular Longevity. 2017;2017:15. doi: 10.1155/2017/6792694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brillantes A-MB, Ondrias K, Scott A, et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77(4):513–23. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 101.Aracena P, Tang W, Hamilton SL, Hidalgo C. Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxidants & redox signaling. 2005;7(7–8):870–81. doi: 10.1089/ars.2005.7.870. [DOI] [PubMed] [Google Scholar]
- 102.Zissimopoulos S, Docrat N, Lai FA. Redox sensitivity of the ryanodine receptor interaction with FK506-binding protein. Journal of Biological Chemistry. 2007. [DOI] [PubMed]
- 103.Kelly GS. Clinical applications of N-acetylcysteine. Alternative medicine review : a journal of clinical therapeutic. 1998;3(2):114–27. [PubMed] [Google Scholar]
- 104.Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-acetylcysteine - a safe antidote for cysteine/glutathione deficiency. Current opinion in pharmacology. 2007;7(4):355–9. doi: 10.1016/j.coph.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalebic T, Kinter A, Poli G, Anderson ME, Meister A, Fauci AS. Suppression of human immunodeficiency virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N-acetylcysteine. Proceedings of the National Academy of Sciences. 1991;88(3):986–90. doi: 10.1073/pnas.88.3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. American journal of respiratory and critical care medicine. 1997;156(6):1897–901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- 107.Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(12):4628–33. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-acetylcysteine on Semen Parameters and Oxidative/Antioxidant Status. Urology. 2009;74(1):73–6. doi: 10.1016/j.urology.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 109.Hardan AY, Fung LK, Libove RA, et al. A Randomized Controlled Pilot Trial of Oral N-Acetylcysteine in Children with Autism. Biological Psychiatry. 2012;71(11):956–61. doi: 10.1016/j.biopsych.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang KM, Hu P, Wang SW, et al. Salbutamol changes the molecular and mechanical properties of canine skeletal muscle. The Journal of physiology. 1996;496(Pt 1):211–20. doi: 10.1113/jphysiol.1996.sp021678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Messina S, Hartley L, Main M, et al. Pilot trial of salbutamol in central core and multi-minicore diseases. Neuropediatrics. 2004;35(5):262–6. doi: 10.1055/s-2004-821173. [DOI] [PubMed] [Google Scholar]
- 112.Kissel JT, McDermott MP, Natarajan R, et al. Pilot trial of albuterol in facioscapulohumeral muscular dystrophy. FSH-DY Group. Neurology. 1998;50(5):1402–6. doi: 10.1212/wnl.50.5.1402. [DOI] [PubMed] [Google Scholar]
- 113.Kissel JT, McDermott MP, Mendell JR, et al. Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. 2001;57(8):1434–40. doi: 10.1212/wnl.57.8.1434. [DOI] [PubMed] [Google Scholar]
- 114.Ma L, Chu W, Chai J, Shen C, Li D, Wang X. ER stress and subsequent activated calpain play a pivotal role in skeletal muscle wasting after severe burn injury. PloS one. 2017;12(10):e0186128. doi: 10.1371/journal.pone.0186128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bannister RA. Dantrolene-Induced Inhibition of Skeletal L-Type Current Requires RyR1 Expression. BioMed Research International. 2013;2013:10. doi: 10.1155/2013/390493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meyler WJ, Wesseling H, Agoston S. The effects fo dantrolene sodium on cardiac and skeletal muscle in rats. European Journal of Pharmacology. 1976;39(1):127–31. doi: 10.1016/0014-2999(76)90120-5. [DOI] [PubMed] [Google Scholar]
- 117.Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene--a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59(4):364–73. doi: 10.1111/j.1365-2044.2004.03658.x. [DOI] [PubMed] [Google Scholar]
- 118.Michalek-Sauberer A, Gilly H. Prophylactic use of dantrolene in a patient with central core disease. Anesthesia and analgesia. 1998;86(4):915–6. doi: 10.1097/00000539-199804000-00047. [DOI] [PubMed] [Google Scholar]
- 119.Cannon SC. Mind the magnesium, in dantrolene suppression of malignant hyperthermia. Proceedings of the National Academy of Sciences. 2017;114(18):4576–8. doi: 10.1073/pnas.1704103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Choi RH, Koenig X, Launikonis BS. Dantrolene requires Mg<sup>2+</sup> to arrest malignant hyperthermia. Proceedings of the National Academy of Sciences. 2017;114(18):4811–5. doi: 10.1073/pnas.1619835114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kushnir A, Marks AR. Ryanodine receptor patents. Recent patents on biotechnology. 2012;6(3):157–66. doi: 10.2174/1872208311206030157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lehnart SE, Mongillo M, Bellinger A, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. The Journal of Clinical Investigation. 2008;118(6):2230–45. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mei Y, Xu L, Kramer HF, Tomberlin GH, Townsend C, Meissner G. Stabilization of the skeletal muscle ryanodine receptor ion channel-FKBP12 complex by the 1,4-benzothiazepine derivative S107. PloS one. 2013;8(1):e54208. doi: 10.1371/journal.pone.0054208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bellinger AM, Reiken S, Carlson C, et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nature Medicine. 2009;15:325. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Crunkhorn S. Fixing the leak. Nature reviews Drug discovery. 2009;8(4):277. doi: 10.1038/nrd2857. [DOI] [PubMed] [Google Scholar]
- 126.Andersson Daniel C, Betzenhauser Matthew J, Reiken S, et al. Ryanodine Receptor Oxidation Causes Intracellular Calcium Leak and Muscle Weakness in Aging. Cell Metabolism. 2011;14(2):196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Capogrosso RF, Mantuano P, Uaesoontrachoon K, et al. Ryanodine channel complex stabilizer compound S48168/ARM210 as a disease modifier in dystrophin-deficient mdx mice: proof-of-concept study and independent validation of efficacy. The FASEB Journal. 2018;32(2):1025–43. doi: 10.1096/fj.201700182RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaneko N. New 1,4-benzothiazepine derivative, K201, demonstrates cardioprotective effects against sudden cardiac cell death and intracellular calcium blocking action. Drug Development Research. 2004;33(4):429–38. [Google Scholar]
- 129.Darcy YL, Diaz-Sylvester PL, Copello JA. K201 (JTV519) is a Ca<sup>2+</sup>−Dependent Blocker of SERCA and a Partial Agonist of Ryanodine Receptors in Striated Muscle. Molecular Pharmacology. 2016;90(2):106–15. doi: 10.1124/mol.115.102277. [DOI] [PubMed] [Google Scholar]
- 130.Chen W, Koop A, Liu Y, et al. Reduced threshold for store overload-induced Ca(2+) release is a common defect of RyR1 mutations associated with malignant hyperthermia and central core disease. The Biochemical journal. 2017;474(16):2749–61. doi: 10.1042/BCJ20170282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lakatta EG. Functional implications of spontaneous sarcoplasmic reticulum Ca2+ release in the heart. Cardiovascular Research. 1992;26(3):193–214. doi: 10.1093/cvr/26.3.193. [DOI] [PubMed] [Google Scholar]
- 132.Chen W, Wang R, Chen B, et al. The ryanodine receptor store-sensing gate controls Ca2+ waves and Ca2+−triggered arrhythmias. Nature Medicine. 2014;20:184. doi: 10.1038/nm.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lanner JT, Georgiou DK, Dagnino-Acosta A, et al. AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation. Nat Med. 2012;18(2):244–51. doi: 10.1038/nm.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pauly M, Chabi B, Favier FB, et al. Combined Strategies for Maintaining Skeletal Muscle Mass and Function in Aging: Myostatin Inactivation and AICAR-Associated Oxidative Metabolism Induction. The journals of gerontology Series A, Biological sciences and medical sciences. 2015;70(9):1077–87. doi: 10.1093/gerona/glu147. [DOI] [PubMed] [Google Scholar]
- 135.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. American journal of physiology Cell physiology. 2004;287(4):C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 136.Illingworth MA, Main M, Pitt M, et al. RYR1-related congenital myopathy with fatigable weakness, responding to pyridostigimine. Neuromuscular Disorders. 2014;24(8):707–12. doi: 10.1016/j.nmd.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 137.Liewluck T, Shen X-M, Milone M, Engel AG. Endplate structure and parameters of neuromuscular transmission in sporadic centronuclear myopathy associated with myasthenia. Neuromuscular Disorders. 2011;21(6):387–95. doi: 10.1016/j.nmd.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lièvre M, Ménard J, Bruckert E, et al. Premature discontinuation of clinical trial for reasons not related to efficacy, safety, or feasibility. BMJ : British Medical Journal. 2001;322(7286):603–6. doi: 10.1136/bmj.322.7286.603. [DOI] [PMC free article] [PubMed] [Google Scholar]