Abstract
Duchenne muscular dystrophy (DMD) is a progressive X-linked degenerative muscle disease due to mutations in the DMD gene. Genetic confirmation has become standard in recent years. Improvements in the standard of care for DMD have led to improved survival. Novel treatments for DMD have focused on reducing the dystrophic mechanism of the muscle disease, modulating utrophin protein expression, and restoring dystrophin protein expression. Among the strategies to reduce the dystrophic mechanisms are 1) inhibiting inflammation, 2) promoting muscle growth and regeneration, 3) reducing fibrosis, and 4) facilitating mitochondrial function. The agents under investigation include a novel steroid, myostatin inhibitors, idebenone, an anti-CTGF antibody, a histone deacetylase inhibitor, and cardiosphere-derived cells. For utrophin modulation, AAV-mediated gene therapy with GALGT2 is currently being investigated to upregulate utrophin expression. Finally, the strategies for dystrophin protein restoration include 1) nonsense readthrough, 2) synthetic antisense oligonucleotides for exon skipping, and 3) AAV-mediated micro/minidystrophin gene delivery. With newer agents, we are witnessing the use of more advanced biotechnological methods. Although these potential breakthroughs provide significant promise, they may also raise new questions regarding treatment effect and safety.
Electronic supplementary material
The online version of this article (10.1007/s13311-018-00687-z) contains supplementary material, which is available to authorized users.
Keywords: Duchenne muscular dystrophy, Dystrophin, Gene therapy
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder that affects approximately 1 in 5000 live male births [1, 2]. It was first described in detail in the 1860s by the French neurologist Guillaume-Benjamin-Amand Duchenne [3]. Patients with Duchenne muscular dystrophy usually exhibit motor symptoms within the first 3 years of life. Most commonly, they may have a “waddling” gait that results from hip-girdle weakness and require the use of their hands when they get up from the floor (Gower’s maneuver). Serum creatine kinase (CK) levels are typically markedly elevated [4].
The disease is due to an absence of the dystrophin protein in the skeletal muscle membrane, and muscles lacking dystrophin are more susceptible to mechanical injury. Absence of dystrophin may be demonstrated by the absence of immunostaining for dystrophin on muscle biopsy. Genetic testing, however, has become more readily available in recent years and has become the standard method of diagnostic confirmation. Typically, genetic testing starts with screening for duplications or deletions either by multiplex ligation-dependent probe amplification (MLPA) or by microarray analysis. If duplication/deletion testing is negative, then sequencing of all 79 exons is performed to detect missense, nonsense, splice site, and small indel mutations [5]. This analysis will not detect intronic mutations and rearrangements, however, and these cases may require muscle biopsy to demonstrate the absence of dystrophin. If available, RNA sequencing may be performed on the muscle tissue to identify the specific intronic mutation or rearrangement.
DMD is considered a multisystem disease [4–6]. As the patient ages, there is progressive muscle weakness, respiratory insufficiency, musculoskeletal deformities, and cardiomyopathy. In addition, cognitive impairment, autism spectrum disorder, and behavior problems are not uncommonly seen but are not progressive. Progressive pulmonary insufficiency and orthopedic issues are a direct result of the progressive skeletal muscle weakness; cardiomyopathy and cognitive/behavioral issues, however, are most likely a result of aberrant dystrophin expression in these tissues. The degree of cardiomyopathy or cognitive/behavioral impairment, however, is variable and is often not correlated with the degree of skeletal muscle involvement. It is not exactly clear what would contribute to this variability; genetic modifiers are likely to play some role, and the location of the mutation within the DMD gene and the effect on specific isoforms of dystrophin may also play some role in determining the phenotypic profile.
Dystrophin and the DMD Gene
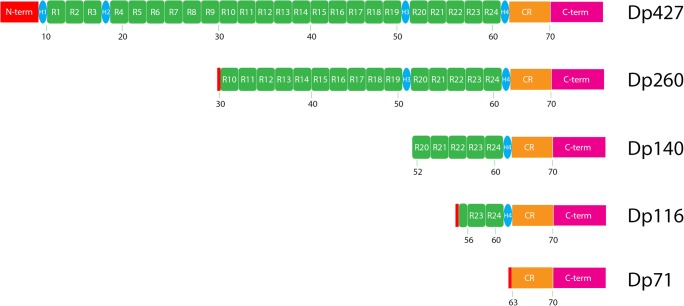
Approximately 125 years after Duchenne described the disease, it was linked to the DMD gene [7] on the X chromosome and demonstrated to encode a 427-kDa protein appropriately named dystrophin [8]. This protein is characterized by an N-terminal actin binding domain, a central rod domain, and a C-terminal domain (see Fig. 1) that binds to a membrane-bound protein complex known as the dystrophin-associated glycoprotein complex. The rod domain is comprised of 24 spectrin-like repeats, interspersed with 4 hinge domains (see Fig. 1).
Fig. 1.
The canonical dystrophin protein, Dp427, and the different isoforms: Dp260, Dp140, Dp116, and Dp71. The numbers under the constructs show the map to the approximate exon encoding that region. N-term = N terminal domain, R# = spectrin repeat number, H# = hinge number, CR = cysteine-rich domain, C-term = C terminal domain
The DMD gene is considered one of the largest genes in the human genome, and many mutations have been reported. De novo mutations appear to be common, with estimates ranging between 12 and 33% of patients with DMD [9–11]. Estimates of the prevalence of different mutation types vary, but recent reports suggest that, among DMD patients, 69% have large deletions, 11% have large duplications, 10% have nonsense mutations, 7% have missense or small indels, and another 3% have intronic or other mutations [12]. Monaco et al. [13] proposed that deletions [within the central rod domain] that preserve the reading frame hypothesis would likely result in a milder Becker muscular dystrophy (BMD) phenotype, whereas deletions that disrupt the reading frame are likely to result in a severe DMD phenotype. This reading frame hypothesis has been shown to be valid in about 91% of 4700 patients entered into the Leiden database that was collecting genotypes and phenotypes of DMD mutations [14].
There are several isoforms of the dystrophin protein that have been described and are driven by different promoters (see Fig. 1). These isoforms include the variants of the canonical Dp427—expressed in lymphocytes, the brain, and muscle and driven by promoters upstream of exon 1 [7, 15]; Dp260—expressed in the retina and driven by a promoter upstream of exon 30 [16]; Dp140—expressed in the brain and kidney and driven by a promoter upstream of exon 45 [17]; Dp116—expressed in Schwann cells and driven by a promoter upstream of exon 56 [18]; and Dp71—ubiquitously expressed and driven by a promoter upstream of exon 63 [19]. Thus, the location of the mutation within the DMD gene may determine the phenotypic profile of the patient through differential expression of these isoforms.
Although the reading frame is useful in predicting the severity of skeletal muscle weakness, there is still some phenotypic variability within the prediction. Genetic modifiers are likely to contribute some role in this phenotypic variability, and SPP1 and LTB4, among others, have been reported to modify the phenotype of DMD [20].
Treatment for Duchenne Muscular Dystrophy
As standards of care for DMD have evolved, survival has also improved [21]. Updated care considerations for DMD have recently been published [4–6]. Among the standards of care discussed, oral glucocorticoids, cardiac management, pulmonary management, vaccinations, physical therapy and orthopedic care, nutritional management, and bone health are often highlighted. The goals of these care considerations are to improve long-term survival, maintain mobility and independence, and improve overall quality of life.
The development of validated outcome measures for DMD has helped to improve clinical trial methodology for newer agents under investigation. These agents can be categorized into 3 strategies: 1) reducing the dystrophic mechanisms of disease, 2) modulating utrophin protein expression, and 3) restoring dystrophin protein expression. Reviewed here are the current approaches that have been approved for use or are still under investigation (Table 1).
Table 1.
Agents under investigation for Duchenne muscular dystrophy
| Category | Agent | Description |
|---|---|---|
| Anti-inflammatory | Vamorolone | Novel steroid analog with a potentially favorable side-effect profile |
| Edasalonexent | NF-κB inhibitor | |
| Myostatin | Domagrozumab | Humanized monoclonal antibody against myostatin, leading to muscle growth/regeneration |
| Talditercept alfa | Human IgG1(Fc)–adnectin fusion that binds myostatin, leading to muscle growth/regeneration | |
| Others | Idebenone | Facilitating mitochondrial function and metabolism |
| Givinostat | HDAC inhibitor, potentially leading to anti-inflammatory, anti-fibrotic, and regenerative effects | |
| Pamrevlumab | Human monoclonal antibody against connective tissue growth factor (CTGF), anti-fibrotic | |
| CAP-1002 | Allogenic cardiosphere-derived cells | |
| Utrophin modulation | rAAVrh74.MCK.GALGT2 | Gene therapy with GALGT2 to modulate utrophin expression |
| Dystrophin protein restoration | Ataluren | Nonsense suppression |
| Golodirsen | Exon 53 skipping, PMO chemistry | |
| Casimersen | Exon 45 skipping, PMO chemistry | |
| SRP-5051 | Exon 51 skipping, PPMO chemistry | |
| NS-065 | Exon 53 skipping, PMO chemistry | |
| WVE-210201 | Exon 51 skipping, stereo-pure phosphorothioate chemistry | |
| DS-5141b | Exon 45 skipping, 2′-4′-ethylene-bridge nucleic acid/2′-OMe-RNA chemistry | |
| rAAVrh74.MHCK7.Micro-dystrophin | Microdystrophin gene therapy, AAVrh74 vector | |
| SGT-001 | Microdystrophin gene therapy, AAV9 vector | |
| PF-06939926 | Minidystrophin gene therapy, AAV9 vector |
Anti-Inflammatory
Glucocorticoid treatment has been the standard of care for patients with DMD [5]. Prednisone 0.75 mg/kg/day and deflazacort 0.9 mg/kg/day are the most commonly recommended steroid regimens, although other dosing regimens are also used [22]. The benefit of steroids is apparent within months of starting treatment [23], and starting steroids at a younger age is associated with improved outcomes [24]. A large-scale observational study conducted through the CINRG network demonstrated that long-term steroid use resulted in improved outcomes including ambulation [25], and another study has demonstrated reduced need for scoliosis surgery [26]. Deflazacort has been associated with less weight gain [27], which may improve its tolerability. A multicenter, randomized, double-blind, placebo-controlled study comparing deflazacort, prednisone, and placebo was completed in 1995 and demonstrated data suggesting possible superiority efficacy of deflazacort over prednisone [28]. Since deflazacort was previously not available in the USA, additional clinical studies were performed to secure Food and Drug Administration (FDA) approval, which was granted in 2017. Steroid use is now a prerequisite for enrollment in most interventional clinical trials for DMD. This premise was used for a post hoc analysis of the placebo arm from the ataluren phase 3 study for DMD, which demonstrated improved outcomes in deflazacort-treated patients compared to prednisone-treated patients [29]. A 5-year, randomized, double-blind study comparing daily deflazacort, daily prednisone, and intermittent (10 days on, 10 days off) prednisone is ongoing [30].
Recently, vamorolone [31] was identified as a novel steroid analog based on membrane-stabilizing and anti-inflammatory properties (including inhibition of NF-κB) without significant immunosuppressive or hormonal effects. Studies are ongoing to establish the safety profile and efficacy of vamorolone [32], looking for potential advantages of the novel steroid analog over currently used steroids. In a similar vein, edasalonexent was identified as an inhibitor of NF-κB that may benefit DMD patients based on its anti-inflammatory properties [33]. A phase 2 study in DMD patients has recently been completed [34], with a phase 3 study currently being planned.
Myostatin
As DMD is a degenerative process with progressive development of necrotic muscle fibers, mechanisms that promote muscle regeneration are a reasonable strategy for treatment. Myostatin (GDF8) was originally identified in mice as a TGF-β superfamily member that demonstrated significant hypertrophy and hyperplasia of muscles [35]. It was subsequently shown that loss of myostatin attenuated the severity of muscular dystrophy in the mdx mouse (a mouse model for Duchenne) [36]. Thus, inhibiting myostatin may serve as an appropriate strategy in treating DMD.
Recently, there have been 2 anti-myostatin programs that are focused on DMD. A large phase 2 study of domagrozumab [37], an intravenous humanized monoclonal antibody against myostatin given monthly, was recently terminated due to lack of efficacy [38]. Talditercept alfa, a weekly, subcutaneous human IgG1(Fc)–adnectin fusion that binds myostatin, is currently being evaluated in an ongoing phase 3 clinical trial [39].
Other Mechanisms
Idebenone is a synthetic short-chain benzoquinone that, like coenzyme Q10, carries energy equivalents within the electron transport chain. Unlike coenzyme Q10, however, idebenone also shuttles energy equivalents from the cytosol to the electron transport chain [40]. Thus, the facilitation of metabolic pathways is the rationale for its potential effect in DMD. A phase 3, randomized, double-blind, placebo-controlled study of idebenone in DMD patients not using glucocorticoids demonstrated improved pulmonary function among the treated population [41]. Whether idebenone will potentially benefit patients who are on steroids is being addressed in an ongoing phase 3, randomized, double-blind, placebo-controlled study of idebenone for DMD patients who are taking glucocorticoids [42].
Histone deacetylase (HDAC) inhibitors have been shown to activate gene expression, and previous studies have suggested that HDAC inhibitors confer a benefit in the mdx mouse [43]. Givinostat is an HDAC inhibitor that is being investigated in DMD patients; the proposed rationale is based on the expression of anti-inflammatory, anti-fibrotic, and proregenerative genes. A phase 3, randomized, double-blind, placebo-controlled study of givinostat in DMD patients is currently ongoing [44].
Pamrevlumab is a fully human monoclonal antibody against connective tissue growth factor (CTGF). The rationale is that the anti-fibrotic properties of pamrevlumab will likely be beneficial to DMD patients. A phase 2, open-label study of pamrevlumab in DMD patients is currently ongoing [45].
CAP-1002 contains allogenic cardiosphere-derived cells (CDCs), and preclinical studies suggest that CAP-1002 improves mdx mice through the secretion of growth factors and exosomes [46]. CAP-1002 has been proposed to act by modulating the immune system, inhibiting fibrosis, and promoting regeneration [46]. A phase 2, randomized, double-blind, placebo-controlled study in nonambulatory boys and men with DMD is currently ongoing [47].
Utrophin Modulation
Utrophin was originally identified as “dystrophin-related protein” (DRP) based on its significant homology to dystrophin [48]. In contrast to dystrophin, however, utrophin is expressed mostly at neuromuscular junctions [49]. It was subsequently shown that dystrophin–utrophin double mutants demonstrated more severe muscle weakness than the dystrophin-only mutant mice (mdx mouse) [50, 51]. Finally, overexpression of utrophin in the mdx mouse appeared to prevent these mice from developing any signs of muscular dystrophy [52]. In a high-throughput screen for small molecules that activate utrophin transcription, ezutromid was identified. A phase 2 study of ezutromid in DMD patients [53], however, demonstrated no efficacy signals [54].
A different approach to modulating utrophin expression is being taken at Nationwide Children’s Hospital. The strategy in this study is to overexpress Galgt2 using adeno-associated virus (AAVrh74)-mediated gene delivery of the GALGT2 gene [55]. Galgt2 normally acts at the synaptic regions to add the terminal GalNAc to an O-linked carbohydrate antigen on the α-dystroglycan near the neuromuscular junctions [56]. Overexpression of GALGT2 results in ectopic expression of synapse-associated proteins, including utrophin, away from the synaptic regions [56]. Thus, overexpression of GALGT2 may ameliorate the phenotype of DMD by upregulating utrophin expression along myofibers. Overexpression of galgt2 protein in mdx mice prevents muscular dystrophy when the GALGT2 gene is introduced transgenically [56] as well as through AAVrh74-mediated gene delivery [57]. A first-in-human study of AAVrh74-mediated GALGT2 gene delivery in DMD boys began recruiting in 2018 [55].
Dystrophin Protein Restoration
A number of strategies are also being employed to restore dystrophin protein expression. Most of these strategies are not designed to restore the native wild-type protein. Nonetheless, expression of the altered dystrophin protein may confer a better phenotype than absence of expression or a truncated, unstable protein.
Dystrophin restoration through nonsense suppression
Ataluren is a small molecule that was identified to suppress nonsense mutations, promoting full-length dystrophin expression in cell culture [58]. This molecule has been studied in humans, most recently in a large phase 3 study of DMD patients with nonsense mutations that showed benefit in a number of outcome measures including the 6-min walk distance (6MWD), particularly among patients whose baseline 6MWDs were between 300 and 400 m [59]. Ataluren has received conditional approval by the European Medicines Agency (EMA), but did not receive approval from the FDA. Additional studies are being conducted to address some of the questions raised by the FDA, including a study to confirm the benefit that was seen among patients with baseline 6MWDs between 300 and 400 m [60] and another study to assess dystrophin expression in the muscle biopsies of ataluren-treated patients [61].
-
2)
Dystrophin restoration through exon skipping
The term “exon skipping” commonly refers to the use of synthetic antisense oligonucleotides (ASO) to inhibit a splice enhancer site to prevent a particular exon from participating in splicing [62]. As predicted by the reading frame rule [13], deletion mutations that shift the reading frame would be predicted to result in a truncated and/or unstable protein. Certain patients, however, may have a deletion where the reading frame could be restored by skipping an additional exon adjacent to the deletion. The altered transcript would be shortened, but this shortened coding sequence would not have a disrupted reading frame, permitting the expression of a stable functional protein [63].
Based on prevalence data from a large database of patients, 13 to 14% of DMD patients had an out-of-frame deletion where the reading frame would be corrected by skipping exon 51 [12, 64], which is more than the number of patients that would be corrected by skipping other exons. Thus, the first clinical trials for exon skipping were conducted in patients with deletions where the reading frame was corrected by (amenable to) skipping exon 51. This included drisapersen (BioMarin - San Rafael, CA) [65], a 2′-O-methyl phosphorothioate ASO, and eteplirsen (Sarepta Therapeutics - Cambridge, MA) [66], a phosphorodiamidate morpholino oligomer (PMO). The data for drisapersen was submitted to the FDA but did not receive approval, and BioMarin has decided that they will no longer pursue regulatory approval for drisapersen. Eteplirsen, however, was approved by the FDA in 2016 based on dystrophin protein biomarker data under an Accelerated Approval pathway, which requires additional data from confirmatory trials to be submitted to the FDA to verify and describe the anticipated clinical benefit. Eteplirsen was the FDA’s first journey into the use of dystrophin quantification as a surrogate outcome measure for DMD. Based on this experience, quantification methods were developed with feedback from the FDA [67]. This experience has also influenced the FDA guidance documents on developing drugs for Duchenne muscular dystrophy [68] and bioanalytical method validation [69].
Sarepta has additional PMOs that are also currently being studied in Duchenne muscular dystrophy patients. Golodirsen, designed to skip exon 53, and casimersen, designed to skip exon 45, are currently being evaluated in a phase 3 double-blind, placebo-controlled study [70]. Based on prevalence data (12, 64), 8.1 to 9.0% of all DMD patients may be a candidate for casimersen, and although 7.7 to 10.1% of DMD patients may be candidates for golodirsen, the number of new patients for golodirsen may only be an additional 5.7 to 8.1% beyond eteplirsen-treated patients, since approximately 2% of DMD patients are exon 52 deletion patients who are candidates for either exon 53 skipping or exon 51 skipping [12].
Preclinical data on peptide-conjugated PMO (PPMO) in mice and nonhuman primates demonstrates increased penetration into muscle tissue, improved potency, and a favorable pharmacokinetic profile over the PMO chemistry, with notable penetration into the heart and diaphragm [71, 72]. SRP-5051, a PPMO targeting exon 51 under development by Sarepta, is currently being studied in a phase 1 clinical trial [73].
Three other exon-skipping programs are also underway. NS-065 (NS Pharma - Paramus, NJ) is another morpholino antisense oligonucleotide (ASO) that has been studied in DMD boys amenable to exon 53 skipping [74]. WVE-210201 (Wave Life Sciences - Singapore) is a stereo-pure phosphorothioate ASO, using the stereoisomer that has been optimized for potency for exon skipping. A phase 1 of WVE-210201 for patients amenable to exon 51 skipping is currently ongoing [75]. DS-5141b (Daiichi Sankyo, Tokyo, Japan) is an ASO comprised of 2′-4′-ethylene-bridge nucleic acid (ENA) and 2′-O-methyl RNA [76] and is designed to skip exon 45. A phase 1 study was recently completed [77].
-
3)
Dystrophin restoration through gene therapy
The large size of the dystrophin protein meant that full gene replacement therapy for DMD was only feasible with large viruses such as adenoviral vectors [78]. The death of Jesse Gelsinger in 1999 during a gene therapy clinical trial for ornithine trans-carbamylase (OTC) deficiency [79], however, raised significant concerns about the safety of gene therapy with an adenoviral vector [80]. England et al., however, reported in 1990 a patient with very mild muscular dystrophy with a deletion of exons 17 to 48 [81]. This patient was still ambulatory at age 61, despite missing more than 46% of the protein sequence, illustrating that very mild phenotypes may be possible even with very large deletions of the dystrophin gene. After 1999, the gene therapy strategy for Duchenne shifted to optimizing miniaturized versions of dystrophin, often nicknamed “minidystrophins” or “microdystrophins” that would be small enough to be packaged in an adeno-associated virus (AAV), which has a payload capacity of ~ 5 kb [82]. Over the past 16 years, the preclinical and clinical investigational experience with AAVs has grown to provide a better understanding of the safety profile of these viruses. This experience has opened the door to a number of gene therapy strategies for various genetic diseases, including the micro/minidystrophin gene delivery programs for DMD.
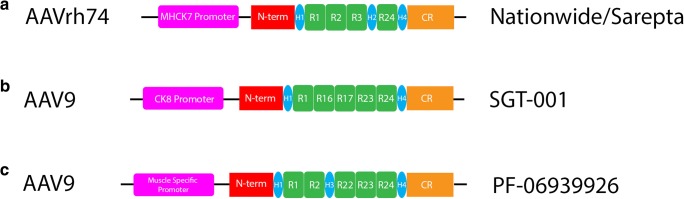
Currently, there are 3 gene therapy programs for DMD that are actively enrolling. All utilize a muscle-specific promoter that is driving expression of different micro/minidystrophins (Fig. 2). The Sarepta program uses an AAVrh74 capsid with an MHCK7 promoter [83, 84]. SGT-001 (Solid Biosciences - Cambridge, MA) is an AAV9 capsid with a CK8 promoter driving microdystrophin expression [85, 86]. PF-06939936 (Pfizer - New York, NY) also uses an AAV9 capsid with a muscle-specific promoter that Pfizer has not publicly disclosed [87, 88]. All 3 programs began dosing in 2018. Although there has been much interest in gene therapy in recent years, patients with immunity to the AAV vector would not be candidates for gene therapy. Some investigators have raised questions about plasmapheresis and other measures to attenuate immunity to the AAV vector, but the author of this review is not aware of any formal protocols under development.
Fig. 2.
The 3 AAV micro/minidystrophin agents currently under investigation. A Sarepta/Nationwide’s agent uses an AAVrh74 capsid, with MHCK7 promoter [84]. B SGT-001 uses an AAV9 capsid, with CK8 promoter [86]. C PF-06939926 uses an AAV9 capsid, with a muscle-specific promoter that has not been publicly disclosed at the time of this review [88]. N-term = N terminal domain, R# = spectrin repeat number, H# = hinge number, CR = cysteine-rich domain
Although the gene therapy strategies are designed to restore an optimized micro/minidystrophin protein, the protein expressed is still not the full dystrophin protein and thus would still be considered an imperfect protein. Thus, with treatment, even in the most optimistic scenario, we would still expect some progression of the disease over time, although the progression would presumably be dramatically slowed by this treatment. Another issue that may arise with the gene therapy strategy is the question of durability. Since the genome of AAVs generally does not integrate into the host genome [89], the micro/minidystrophin transgene would persist in host cells as episome and would not be replicated during mitosis. Thus, in any tissue with cell division or turnover (e.g., mildly dystrophic muscle), the transgene may eventually become “diluted” or lost. Since dosing would result in immunity to the AAV vector, redosing with gene therapy would not be possible unless protocols that avoid the host immune system are available. Alternatively, some have raised the possibility of the rare occurrence of AAV vector integration [90]; if this happens at any significant frequency, it would raise the concern of altering the expression of endogenous, chromosomal genes.
Conclusion
Dystrophin-restoring therapies have become an important strategy in the treatment of DMD. Synthetic antisense oligonucleotide-mediated exon skipping continues to be a significant strategy, with newer and possibly more pharmacodynamically favorable chemistries under investigation. The latest development to enter clinical trials is gene replacement therapy with micro/minidystrophin. Although there is great interest in this therapy, there are still questions regarding immunity, host integration, and durability of these therapies. Even in the most optimistic scenarios, however, dystrophin-restoring therapies will still exhibit some disease progression. Other strategies for treating DMD are also under investigation, including anti-inflammatory mechanism, promoting muscle growth and regeneration, and anti-fibrotic mechanisms. These strategies remain important as they will likely remain complementary treatments to any dystrophin-restoring therapies.
With validated outcome measures for ambulatory DMD patients, we are now able to test new treatments in well-designed clinical trials, and the number of studies has grown significantly over the past 10 years. The growth in the number of clinical trials, however, has created a relative shortage of potential research subjects that are still ambulatory and able to participate in these studies. Outcome measures for younger patients as well as older, nonambulatory patients are also being developed and validated to help broaden the spectrum of DMD patients that can be studied.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Electronic Supplementary Material
(PDF 1225 kb)
References
- 1.Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol. 2012;71(3):304–13. doi: 10.1002/ana.23528. [DOI] [PubMed] [Google Scholar]
- 2.Moat SJ, Bradley DM, Salmon R, Clarke A, Hartley L. Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK) European journal of human genetics : EJHG. 2013;21(10):1049–53. doi: 10.1038/ejhg.2012.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duchenne G-B-A. Paraplegie hypertrophique de l'enfance de cause cerebrale1861.
- 4.Birnkrant DJ, Bushby K, Bann CM, Alman BA, Apkon SD, Blackwell A, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347–61. doi: 10.1016/S1474-4422(18)30025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17(3):251–67. doi: 10.1016/S1474-4422(18)30024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Colvin MK, et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018;17(5):445–55. doi: 10.1016/S1474-4422(18)30026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50(3):509–17. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–28. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 9.Haldane JBS. The rate of spontaneous mutation of a human gene. Journal of Genetics. 1935;31. [DOI] [PubMed]
- 10.Lane RJ, Robinow M, Roses AD. The genetic status of mothers of isolated cases of Duchenne muscular dystrophy. Journal of medical genetics. 1983;20(1):1–11. doi: 10.1136/jmg.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo A, Barbujani G, Mostacciuolo ML, Herrmann FH, Spiegler AW, Galluzzi G, et al. Sporadic cases in Duchenne muscular dystrophy. A reappraisal through segregation analysis on 988 sibships. Human genetics. 1987;76(3):230–5. doi: 10.1007/BF00283613. [DOI] [PubMed] [Google Scholar]
- 12.Bladen CL, Salgado D, Monges S, Foncuberta ME, Kekou K, Kosma K, et al. The TREAT-NMD DMD Global Database: analysis of more than 7,000 Duchenne muscular dystrophy mutations. Hum Mutat. 2015;36(4):395–402. doi: 10.1002/humu.22758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2(1):90–5. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- 14.Aartsma-Rus A, Van Deutekom JC, Fokkema IF, Van Ommen GJ, Den Dunnen JT. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34(2):135–44. doi: 10.1002/mus.20586. [DOI] [PubMed] [Google Scholar]
- 15.Monaco AP, Neve RL, Colletti-Feener C, Bertelson CJ, Kurnit DM, Kunkel LM. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323(6089):646–50. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- 16.Pillers DA, Bulman DE, Weleber RG, Sigesmund DA, Musarella MA, Powell BR, et al. Dystrophin expression in the human retina is required for normal function as defined by electroretinography. Nature genetics. 1993;4(1):82–6. doi: 10.1038/ng0593-82. [DOI] [PubMed] [Google Scholar]
- 17.Lidov HG, Selig S, Kunkel LM. Dp140: a novel 140 kDa CNS transcript from the dystrophin locus. Human molecular genetics. 1995;4(3):329–35. doi: 10.1093/hmg/4.3.329. [DOI] [PubMed] [Google Scholar]
- 18.Byers TJ, Lidov HG, Kunkel LM. An alternative dystrophin transcript specific to peripheral nerve. Nature genetics. 1993;4(1):77–81. doi: 10.1038/ng0593-77. [DOI] [PubMed] [Google Scholar]
- 19.Tinsley JM, Blake DJ, Davies KE. Apo-dystrophin-3: a 2.2kb transcript from the DMD locus encoding the dystrophin glycoprotein binding site. Human molecular genetics. 1993;2(5):521–4. doi: 10.1093/hmg/2.5.521. [DOI] [PubMed] [Google Scholar]
- 20.Vo AH, McNally EM. Modifier genes and their effect on Duchenne muscular dystrophy. Current opinion in neurology. 2015;28(5):528–34. doi: 10.1097/WCO.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passamano L, Taglia A, Palladino A, Viggiano E, D'Ambrosio P, Scutifero M, et al. Improvement of survival in Duchenne Muscular Dystrophy: retrospective analysis of 835 patients. Acta myologica : myopathies and cardiomyopathies : official journal of the Mediterranean Society of Myology. 2012;31(2):121–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Griggs RC, Herr BE, Reha A, Elfring G, Atkinson L, Cwik V, et al. Corticosteroids in Duchenne muscular dystrophy: major variations in practice. Muscle Nerve. 2013;48(1):27–31. doi: 10.1002/mus.23831. [DOI] [PubMed] [Google Scholar]
- 23.Griggs RC, Moxley RT, 3rd, Mendell JR, Fenichel GM, Brooke MH, Pestronk A, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Archives of neurology. 1991;48(4):383–8. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 24.Ricotti V, Ridout DA, Pane M, Main M, Mayhew A, Mercuri E, et al. The NorthStar Ambulatory Assessment in Duchenne muscular dystrophy: considerations for the design of clinical trials. Journal of neurology, neurosurgery, and psychiatry. 2016;87(2):149–55. doi: 10.1136/jnnp-2014-309405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henricson EK, Abresch RT, Cnaan A, Hu F, Duong T, Arrieta A, et al. The cooperative international neuromuscular research group Duchenne natural history study: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve. 2013;48(1):55–67. doi: 10.1002/mus.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebel DE, Corston JA, McAdam LC, Biggar WD, Alman BA. Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne muscular dystrophy: long-term follow-up. The Journal of bone and joint surgery American volume. 2013;95(12):1057–61. doi: 10.2106/JBJS.L.01577. [DOI] [PubMed] [Google Scholar]
- 27.Bonifati MD, Ruzza G, Bonometto P, Berardinelli A, Gorni K, Orcesi S, et al. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. 2000;23(9):1344–7. doi: 10.1002/1097-4598(200009)23:9<1344::AID-MUS4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Griggs RC, Miller JP, Greenberg CR, Fehlings DL, Pestronk A, Mendell JR, et al. Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne muscular dystrophy. Neurology. 2016;87(20):2123–31. doi: 10.1212/WNL.0000000000003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shieh PB, McIntosh J, Jin F, Souza M, Elfring G, Narayanan S, et al. Deflazacort vs prednisone/prednisolone for maintaining motor function and delaying loss of ambulation: A post hoc analysis from the ACT DMD trial. Muscle Nerve. 2018. [DOI] [PMC free article] [PubMed]
- 30.Finding the Optimum Regimen for Duchenne Muscular Dystrophy (FOR-DMD). https://clinicaltrials.gov/ct2/show/NCT01603407.
- 31.Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, et al. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO molecular medicine. 2013;5(10):1569–85. doi: 10.1002/emmm.201302621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A Study to Assess the Efficacy and Safety of Vamorolone in Boys With Duchenne Muscular Dystrophy (DMD). https://clinicaltrials.gov/ct2/show/NCT03439670.
- 33.Donovan JM, Zimmer M, Offman E, Grant T, Jirousek M. A Novel NF-kappaB Inhibitor, Edasalonexent (CAT-1004), in Development as a Disease-Modifying Treatment for Patients With Duchenne Muscular Dystrophy: Phase 1 Safety, Pharmacokinetics, and Pharmacodynamics in Adult Subjects. Journal of clinical pharmacology. 2017;57(5):627–39. doi: 10.1002/jcph.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phase 1/2 Study in Boys With Duchenne Muscular Dystrophy (MoveDMD®). https://clinicaltrials.gov/ct2/show/NCT02439216.
- 35.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 36.Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol. 2002;52(6):832–6. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- 37.A Phase 2 Study to Evaluate the Safety, Efficacy, Pharmacokinetics and Pharmacodynamics of PF-06252616 in Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT02310763.
- 38.Pfizer terminates domagrozumab PF-06252616 clinical studies for the treatment of Duchenne Muscular Dystrophy [press release]. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_terminates_domagrozumab_pf_06252616_clinical_studies_for_the_treatment_of_duchenne_muscular_dystrophy, 30 August 2018 2018.
- 39.Clinical Trial to Evaluate the Efficacy, Safety, and Tolerability of RO7239361 in Ambulatory Boys With Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT03039686.
- 40.Gueven N, Woolley K, Smith J. Border between natural product and drug: comparison of the related benzoquinones idebenone and coenzyme Q10. Redox biology. 2015;4:289–95. doi: 10.1016/j.redox.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buyse GM, Voit T, Schara U, Straathof CS, D'Angelo MG, Bernert G, et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): a double-blind randomised placebo-controlled phase 3 trial. Lancet. 2015;385(9979):1748–57. doi: 10.1016/S0140-6736(15)60025-3. [DOI] [PubMed] [Google Scholar]
- 42.A Phase III Double-blind Study With Idebenone in Patients With Duchenne Muscular Dystrophy (DMD) Taking Glucocorticoid Steroids (SIDEROS). https://clinicaltrials.gov/ct2/show/NCT02814019.
- 43.Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V, et al. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nature medicine. 2006;12(10):1147–50. doi: 10.1038/nm1479. [DOI] [PubMed] [Google Scholar]
- 44.Clinical Study to Evaluate the Efficacy and Safety of Givinostat in Ambulant Patients With Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT02851797.
- 45.Trial of Pamrevlumab (FG-3019), in Non-Ambulatory Subjects With Duchenne Muscular Dystrophy (DMD). https://clinicaltrials.gov/ct2/show/NCT02606136.
- 46.Aminzadeh MA, Rogers RG, Fournier M, Tobin RE, Guan X, Childers MK, et al. Exosome-Mediated Benefits of Cell Therapy in Mouse and Human Models of Duchenne Muscular Dystrophy. Stem cell reports. 2018;10(3):942–55. doi: 10.1016/j.stemcr.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.A Study of CAP-1002 in Ambulatory and Non-Ambulatory Patients With Duchenne Muscular Dystrophy (HOPE-2). https://clinicaltrials.gov/ct2/show/NCT03406780.
- 48.Tinsley JM, Blake DJ, Roche A, Fairbrother U, Riss J, Byth BC, et al. Primary structure of dystrophin-related protein. Nature. 1992;360(6404):591–3. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- 49.Ohlendieck K, Ervasti JM, Matsumura K, Kahl SD, Leveille CJ, Campbell KP. Dystrophin-related protein is localized to neuromuscular junctions of adult skeletal muscle. Neuron. 1991;7(3):499–508. doi: 10.1016/0896-6273(91)90301-F. [DOI] [PubMed] [Google Scholar]
- 50.Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90(4):717–27. doi: 10.1016/S0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 51.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90(4):729–38. doi: 10.1016/S0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 52.Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, et al. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nature medicine. 1998;4(12):1441–4. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- 53.PoC Study to Assess Activity and Safety of SMT C1100 (Ezutromid) in Boys With DMD (PhaseOut DMD). https://clinicaltrials.gov/ct2/show/NCT02858362.
- 54.Summit Announces PhaseOut DMD Did Not Meet Primary Endpoint [press release]. https://www.summitplc.com/wp-content/uploads/2018/08/2018_RNS_30-PhaseOut-Full-Results-FINAL.pdf, 27 June 2018 2018.
- 55.Gene Transfer Clinical Trial to Deliver rAAVrh74.MCK.GALGT2 for Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT03333590. [DOI] [PMC free article] [PubMed]
- 56.Nguyen HH, Jayasinha V, Xia B, Hoyte K, Martin PT. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(8):5616–21. doi: 10.1073/pnas.082613599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin PT, Xu R, Rodino-Klapac LR, Oglesbay E, Camboni M, Montgomery CL, et al. Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. American journal of physiology Cell physiology. 2009;296(3):C476–88. doi: 10.1152/ajpcell.00456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447(7140):87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 59.McDonald CM, Campbell C, Torricelli RE, Finkel RS, Flanigan KM, Goemans N, et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10101):1489–98. doi: 10.1016/S0140-6736(17)31611-2. [DOI] [PubMed] [Google Scholar]
- 60.Long-Term Outcomes of Ataluren in Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/record/NCT03179631.
- 61.Phase 2 Study to Assess Dystrophin Levels in Subjects With Nonsense Mutation Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT03648827.
- 62.Mann CJ, Honeyman K, Cheng AJ, Ly T, Lloyd F, Fletcher S, et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(1):42–7. doi: 10.1073/pnas.98.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aartsma-Rus A, Janson AA, Kaman WE, Bremmer-Bout M, den Dunnen JT, Baas F, et al. Therapeutic antisense-induced exon skipping in cultured muscle cells from six different DMD patients. Human Molecular Genetics. 2003;12(8):907–14. doi: 10.1093/hmg/ddg100. [DOI] [PubMed] [Google Scholar]
- 64.Aartsma-Rus A, Fokkema I, Verschuuren J, Ginjaar I, van Deutekom J, van Ommen GJ, et al. Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations. Hum Mutat. 2009;30(3):293–9. doi: 10.1002/humu.20918. [DOI] [PubMed] [Google Scholar]
- 65.Goemans N, Mercuri E, Belousova E, Komaki H, Dubrovsky A, McDonald CM, et al. A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy. Neuromuscul Disord. 2018;28(1):4–15. doi: 10.1016/j.nmd.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Mendell JR, Goemans N, Lowes LP, Alfano LN, Berry K, Shao J, et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol. 2016;79(2):257–71. doi: 10.1002/ana.24555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Charleston JS, Schnell FJ, Dworzak J, Donoghue C, Lewis S, Chen L, et al. Eteplirsen treatment for Duchenne muscular dystrophy: Exon skipping and dystrophin production. Neurology. 2018;90(24):e2146–e54. doi: 10.1212/WNL.0000000000005680. [DOI] [PubMed] [Google Scholar]
- 68.Duchenne Muscular Dystrophy and Related Dystrophinopathies: Developing Drugs for Treatment - Guidance for Industry. http://www.fda.gov/downloads/drugs/GuidanceComplianceRegulatoryInfo/guidance/ucm450229.pdf: Food and Drug Administration; 2018.
- 69.Bioanalytical Method Validation - Guidance for Industry. http://www.fda.gov/downloads/drugs/guidances/ucm070107.pdf: Food and Drug Administration; 2018.
- 70.Study of SRP-4045 and SRP-4053 in DMD Patients (ESSENCE). https://clinicaltrials.gov/ct2/show/NCT02500381.
- 71.Passini MA. Development of Oligonucleotides for the Treatment of Duchenne Muscular Dystrophy (DMD). RNA Meeting, Cold Spring Harbor Laboratory; Cold Spring Harbor, New York2017.
- 72.Passini MAG, L.; Wood, J.A.; Yao, M.; Estrella, N.L.; Treleaven, C.M.; Charleston, J.S.; Rutkowski, J.V.; Hanson, G.J. Development of PPMO for the Treatment of DMD. 13th Annual Meeting of the Oligonucleotide Therapeutics Society; Bordeaux, France2017.
- 73.A Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of a Single Dose of SRP-5051 in Patients With Duchenne Muscular Dystrophy (DMD). https://clinicaltrials.gov/ct2/show/NCT03375255.
- 74.Safety and Dose Finding Study of NS-065/NCNP-01 in Boys With Duchenne Muscular Dystrophy (DMD). https://clinicaltrials.gov/ct2/show/NCT02740972.
- 75.Safety and Tolerability of WVE-210201 in Patients With Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT03508947.
- 76.Takaishi KK, M.; Ito, K.; Kanda, A.; Takakusa, H.; Miida, H.; Masuda, T.; Nakamura, A.; Onishi, Y.; Onoda, T.; Kazuki, Y.; Oshimura, M.; Takeshima, Y.; Matsuo, M.; Koizumi, M. . Stunning pharmacological properties of DS-5141b, an antisense oligonucleotide consisting of 2’-O,4’-C-ethylene-bridged nucleic acids and 2’-O-methyl RNA, on dystrophin mRNA exon skipping. 22nd International Congress of the World Muscle Society; San Malo, France 2017.
- 77.Study of DS-5141b in Patients With Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT02667483.
- 78.Hartigan-O'Connor D, Chamberlain JS. Progress toward gene therapy of Duchenne muscular dystrophy. Seminars in neurology. 1999;19(3):323–32. doi: 10.1055/s-2008-1040848. [DOI] [PubMed] [Google Scholar]
- 79.Stolberg SG. The biotech death of Jesse Gelsinger. The New York times magazine. 1999;136-40:49–50. [PubMed] [Google Scholar]
- 80.Assessment of adenoviral vector safety and toxicity: report of the National Institutes of Health Recombinant DNA Advisory Committee. Hum Gene Ther. 2002;13(1):3–13. [DOI] [PubMed]
- 81.England SB, Nicholson LV, Johnson MA, Forrest SM, Love DR, Zubrzycka-Gaarn EE, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343(6254):180–2. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 82.Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nature medicine. 2002;8(3):253–61. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 83.Systemic Gene Delivery Clinical Trial for Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT03375164.
- 84.Mendell J. System Delivery of AAVrh74.MHCK7.Micro-dystrophin for Duchenne Muscular Dystroph (Preliminary Results from Phase I/II Clinical Trial). Sarepta Therapeutics R&D Day2018.
- 85.Microdystrophin Gene Transfer Study in Adolescents and Children With DMD (IGNITE DMD). https://clinicaltrials.gov/ct2/show/NCT03368742.
- 86.Schneider JSG, J.P.; Brown, K.J.; Golebiowski, D.; Shanks, C.; Ricotti, V.; Quiroz, J.; Morris, C.A. SGT-001 Microdystrophin Gene Therapy for Duchenne Muscular Dystrophy. 22nd International Annual Congress of the World Muscle Society; Saint-Malo, France2017.
- 87.A Study to Evaluate the Safety and Tolerability of PF-06939926 Gene Therapy in Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT03362502.
- 88.Moorehead T. Pfizer PF-06939926: Gene therapy safety and tolerability study in Duchenne Muscular Dystrophy (DMD). Parent Project Muscular Dystrophy Annual Conference; Scottsdale, Arizona2018.
- 89.Gardner JP, Zhu H, Colosi PC, Kurtzman GJ, Scadden DT. Robust, but transient expression of adeno-associated virus-transduced genes during human T lymphopoiesis. Blood. 1997;90(12):4854–64. [PubMed] [Google Scholar]
- 90.Deyle DR, Russell DW. Adeno-associated virus vector integration. Current opinion in molecular therapeutics. 2009;11(4):442–7. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)