Abstract
BACKGROUND/OBJECTIVES
Reducing the number of adipocytes by inducing apoptosis of mature adipocytes as well as suppressing differentiation of preadipocytes plays an important role in preventing obesity. This study examines the anti-adipogenic and pro-apoptotic effect of red pepper seed water extract (RPS) prepared at 4℃ (RPS4) in 3T3-L1 cells.
MATERIALS/METHODS
Effect of RPS4 or its fractions on lipid accumulation was determined in 3T3-L1 cells using oil red O (ORO) staining. The expressions of AMP-activated protein kinase (AMPK) and adipogenic associated proteins [peroxisome proliferator-activated receptor-γ (PPAR-γ), CCAAT/enhancer-binding proteins α (C/EBP α), sterol regulatory element binding protein-1c (SREBP-1c), fatty acid synthase (FAS), and acetyl-CoA carboxylase (ACC)] were measured in 3T3-L1 cells treated with RPS4. Apoptosis and the expression of Akt and Bcl-2 family proteins [B-cell lymphoma 2 (Bcl-2), Bcl-2-associated death promoter (Bad), Bcl-2 like protein 4 (Bax), Bal-2 homologous antagonist/killer (Bak)] were measured in mature 3T3-L1 cells treated with RPS4.
RESULTS
Treatment of RPS4 (0–75 µg/mL) or its fractions (0–50 µg/mL) for 24 h did not have an apparent cytotoxicity on pre and mature 3T3-L1 cells. RPS4 significantly suppressed differentiation and cellular lipid accumulation by increasing the phosphorylation of AMPK and reducing the expression of PPAR-γ, C/EBP α, SREBP-1c, FAS, and ACC. In addition, all fractions except ethyl acetate fraction significantly suppressed cellular lipid accumulation. RPS4 induced the apoptosis of mature adipocytes by hypophosphorylating Akt, increasing the expression of the pro-apoptotic proteins, Bak, Bax, and Bad, and reducing the expression of the anti-apoptotic proteins, Bcl-2 and p-Bad.
CONCLUSIONS
These finding suggest that RPS4 can reduce the numbers as well as the size of adipocytes and might useful for preventing and treating obesity.
Keywords: Capsicum, adipocyte, adipogenesis, apoptosis, lipid metabolism
INTRODUCTION
Excess body fat may damage health and is associated with diverse diseases, including hypertension, type II diabetes, coronary heart disease, respiratory complications, osteoarthritis, and cancer [1]. The mass of adipose tissue depends on the processes governing the size and number of adipocytes [2,3]. Increased proliferation and differentiation increase the number of adipocytes, whereas the loss of accumulated lipids by lipolysis and/or apoptosis of mature fat cells decreases the mass of adipose tissue [3,4].
Because of serious adverse effects of anti-obesity drugs, many naturally occurring food materials and phytochemicals can be beneficial in the treatment of obesity [5,6,7,8,9]. Peppers (Capsicum annuum L.) are the most popular spices consumed throughout the world and grow in Asia, Africa, and the Mediterranean regions. The content of capsaicinoids, a group of phenolic substances, differs with the varieties and parts of the pepper. The placental tissue, the inner wall that holds the seeds and the fruit of peppers, is high in capsaicinoids [10]. Human and animal studies have found that capsaicinoids increase energy expenditure [11] and reduce energy intake through affecting appetite [12]. However, in the pepper seeds, capsaicinods are not produced and the content of it is negligible [13,14]. Pepper seeds are by-products produced in abundance during pepper powder or paste processing. Phenolic compounds in pepper seeds are reported to have antioxidant, antifungal, and antiproliferative properties [15,16,17].
Capsicoside A and G were isolated from the pepper seed ethanol extract [18]. The inhibitory effects of capsicoside G on differentiation of 3T3-L1 preadipocytes [19] and weight gain of high-fat diet-fed animals [20,21] were demonstrated. However, the molecular mechanism underlying pepper seed mediated apoptosis of mature adipocytes is unknown. For some time, it has been believed that the number of adipocytes in adults remains constant. Therefore, the therapeutic targets for obesity are increasing energy expenditure, reducing intake, or inhibiting adipogenesis. However, the apoptosis of human adipocytes apparently can be triggered by tumor necrosis factor alpha (TNF-α) [4]. As a result, inducing apoptosis of adipocyte has grabbed attention as a therapeutic target to prevent or treat obesity. We have demonstrated RPS, extracted at 60℃ (RPS60), inhibits the early phase of adipogenesis by activating AMPK [22]. However, the RPS60 did not show any effect on apoptosis (data not shown). Here, we prepared RPS at 4℃ (RPS4) and demonstrated its pro-apoptotic effect, as well as a more potent anti-adipogenic effect, at a lower concentration compared to RPS60. We also elucidated its underlying mechanism of pro- apoptotic action.
MATERIALS AND METHODS
Preparation of red pepper seed extracts and bioassay guided fractionation
The seeds of red pepper cultivated in Republic of Korea were ground. The red pepper seed powder was added with water at a ratio of 1 part powder to 9 parts water (1:9, w/w) and extracted in a cold lap chamber at 4℃ for 24 h. After two times of centrifuging extract at 4,700 rpm for 20 min, supernatant was filtered under reduced pressure. The filtrate was evaporated and freeze-dried until used. The yields obtained were 11.4 %.
The water extract (80 g) was suspended in distilled water and solvent fractionation was carried out using different solvents (n-hexane, chloroform, ethyl acetate and butanol) with increasing polarity to collect the corresponding fractionates separately. All the fractionates were evaporated and freeze-dried: n-hexane (RPS4-H, 15.7 g: 19.6%), chloroform (RPS4-Cl, 15.7 g: 21.6%), ethylacetate (RPS4-EA, 2.0 g: 2.0%), butanol (RPS4-B, 13.1 g: 16.4%) and water layer (RPS4-W, 30.2 g: 37.7%).
Cell culture
3T3-L1 mouse embryo fibroblasts were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in Dulbecco's modified eagle's medium (DMEM; Gibco, Carlsbad, CA, USA), containing penicillin 100 units/mL, streptomycin 100 µg/mL and 10% newborn calf serum (NCS, Gibco, Carlsbad, CA, USA), and maintained in a humidified incubator at 37℃ and 5% CO2.
Cell viability
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Biosesang, Seongnam-si, Gyeonggi-do, Republic of Korea) assay was used to find out the effect of RPS4 and its fraction on cell viability. Briefly, pre- and mature adipocyte of 3T3-L1 cells were cultured and treated with RPS4 (0–75 µg/mL) or each fractionate (0–50 µg/mL) for 24 h in 96-well plates. Then 100 µL MTT solution (2 mg/mL) was added to each well followed by incubation for 4 h at 37℃. Cells were then exposed to dimethyl sulfoxide (DMSO, 99% v/v, Sigma, St. Louis, MO, USA) and the absorbance was measured at 540 nm using an ELISA.
Differentiation of 3T3-L1 preadipocytes and Oil Red O (ORO) staining
Determination of differentiation was performed as described previously [6]. In brief, 3T3-L1 preadipocytes were cultured until confluent, stimulated with DMEM containing 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA), isobutylmethylxanthine (0.5 mM IBMX, Sigma, St. Louis, MO, USA), insulin (5 mg/mL, Sigma, St. Louis, MO, USA), and dexamethasone (1 µM, Sigma, St. Louis, MO, USA), and maintained in 10% FBS DMEM containing insulin for two more days. Then, cells were cultured with 10% FBS DMEM medium for an additional 4 days. Samples were dissolved in the medium during differentiation. To measure lipid droplet cells, cells were performed using ORO staining on day 8 [6]. Cells were washed, fixed, and then stained with ORO (Sigma, St. Louis, MO, USA) for 1 h. Thereafter, the stained cells were washed and were photographed by a microscope (Olympus CKX41, Shinjuku, Tokyo, Japan) at 10x magnification, and were quantified by ELISA (540 nm).
Apoptosis
The Muse™ Annexin V & Dead Cell Kit (Merck KGaA, Darmstadt, Germany) was used for the assessment of apoptosis. The mature adipocytes were incubated with PBS or RPS4 for 24 h. Thereafter, the treatment medium was removed. After washing with PBS, the cells were collected using trypsin-EDTA as described previously [6]. The harvested cells were centrifuged at 1,400 rpm for 5 min, and re-suspended with the complete medium. Muse™ Annexin V & Dead Cell reagent (100 µL) was added to each of the re-suspended cells, which were incubated for 20 min. The re-suspended cells were assayed by Muse Cell Analyzer.
Immunoblotting
After washing with cold PBS, the 3T3-L1 adipocytes were scraped into lysis buffer (10 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton × 100, 10% glycerol, 0.1% sodium dodecyl sulfate (SDS), 0.5% deoxycholate, 1mM PMSF, 5% protease inhibitor cocktail, and pH 7.4) and maintained for 10 min. After centrifuging of the lysis mixture at 13000 rpm for 20 min at 4℃, and the supernatant was collected as the lysate. A Bradford protein assay (Sigma, St. Louis, MO, USA) was used to measure the protein concentration. The 20 µg of lysate protein was added with buffer containing 60 mM Tris-HCl (pH 6.8), 25% glycerol, 2% SDS, 10% bromophenol, and 5% 2-mercaptoethanol, and then heated at 95℃ for 10 min followed by separation using SDS-polyacrylamide gel electrophoresis. The separated proteins were transferred onto a nitrocellulose membrane (Millipore, Billerica, MA, USA) in a transblot chamber with Trans buffer (0.025 M Tris-HCl, 0.192 M glycine, and 20% MeOH). The membranes were blocked for 1 h at 4℃ with 5% nonfat milk in Tris-buffered saline and Tween 20 (TBS-T) buffer [10 mM Tris-HCl (pH 6.8), 100 mM NaCl, and 0.1% Tween 20]. The membranes were then incubated overnight with primary antibodies [PPAR-γ, FAS, ACC, p-ACC, AMPK, p-AMPK, Bak, Bax, Bcl-2, Bad, and p-Bad; Cell Signaling Technology., Beverly, MA, USA (1:1,000); C/EBP α, SREBP-1c, and β-actin; Santa Cruz Biotechnology Inc., Dallas, TX, USA (1:1000)]. Following a 3 times wash with TBS-T buffer, the membranes were incubated with horseradish peroxidase conjugated secondary antibody [goat anti-rabbit or goat anti-mouse (1:2,000)] for 1 h. Bands were detected by adding chemiluminescent substrate (IMGENEX, San Diego, CA, USA). Protein expression was quantified using UVP (imaging system for chemiluminescent Western blot, Upland, CA, USA) and Vision Work image analysis software (Analysis Software, Upland, CA, USA).
Statistical analysis
Results were expressed as the Mean±standard error (SE) of three independent experiments. Statistics were analyzed using the SPSS 23.0 statistical program (SPSS Inc., Chicago, IL, USA). Comparisons among groups were based on one-way ANOVA followed by Duncan's multiple range test. A P-value < 0.05 was considered statistically significant.
RESULTS
Effect of RPS4 on viability of pre- and mature adipocytes
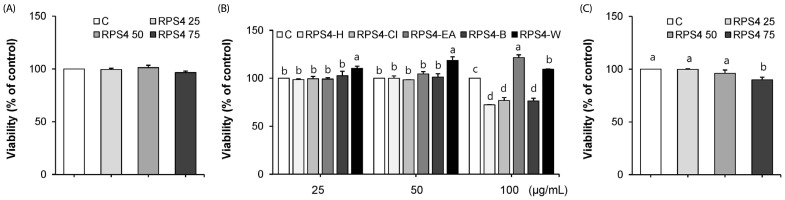
Pre and mature adipocytes were exposed to different concentrations of RPS4 (0–75 µg/mL) or fractionates (0–50 µg/mL) for 24 h and intracellular toxicity was determined by MTT assay. As shown in Fig. 1A and B, there was no toxic effect on preadipocytes after 24 h treatment with either 75 µg/mL RPS4 or 50 µg/mL of each of the fractionates. However, RPS4-H, RPS4-Cl, and RPS4-B at a concentration of 100 µg/mL, significantly reduced viability of preadipocytes compared to control (Suppl Fig. 2). The viability of mature adipocytes after 24 h treatment of RPS4 at the concentration of 75 µg/mL was reduced to 90.0% of that of the control (Fig. 1C).
Fig. 1. Effect of red pepper seed water extract (RPS4) and its fractions on the viability in preadipocytes (A, B) and mature adipocytes (C).
Cells were treated with various concentrations of RPS4 (A, C) or its fraction (B) for 24 h. C, PBS or DMSO; RPS4 25, red pepper seed water extract (4℃) 25 µg/mL; RPS4 50, red pepper seed water extract (4℃) 50 µg/mL; RPS4 75, red pepper seed water extract (4℃) 75 µg/mL; RPS4-H, n-hexane fractionate of red pepper seed water extract (4℃) ; RPS4-Cl, chloroform fractionate of red pepper seed water extract (4℃); RPS4-EA, ethylacetate fractionate of red pepper seed water extract (4℃); RPS4-B, butanol fractionate of red pepper seed water extract (4℃); RPS4-W, water fractionate of red pepper seed water extract (4℃).Values are Mean±SE. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05).
RPS4 decreased the differentiation and TG accumulation in 3T3-L1 cells
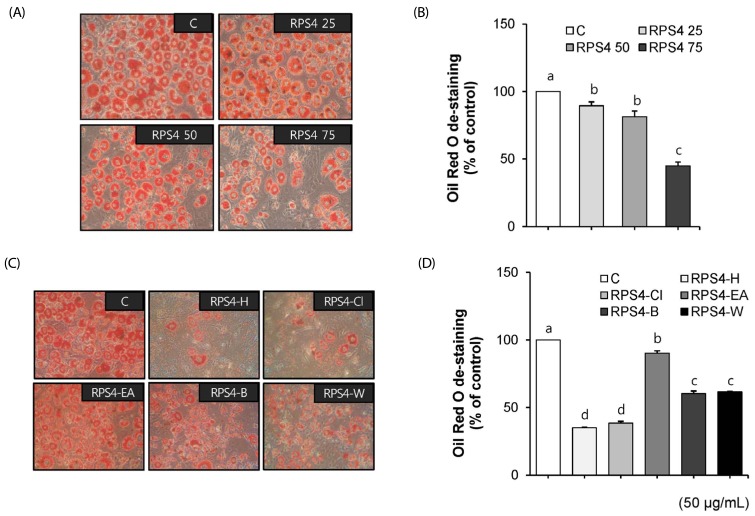
On the eighth day of differentiation, the effect of RPS4 or fractions on the differentiation of 3T3-L1 cells was examined using ORO staining. RPS4 reduced adipocyte differentiation and TG accumulation compared to the control, as indicated by decreased ORO staining (Fig. 2A). After dissolving lipid droplets in isopropanol, the absorbance quantified by ELISA at 540nm also indicated that RPS4 dose-dependently decreased TG accumulation (P < 0.05) (Fig. 2B). Furthermore, the fractions, except RPS4-EA reduced adipocyte differentiation. However, RPS4-H and RPS4-Cl reduced TG accumulation more effectively compared to RPS4-B and RPS4-W (Fig. 2C & D).
Fig. 2. Effect of red pepper seed water extract (RPS4) and its fractions on lipid accumulation in 3T3-L1 cells.
The 3T3-L1 cells treated with PBS, DMSO, 25, 50, or 75 µg/mL RPS4 or 50 µg/mL its fractions during the differentiation. After 8 days, 3T3-L1 cells were stained using Oil Red O (A) and measured lipid droplet by ELISA (B). C, PBS or DMSO; RPS4 25, red pepper seed water extract (4℃) 25 µg/mL; RPS4 50, red pepper seed water extract (4℃) 50 µg/mL; RPS4 75, red pepper seed water extract (4℃) 75 µg/mL; RPS4-H, n-hexane fractionate of red pepper seed water extract (4℃) ; RPS4-Cl, chloroform fractionate of red pepper seed water extract (4℃); RPS4-EA, ethylacetate fractionate of red pepper seed water extract (4℃); RPS4-B, butanol fractionate of red pepper seed water extract (4℃); RPS4-W, water fractionate of red pepper seed water extract (4℃). Values are Mean±SE. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05).
RPS4 activated AMPK and decreased adipocyte-associated protein expression in 3T3-L1 cells
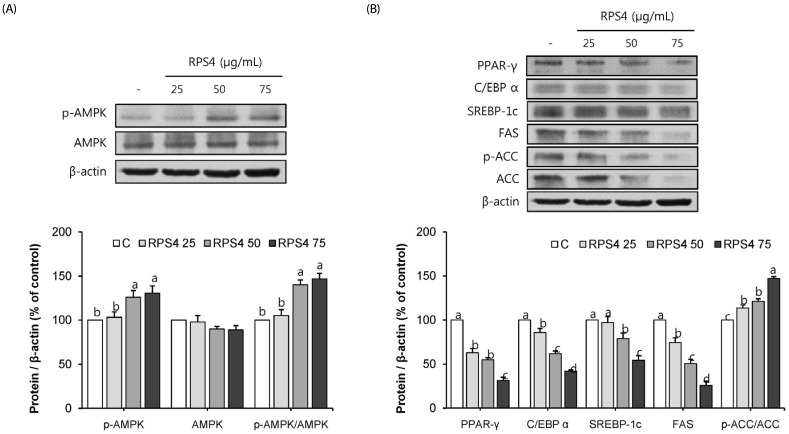
RPS4 significantly increased the phosphorylation of AMPK. 50 and 75 µg/mL RPS4 increased the phosphorylation of AMPK, thereby increasing p-AMPK/AMPK level by 140 and 147%, respectively compared to the control (Fig. 3A). The expressions of differentiation transcriptional factors PPAR-γ, C/EBPα, and SREBP-1c were dose-dependently down regulated by RPS4 treatment. Also, the expression of adipogenic marker proteins, FAS and ACC were down regulated by RPS4. RPS4 at the concentration of 75 µg/mL reduced PPAR-γ, C/EBPα, SREBP-1c, FAS and ACC up to 31%, 42%, 54%, 25% and 21% of that of the control, respectively. Moreover, it increased p-ACC/ACC up to 147% of that of the control (Fig. 3B). Taken together, these data indicate that RPS4 induced AMPK phosphorylation and decreased adipogenic-related proteins during differentiation.
Fig. 3. Effect of red pepper seed water extract (RPS4) on the expression of AMPK (A) and adipogenic associated proteins (B) in 3T3-L1 adipocytes.
C, PBS; RPS4 25, red pepper seed water extract (4℃) 25 µg/mL; RPS4 50, red pepper seed water extract (4℃) 50 µg/mL; RPS4 75, red pepper seed water extract (4℃). Values are Mean±SE. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05).
RPS4 induced apoptosis in mature 3T3-L1 cells
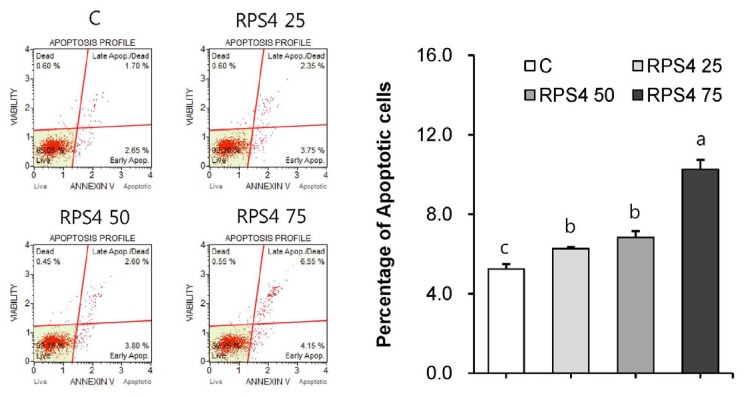
Next, to examine whether RPS4 is able to induce the reduction in cell number via apoptosis, we measured apoptosis in mature adipocytes by Muse™ Annexin V & Dead Cell Kit. As shown in Fig. 4, flow cytometric analysis showed that treatment of cells with RPS4 increased cells in the early stage of apoptosis [lower-right quadrant: Annexin V-PE(+) and Dead Cell Marker(−)] and in the late stage of apoptosis or dead by apoptotic mechanism [upper-right quadrant: Annexin V-PE(+) and Dead Cell Marker(+)].
Fig. 4. Effect of red pepper seed water extract (RPS4) on apoptosis in mature adipocytes.
Mature adipocytes were incubated with various concentration of PBS, 25, 50, 75 µg/mL of RPS4 for 24 h and then cell apoptosis was evaluated by Muse Cell Analyzer. C, PBS; RPS4 25, red pepper seed water extract (4℃) 25 µg/mL; RPS4 50, red pepper seed water extract (4℃) 50 µg/mL; RPS4 75, red pepper seed water extract (4℃). Values are Mean±SE. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05).
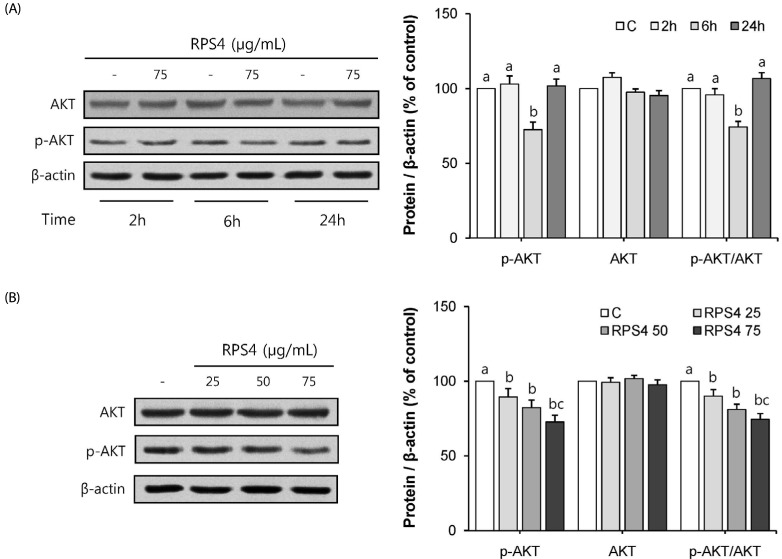
RPS4 caused hypophosphorylation of Akt in mature 3T3-L1 cells
Mature 3T3-L1 cells were treated with 75 µg/mL RPS4 for 2, 6, or 24 h. As shown in Fig. 5A, RPS4 induced hypophosphorylation of Akt was observed compared to control group after 6h treatment, with partial recovery after 24 h. Expression of p-Akt was measured in mature 3T3-L1 cells treated with different concentrations of RPS4 for 6h. RPS4 showed a dose dependent decrease in Akt phosphorylation after a 6 h treatment (Fig. 5B).
Fig. 5. Effect of red pepper seed water extract (RPS4) on the expression of Akt and p-Akt in mature adipocytes.
(A) Mature adipocytes were treated with RPS4 for 2, 6, or 24 h; (B) Mature adipocytes were treated with various concentration RPS4 for 6 h. C, PBS; RPS4 25, red pepper seed water extract (4℃) 25 µg/mL; RPS4 50, red pepper seed water extract (4℃) 50 µg/mL; RPS4 75, red pepper seed water extract (4℃) 75 µg/mL. Values are Mean±SE. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05).
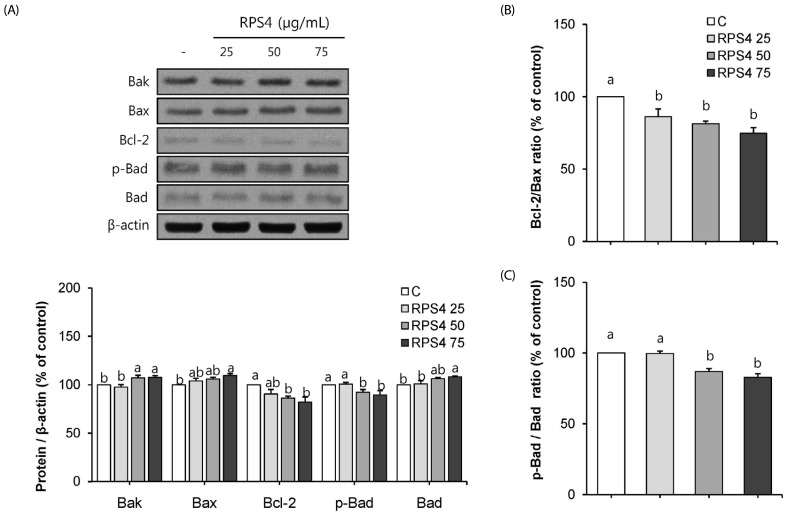
RPS4 induced apoptosis was regulated by the Bcl-2 family proteins in mature 3T3-L1 cells
To assess whether RPS4 is able to modulate the Bcl-2 family protein expressions, we treated with PBS, 25, 50, or 75 µg/mL of RPS4 in mature 3T3-L1 cells for 24 h. As expected, RPS4 showed increases in the expressions of pro-apoptotic proteins, Bak, Bax, and Bad, whereas showed decreases in the expressions of anti-apoptotic proteins Bcl-2 and p-Bad. (Fig. 6A). RPS4 induced increases or decreases in the protein expression were broadly dose-dependent. Additionally, RPS4 treatment decreased the expression ratios of Bcl-2/Bax (Fig. 6B) and p-Bad/Bad (Fig. 6C).
Fig. 6. Effect of red pepper seed water extract (RPS4) on the expression of Bcl-2 family proteins in mature adipocytes.
(A) Bcl-2 family protein, (B) Bcl-2/Bax and (C) p-Bad/Bad ratio. After differentiation, cells treated with PBS, 25, 50, 75 µg/mL of RPS4 for 24 h to measure the expression of Bcl-2 family proteins using Western blotting. C, PBS; RPS4 25, red pepper seed water extract (4℃) 25 µg/mL; RPS4 50, red pepper seed water extract (4℃) 50 µg/mL; RPS4 75, red pepper seed water extract (4℃) 75 µg/mL. Values are Mean±SE. Stand error bars represent three independent experiments and each experiment was triplicated. Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05).
DISCUSSION
Two anti-adipogenic furostanol saponins, capsicoside A and G were isolated from the water fraction of defatted pepper seed ethanol extract [18]. Their anti-adipogenic activity was evaluated in terms of how they inhibited lipid accumulation during 3T3-L1 adipocyte differentiation. However, the molecular mechanism underlying the pepper seed mediated apoptosis of mature adipocytes is unknown. RPS60, whose anti-adipogenic effect and underlying mechanism of action are known [22], failed to show an apoptotic effect in mature 3T3-L1 cells (data not shown). In this study, we prepared RPS4 and its solvent fractionates, and demonstrated their anti-adipogenic effect. Moreover, we first demonstrated that RPS induced apoptosis partially through a mitochondria-dependent pathway in mature 3T3-L1 cells.
First, we examined the inhibitory effect of RPS4 on the lipid accumulation. A significant decrease in lipid accumulation was found in RPS4 treated 3T3-L1 cells (Fig. 2A and B). Furthermore, although RPS4-B and RPS4-W showed inhibitory effect on TG accumulation, RPS4-H and RPS4-Cl showed potent inhibitory effect on adipogenesis compared to RPS4-B and RPS4-W (Fig. 2C and D). Therefore, we can speculate the presence of polar and non-polar active compounds in RPS4 and that non-polar active compounds are more anti-adipogenic than polar active compounds. Meanwhile, capsicoside A and G, the most active compounds in defatted pepper seeds, were isolated from water fraction of ethanol extract [18].
In the previous study, since RPS60 inhibited the differentiation of 3T3-L1 cells by activating AMPK [22], the effect of RPS4 on the activation of AMPK was established (Fig. 3A). Like RPS60, RPS4 significantly increased the phosphorylation of AMPK. AMPK suppresses fatty acid synthesis by inactivating ACC [19,23] and down regulating the expression of FAS [24]. Additionally, AMPK inhibits fatty acid synthesis at the transcriptional level by down regulating SREBP-1c, the transcription factors of enzymes involved in fatty acid synthesis [25,26]. Furthermore, we demonstrated that compound C, the inhibitor of AMPK, reversed RPS60 induced decrease in not only SREBP 1-c but also PPAR-γ and C/EBP-α expression [22]. In this study, RPS4 reduced the expression of differentiation transcriptional factors (PPAR-γ, C/EBPα, and SREBP-1c) and adipogenic marker enzymes (ACC and FAS) (Fig. 3B). Therefore, our study indicates that RPS4 inhibited the differentiation of 3T3-L1 adipocytes by activating AMPK and its downstream adipogenic-associated proteins.
We have found that the anti-adipogenic effect of RPS4 is more obvious than that of RPS60 in terms of the percentage of the control [22]. 75 µg/mL RPS4 reduced lipid accumulation up to 45% of that of the control, which was less than the 100 µg/mL RPS60 did (up to 51.9% of that of the control). To evaluate the difference in chemical profiles between RPS4 and RPS60, thinlayer chromatography (TLC) was conducted using methanolchloroform- water (5:5:1) as the eluent. RPS4 exhibited rather different TLC profile compared to RPS 60, although the chemical profiles are not completely identified yet (Suppl Fig. 1). RPS4 clearly displayed visible spots a and b as the main components, however, spots b was not observed in the RPS60. These results indicate that temperature can affect the chemical composition or extraction efficiency in the RPS extraction process. In addition, we compared the yields (% obtained) of each fraction of RPS4 and RPS60. The percentage yield of each fraction was rather different between RPS4 and RPS60 (Suppl Table. 1). In addition, the viability of preadipocytes treated with 100 µg/mL of RPS4-H, RPS4-Cl or RPS4-B was significantly lower than that of the control; whereas, all the fractions of RPS 60 at a concentration of 100 µg/mL had no effect on the viability of preadipocytes (Suppl Fig. 2).
Capsicinoids and capsicoside G are representative anti-adipogenic compounds isolated from red pepper. However, unlike the pepper placental tissue and fruit, which contain substantial amounts of capsaicinoids, no capsaicinoids were detected in pepper seeds, although its main component was phenolic compounds [14]. In addition, capsicoside G is extracted by method relatively different from our RPS4 [19,20,21].
Research on adipocyte apoptosis has been limited owing to the remarkable ability of adipocytes to resist apoptosis. However, according to in vivo and in vitro evidence, the loss of mature fat cells by apoptosis might play a role in a decreasing adipose tissue mass [27,28]. Capsaicin, a major pungent ingredient in red pepper has been demonstrated to induce apoptosis in 3T3-L1 preadipocytes which mediated through the activation of caspase-3, Bax, and Bak and the down-regulation of Bcl-2 [5]. Our flow cytometric analysis result showed that RPS4 treatment for 24 h induced apoptosis in mature 3T3-L1 cells in a concentration dependent manner (Fig. 4). We hypothesized that RPS4 can induce apoptosis at the mitochondrial level which is regulated by the members of the Bcl-2 family. It has been shown that phosphorylated Akt suppresses apoptosis by inhibiting the mitochondrial-induced apoptosis pathway [8,29,30,31]. Phosphorylated Akt inhibits the pro-apoptotic Bcl-2 family protein Bad by phosphorylation. In the present study, RPS4 suppressed the phosphorylation of Akt in the mature 3T3-L1 cells following 6 h of exposure, but Akt phosphorylation was recovered following 24 h of exposure (Fig. 5). In other studies, Akt phosphorylation partially recovered after a brief exposure. The expression of p-Akt was inhibited by the treatment of LY294002, the inhibitor of PI3K/Akt, for 6 h, and partially recovered at 24 h [32]. In addition, although, an ethanol extract of St. John's wort reduced p-Akt expression after treatment for 2 h with partial recovery at 6 h, the expression of Bad remained inhibited after 6 h [33]. In this study, Bad remained inhibited after 24 h of RPS4 exposure (Fig. 6A) and reduced the ratio of p-Bad/Bad expression (Fig. 6C). We also found that RPS4 increased the protein expression of Bak and Bax, whereas it decreased the expression of Bcl-2 (Fig. 6A). We speculate that hypophosphorylation of Bad by RPS4 allowed the formation of a Bad-Bcl-2 heterodimer, which inactivated Bcl-2 and led to Bax/Bak-triggered apoptosis [34,35]. Further, our results revealed that RPS4 decreased the expression ratio of Bcl-2/Bax (Fig. 6B). The ratio of Bcl-2/Bax plays an important role in determining whether cells will undergo apoptosis under cell death-promoting conditions [36,37].
Activated AMPK is known to mediate pro-apoptosis by activating a JNK (c-Jun N terminal kinases) pathway [38], regulating p53 [39], inhibiting mTOR (mammalian target of rapamycin) [40], and directly upregulating pro-apoptotic proteins by inactivating Akt [41] in the different cell lines. However, since AMPK involvement in apoptosis is still controversial, more investigations are needed to elucidate the importance of AMPK in apoptosis of adipocytes.
As mentioned earlier, we have suggested that treatment with RPS60 could inhibit adipocyte differentiation during the early phase of adipogenesis. However, unlike RPS4, RPS60 has not shown a pro-apoptotic effect on mature adipocytes (data not shown). Therefore, given the different response of mature adipocytes to apoptosis, further study need to elucidate the exact compounds in our RPS4 that cause the pro-apoptotic and anti-adipogenic effects.
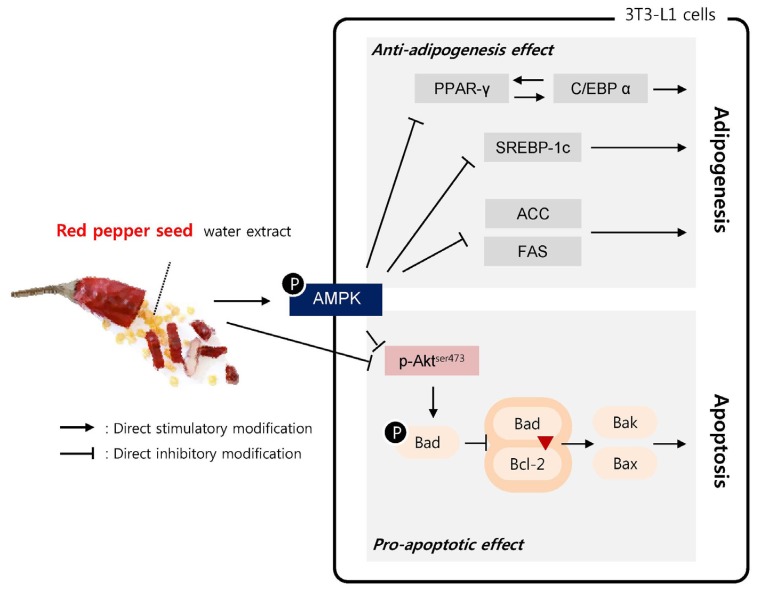
Taken together, RPS4 inhibits preadipocyte differentiation by reducing the expression of adipocyte associated proteins, and induces apoptosis of mature 3T3-L1 cells by decreasing pro-apoptotic protein expression and increasing anti-apoptotic protein expression (Fig. 7). Our findings suggest that RPS4 can reduce the numbers as well as the size of adipocytes and can be developed as a therapeutic agent for treating obesity.
Fig. 7. Proposed working model: PRS4 suppresses adipogenisis and induces apoptosis through AMPK pathways.
Footnotes
This work was supported by the Korea Institute of Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (115015-03-1-HD020).
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
SUPPLEMENTARY MATERIALS
TLC profile of red pepper seed water extract.
Yield of fractions of red pepper seed water extract.
Effect of red pepper seed water extracts and fractions on the viability in preadipocytes.
References
- 1.Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45–52. doi: 10.1111/j.1749-6632.2011.06096.x. [DOI] [PubMed] [Google Scholar]
- 2.Warnke I, Goralczyk R, Fuhrer E, Schwager J. Dietary constituents reduce lipid accumulation in murine C3H10 T1/2 adipocytes: a novel fluorescent method to quantify fat droplets. Nutr Metab (Lond) 2011;8:30. doi: 10.1186/1743-7075-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang JY, Della-Fera MA, Hartzell DL, Nelson-Dooley C, Hausman DB, Baile CA. Esculetin induces apoptosis and inhibits adipogenesis in 3T3-L1 cells. Obesity (Silver Spring) 2006;14:1691–1699. doi: 10.1038/oby.2006.194. [DOI] [PubMed] [Google Scholar]
- 4.Prins JB, O'Rahilly S. Regulation of adipose cell number in man. Clin Sci (Lond) 1997;92:3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CL, Yen GC. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J Agric Food Chem. 2007;55:1730–1736. doi: 10.1021/jf062912b. [DOI] [PubMed] [Google Scholar]
- 6.Rhyu J, Kim MS, You MK, Bang MA, Kim HA. Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes. Nutr Res Pract. 2014;8:33–39. doi: 10.4162/nrp.2014.8.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trigueros L, Peña S, Ugidos AV, Sayas-Barberá E, Pérez-Álvarez JA, Sendra E. Food ingredients as anti-obesity agents: a review. Crit Rev Food Sci Nutr. 2013;53:929–942. doi: 10.1080/10408398.2011.574215. [DOI] [PubMed] [Google Scholar]
- 8.Yao A, Shen Y, Wang A, Chen S, Zhang H, Chen F, Chen Z, Wei H, Zou Z, Shan Y, Zhang X. Sulforaphane induces apoptosis in adipocytes via Akt/p70s6k1/Bad inhibition and ERK activation. Biochem Biophys Res Commun. 2015;465:696–701. doi: 10.1016/j.bbrc.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Yamashita Y, Yasuda M, Yamamoto N, Ashida H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Food Funct. 2015;6:135–145. doi: 10.1039/c4fo00525b. [DOI] [PubMed] [Google Scholar]
- 10.Estrada B, Bernal MA, Díaz J, Pomar F, Merino F. Capsaicinoids in vegetative organs of Capsicum annuum L. in relation to fruiting. J Agric Food Chem. 2002;50:1188–1191. doi: 10.1021/jf011270j. [DOI] [PubMed] [Google Scholar]
- 11.Imaizumi K, Sato S, Kumazawa M, Arai N, Aritoshi S, Akimoto S, Sakakibara Y, Kawashima Y, Tachiyashiki K. Capsaicinoids-induced changes of plasma glucose, free fatty acid and glycerol concentrations in rats. J Toxicol Sci. 2011;36:109–116. doi: 10.2131/jts.36.109. [DOI] [PubMed] [Google Scholar]
- 12.Westerterp-Plantenga MS, Smeets A, Lejeune MP. Sensory and gastrointestinal satiety effects of capsaicin on food intake. Int J Obes. 2005;29:682–688. doi: 10.1038/sj.ijo.0802862. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa K, Janos T, Sakamoto S, Nunomura O. The contents of capsaicinoids and their phenolic intermediates in various tissues of the plants of Capsicum annuum L. Capsicum Eggplant Newsl. 1998;17:222–225. [Google Scholar]
- 14.Kim HA, Kim MS, Kim SH, Kim YK. Pepper seed extract suppresses invasion and migration of human breast cancer cells. Nutr Cancer. 2014;66:159–165. doi: 10.1080/01635581.2014.853814. [DOI] [PubMed] [Google Scholar]
- 15.Diz MS, Carvalho AO, Ribeiro SF, Da Cunha M, Beltramini L, Rodrigues R, Nascimento VV, Machado OL, Gomes VM. Characterisation, immunolocalisation and antifungal activity of a lipid transfer protein from chili pepper (Capsicum annuum) seeds with novel α -amylase inhibitory properties. Physiol Plant. 2011;142:233–246. doi: 10.1111/j.1399-3054.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 16.Hisatomi E, Matsui M, Kubota K, Kobayashi A. Antioxidative activity in the pericarp and seed of Japanese pepper (Xanthoxylum piperitum DC) J Agric Food Chem. 2000;48:4924–4928. doi: 10.1021/jf000252j. [DOI] [PubMed] [Google Scholar]
- 17.Jeon G, Choi Y, Lee SM, Kim Y, Jeong HS, Lee J. Anti-obesity activity of methanol extract from hot pepper (Capsicum annuum L.) seeds in 3T3-L1 adipocyte. Food Sci Biotechnol. 2010;19:1123–1127. [Google Scholar]
- 18.Sung JH, Bang MH, Lee JS. Bioassay-guided isolation of anti-adipogenic compounds from defatted pepper (Capsicum annuum L.) seeds. J Funct Foods. 2015;14:670–675. [Google Scholar]
- 19.Sung J, Lee J. Capsicoside G, a furostanol saponin from pepper (Capsicum annuum L.) seeds, suppresses adipogenesis through activation of AMP-activated protein kinase in 3T3-L1 cells. J Funct Foods. 2016;20:148–158. [Google Scholar]
- 20.Sung J, Jeong HS, Lee J. Effect of the capsicoside G-rich Fraction from pepper (Capsicum annuum L.) seeds on high-fat diet-induced obesity in mice. Phytother Res. 2016;30:1848–1855. doi: 10.1002/ptr.5692. [DOI] [PubMed] [Google Scholar]
- 21.Sung J, Yang J, Kim Y, Kim M, Jeong HS, Lee J. Effect of defatted pepper (Capsicum annuum L.) seed extracts on high-fat diet-induced obesity in C57BL/6J mice. Food Sci Biotechnol. 2016;25:1457–1461. doi: 10.1007/s10068-016-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim HJ, You MK, Wang Z, Kim HA. Red pepper seed inhibits differentiation of 3T3-L1 cells during the early phase of adipogenesis via the activation of AMPK. Am J Chin Med. 2018;46:107–118. doi: 10.1142/S0192415X18500064. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–1070. doi: 10.1042/bst0301064. [DOI] [PubMed] [Google Scholar]
- 24.Foretz M, Carling D, Guichard C, Ferré P, Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;273:14767–14771. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- 25.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowell P, Flexner C, Kwiterovich PO, Lane MD. Suppression of preadipocyte differentiation and promotion of adipocyte death by HIV protease inhibitors. J Biol Chem. 2000;275:41325–41332. doi: 10.1074/jbc.M006474200. [DOI] [PubMed] [Google Scholar]
- 28.Qian H, Hausman DB, Compton MM, Martin RJ, Della-Fera MA, Hartzell DL, Baile CA. TNFα induces and insulin inhibits caspase 3-dependent adipocyte apoptosis. Biochem Biophys Res Commun. 2001;284:1176–1183. doi: 10.1006/bbrc.2001.5100. [DOI] [PubMed] [Google Scholar]
- 29.Harrison ME, Power Coombs MR, Delaney LM, Hoskin DW. Exposure of breast cancer cells to a subcytotoxic dose of apigenin causes growth inhibition, oxidative stress, and hypophosphorylation of Akt. Exp Mol Pathol. 2014;97:211–217. doi: 10.1016/j.yexmp.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 31.Merry DE, Korsmeyer SJ. Bcl-2 gene family in the nervous system. Annu Rev Neurosci. 1997;20:245–267. doi: 10.1146/annurev.neuro.20.1.245. [DOI] [PubMed] [Google Scholar]
- 32.Giannelli G, Sgarra C, Porcelli L, Azzariti A, Antonaci S, Paradiso A. EGFR and VEGFR as potential target for biological therapies in HCC cells. Cancer Lett. 2008;262:257–264. doi: 10.1016/j.canlet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 33.You MK, Kim HJ, Kook JH, Kim HA. St. John's Wort regulates proliferation and apoptosis in MCF-7 human breast cancer cells by inhibiting AMPK/mTOR and activating the mitochondrial pathway. Int J Mol Sci. 2018;19:996. doi: 10.3390/ijms19040966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 35.Rácz B, Gasz B, Gallyas F, Jr, Kiss P, Tamás A, Szántó Z, Lubics A, Lengvári I, Tóth G, Hegyi O, Roth E, Reglodi D. PKA-Bad-14-3-3 and Akt-Bad-14-3-3 signaling pathways are involved in the protective effects of PACAP against ischemia/reperfusion-induced cardiomyocyte apoptosis. Regul Pept. 2008;145:105–115. doi: 10.1016/j.regpep.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 37.Xiao H, Wang J, Yuan L, Xiao C, Wang Y, Liu X. Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways. J Agric Food Chem. 2013;61:1509–1520. doi: 10.1021/jf3050268. [DOI] [PubMed] [Google Scholar]
- 38.Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, Foufelle F, Hue L. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS Lett. 2002;526:38–42. doi: 10.1016/s0014-5793(02)03110-1. [DOI] [PubMed] [Google Scholar]
- 39.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 40.Meynet O, Zunino B, Happo L, Pradelli LA, Chiche J, Jacquin MA, Mondragón L, Tanti JF, Taillan B, Garnier G, Reverso-Meinietti J, Mounier N, Michiels JF, Michalak EM, Carles M, Scott CL, Ricci JE. Caloric restriction modulates Mcl-1 expression and sensitizes lymphomas to BH3 mimetic in mice. Blood. 2013;122:2402–2411. doi: 10.1182/blood-2013-01-478651. [DOI] [PubMed] [Google Scholar]
- 41.Concannon CG, Tuffy LP, Weisová P, Bonner HP, Dávila D, Bonner C, Devocelle MC, Strasser A, Ward MW, Prehn JH. AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J Cell Biol. 2010;189:83–94. doi: 10.1083/jcb.200909166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TLC profile of red pepper seed water extract.
Yield of fractions of red pepper seed water extract.
Effect of red pepper seed water extracts and fractions on the viability in preadipocytes.