Abstract
BACKGROUND/OBJECTIVES
Propolis has a rich source of bioactive compounds and has renal and hepatic protective properties. The purpose of this study was to investigate the beneficial effect of hydro-ethanolic extract of propolis against paracetamol-induced liver damage and impairment of kidney function, as well as hematological changes in rats.
MATERIALS AND METHODS
Six groups of rats were used; the first group was served as a control; the second and third groups were treated by propolis extract at a dose of 50 and 100 mg/kg.B.WT. respectively; the fourth group was treated by paracetamol (200 mg/kg.B.WT.); the fifth group was treated by propolis (50 mg/kg.B.WT.) for eight days and then received similar dose of propolis for following seven days with paracetamol at a dose of 200 mg/kg.B.WT. daily for the seven days; and the sixth group was treated with propolis (100 mg/kg.B.WT.) for eight days and then received similar dose of propolis for following seven days with paracetamol at a dose of 200 mg/kg.B.WT. daily for the seven days. All the animals were treated for a period of 15 days. At the end of the experimental period, blood samples were collected for measurement of the liver enzymes, serum albumin, protein and creatinine, blood urea nitrogen, hematological parameters, and urine volume, protein and albumin.
RESULTS
Paracetamol over dose significantly lowered hemoglobin, serum total protein, albumin, and uric acid, while it significantly increased blood creatinine, blood urea nitrogen, alanine aminotransferase, aspartate aminotransferase and lactate dehydrogenase activities, white blood cells, and platelet count as compared to the control. However, these alterations were significantly attenuated by the use of propolis extract and the effect was dose dependent. Interestingly, propolis prevented paracetamol induced proteinuria, low hemoglobin and body weight loss.
CONCLUSIONS
Propolis significantly prevented paracetamol induced renal, hepatic and hematological toxicity and might be useful in the management of liver and renal diseases particularly proteinuria.
Keywords: Propolis, paracetamol, kidney, liver, proteinuria
INTRODUCTION
It is well known that high doses of paracetamol could cause severe hepatic failure by the formation of N-acetyl-p-benzoquinone imine by cytochrome P450 and generation of reactive oxygen species [1,2]. In addition to the involvement of cytochrome P450s in paracetamol toxicity, a prostaglandin synthase/cyclooxygenase convert paracetamol to N-acetyl-p-benzoquinone imine [3]. These metabolites can be detoxified by glutathione pathway when acceptable doses are used. However, in paracetamol toxicity, glutathione intoxication is not enough to detoxify the high production of N-acetyl-p-benzoquinone imine [4,5]. Basically, the induction of cytochrome P450 isoforms (2E1, 3A4, and 1A2), depletion of intracellular glutathione and oxidative stress are involved in the pathogenesis of paracetamol induced liver injury. Therefore, inhibition of excessive amount of N-acetyl-p-benzoquinone imine formation and the use of N-acetyl cysteine reverses oxidative stress and paracetamol toxicity [6]. N-acetylcysteine increases glutathione pathway that mitigates hepatotoxicity by scavenging the reactive metabolites of paracetamol, and reducing oxidative stress [6].
Paracetamol binds quinone reductase 2 in the kidney and liver, which modulates reactive oxygen species generation. Therefore, it was suggested that the mechanism intensifying paracetamol toxicity is via quinone reductase 2 mediated superoxide production [7]. In kidney damage caused by paracetamol overdose, it was suggested that the process depends on the activation of caspase-9 and caspase-3, and inhibition of nitric oxide overproduction and optimizing the antioxidant capacity protect kidney damage caused by paracetamol toxicity [8]. Furthermore, paracetamol inhibits mitochondrial respiration in kidney cells [9].
Propolis is a glue material that is collected by honeybees from the buds and exudates of various plants. It contains essential and aromatic oils, resins, waxes, pollens and various organic substances. Furthermore, propolis contains a number of bioactive substances such as polyphenols, flavonoids, aromatic acids, and diterpenic and phenolic acids. These ingredients might be responsible for the biological activities of propolis such as protective effects in renal and hepatic injuries, and antimicrobial, anticancer, anti-inflammatory, antioxidant, and anti-ulcer activities [10,11,12,13].
Propolis has a marked protective effect in kidney and liver injury [14,15,16,17]. Therefore, the present study was conducted to evaluate the preventive effects of 50–100 mg/kg of hydroethanolic extract of propolis in liver and kidney damage induced by paracetamol toxicity in albino rats. The effect of propolis on paracetamol induced proteinuria and and low hemoglobin was studied.
MATERIALS AND METHODS
Experimental animals
Adult male Wistar rats (150–220 g) were obtained from the Animal House Breeding Center, Department of Biology, Faculty of Sciences, Fes, Morocco and were used for the experiments. Animals were housed under standard environmental conditions (25 ± 1℃, 55 ± 5% humidity and 12 h/ 12 h light/dark cycle) and were maintained with free access to water and laboratory rat chow. All the experiments were conducted in accordance with the internationally accepted principles for the care and use of laboratory animals. The study protocol for all animal experiments was approved by the Animal Facility and the Laboratory of Physiology-Pharmacology & Environmental Health, the Faculty of Science, Sidi Mohamed Ben Abdallah University, Fez (USMBA-PPSE ACU2017-05).
Collection and extraction of propolis
The hydro-ethanolic extract of propolis was prepared from propolis obtained from colonies of honeybees in the region of Outat el Haj, Morocco. The collected propolis was frozen at −20℃ and ground in a chilled mortar. The ground powder (30 g) was then extracted with the use of 100 mL of ethanol 70% at ambient temperature and maceration under agitation for one week. The solution was then filtered through a Whatman filter paper and concentrated in a rotary evaporator under reduced pressure to get a solid residue. The residue was dissolved in a ethanol and stored at −20℃ until use. During the experiment, distilled water was added to obtain the required propolis concentration that was given to the animals daily by gavage for a total of 15 days.
Experimental design
Animals were housed in metabolic cages 3 days prior to the start of the experiment for adaptation and they were divided into six groups, six animals each. Experimental toxicity of paracetamol was induced by oral administration of 200 mg/kg corresponding to 2/3 the lethal dose (50%) to the rats.
Group 1; (Control group); the animals received 10 mL/kg. B.WT. of distilled water for 15 days.
Group 2; the animals received hydro-ethanolic extract of propolis at a dose of 50 mg/kg.B.WT. daily by gavage for 15 days.
Group 3; the animals received hydro-ethanolic extract of propolis at a dose of 100 mg/kg.B.WT. daily by gavage for 15 days.
Group 4; the animals received paracetamol at a dose of 200 mg/kg.B.WT. daily by gavage for 7 days.
Group 5; the animals received hydro-ethanolic extract of propolis (50 mg/kg.B.WT.) for eight days and then received similar dose of propolis for following seven days with paracetamol at a dose of 200 mg/kg.B.WT. daily by gavage for the seven days
Group 6; the animals received hydro-ethanolic extract of propolis (100 mg/kg.B.WT.) for eight days and then received similar dose of propolis for following seven days with paracetamol at a dose of 200 mg/kg.B.WT. daily by gavages for the seven days.
Urine collection and analysis
The animals were kept in metabolic cages individually for collection of 24-h urine samples on days 0, 7 and 15 of treatment. Urine volume was measured immediately after collection. Urine samples collected on day 15 were stored at −20℃ for determination of various parameters. Urine was analyzed for creatinine, urea, protein, uric acid and albumin levels.
Blood tests
After 15 days of the experiment, blood samples were collected from the anaesthetized animals in all groups by the retro-orbital puncture. Blood was analyzed for creatinine, blood urea nitrogen (BUN), uric acid, proteins and albumin levels. Hepatic function was evaluated by measuring serum alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH).
Hematological parameters including red blood cell (RBC), white blood cell (WBC), platelets (PLT), hemoglobin (Hb) and hematocrit (Ht) values were determined by standard methods on automated hematology analyzer.
Statistical analysis
All data expressed are Mean±SD. The statistical comparisons between groups were performed by one-way analysis of variance (ANOVA) followed by post hoc Tukey's Multiple Comparison Test using Graph Pad Prism 5 software. P < 0.05 was considered statistically significant.
RESULTS
Effect of paracetamol and propolis on hematological parameters
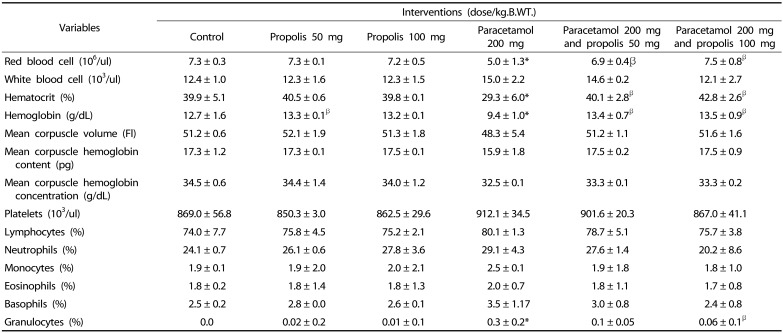
The results of the hematological analysis in the control and treatment groups are presented in the Table 1. The results showed that paracetamol causes a significant reduction in RBCs count, Hb concentration and Ht. However, it caused an elevation of WBC and platelet, which was statistically insignificant as compared to the control group. The co-administration of propolis with paracetamol reversed these changes and significantly attenuated the toxic effects of paracetamol.
Table 1. Effect of paracetamol overdose and propolis on hematological parameters.
* P < 0.05 as compared to the control group
β P < 0.05 as compared to paracetamol treated group (200 mg/kg.B.WT.)
Effect of paracetamol and propolis on kidney function and proteinuria
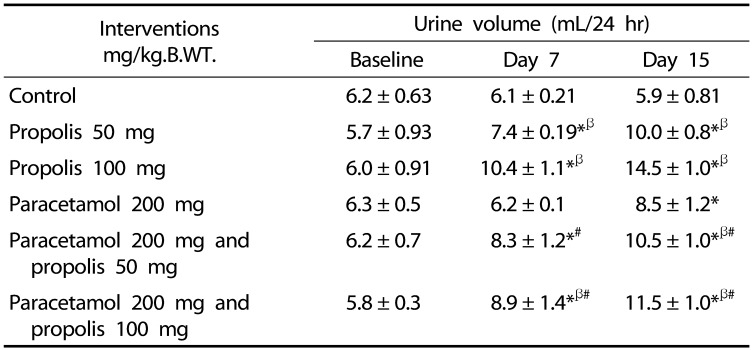
Paracetamol treatment caused a significant increase in the urinary volume. On day 15, urine volume was 5.96 ± 0.810 mL/day in the control group and 8.52 ± 1.23 mL/day in the paracetamol group. The use of propolis further increased urinary output when compared to the paracetamol and normal groups during the first and second week of the experiment (Table 2).
Table 2. Effect of the interventions on the urine volume.
* P < 0.05 as compared to the control group during the same time interval
β P < 0.05 as compared to baseline within the same intervention
# P < 0.05 as compared to paracetamol (200 mg/kg.B.WT.)
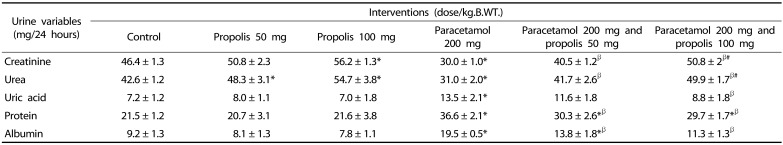
The effects of the interventions on urinary excretion of uric acid and albumin were summarized in Table 3. Paracetamol significantly increased the urinary excretion of uric acid and albumin compared to the control, whereas propolis decreased urinary uric acid and albumin; this effect was a dose dependent.
Table 3. Effects of paracetamol and propolis on 24-hour urinary excretion of creatinine, urea, uric acid, total protein, albumin on day 15 after commencement of the intervention.
* P < 0.05 as compared to the control group
β P < 0.05 as compared to paracetamol treated group (200 mg/kg.B.WT.)
# P < 0.05 as compared to the group treated by paracetamol 200 mg/kg.B.WT. and propolis 50 mg/kg.B.WT.
The results showed that paracetamol overdose causes a significant decreased in the urinary excretion of creatinine and urea while it significantly increases the urinary excretion of proteins as compared to the control. However, propolis decreased the urinary excretion of proteins as compared to the control group and increased urinary excretion of creatinine and urea as compared to the paracetamol group (Table 3).
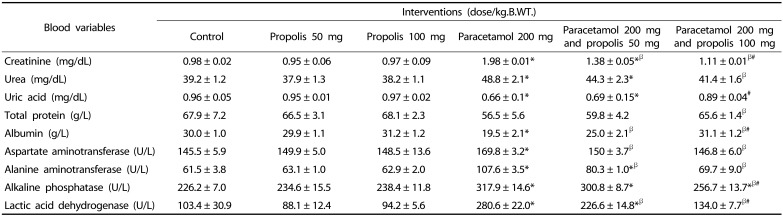
The effects of the interventions on serum levels of creatinine, blood urea, uric acid, total protein and albumin are shown in the Table 4. Paracetamol overdose induced kidney damage that was evident by a significant increase of creatinine and blood urea. It significantly decreased the uric acid level as compared to the control. However, co-administration of propolis with the high dose of paracetamol significantly prevented paracetamol-induced elevation of blood urea and creatinine.
Table 4. Effects of paracetamol overdose and propolis on plasma level of creatinine, BUN, uric acid, total protein, albumin and liver enzymes on day 15 after commencement of the interventions' administration.
* P < 0.05 as compared to the control group
β P < 0.05 as compared to paracetamol treated group (200 mg/kg.B.WT.)
# P < 0.05 as compared to the group treated by paracetamol 200 mg/kg.B.WT. and propolis 50 mg/kg.B.WT.
BUN: blood urea nitrogen
Effect of propolis and paracetamol on liver function
Regarding the liver function test, paracetamol over dose causes a significant elevation in the serum levels of the liver enzymes; serum ALT, AST, ALP and LDH, and a significant lowering of serum proteins and albumin as compared to the control (Table 4). However, oral administration of propolis significantly attenuated the elevation in the liver enzyme activity in paracetamol treated rats, and the higher dose of propolis almost normalized elevated liver enzymes level.
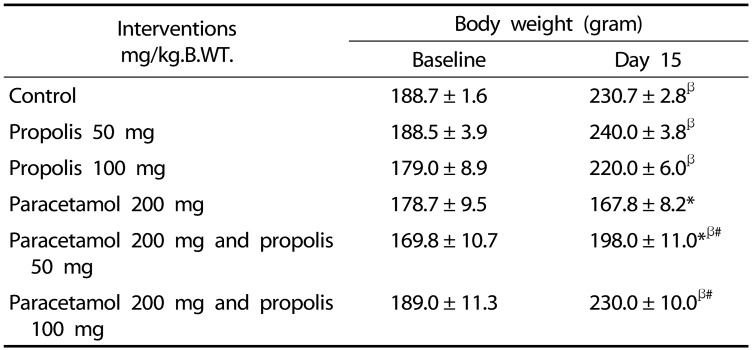
Effect of propolis and paracetamol on body weight
There was no significant difference in the body weight among the groups before the commencement of the study (Table 5). The results showed that paracetamol overdose causes a significant reduction in rats' body weight as compared to the control group (167.89 ± 6.97 vs 230.78 ± 4.57, P < 0.001). In addition, the body weight of the control rats and the paracetamol treated rats that received propolis at a dose of 50 and 100 mg/kg was significantly increased as compared to the paracetamol treated rats.
Table 5. Effect of the interventions on the body weight.
* P < 0.05 as compared to the control group during the same time interval
β P < 0.05 as compared to baseline within the same intervention
# P < 0.05 as compared to paracetamol (200 mg/kg.B.WT.)
DISCUSSION
The results showed that concomitant administration of propolis along with the paracetamol significantly mitigated the adverse effect of paracetamol on kidney and liver function. Propolis prevented proteinuria, anemia, elevation of liver enzymes, and body weight loss. It was known that cytochrome P450 isoforms, depleted glutathione, and excessive reactive oxygen species play a role in the pathogenesis of paracetamol induced liver injury. Interestingly, propolis inhibited various cytochrome 450 isofoms [18,19]. Therefore, propolis might attenuate the adverse effect of paracetamol toxicity through inhibition of cytochrome P450, which results in the reduction of N-acetyl-p-benzoquinone imine formation that keeps the glutathione pathway active.
The increase of the liver enzymes by high dose of paracetamol is similar to findings of other studies [5,20]. As albumin is mainly synthesized in the liver, the reduction of serum albumin and total protein by paracetamol overdose is another indication of liver injury. This effect might be attributed to the proteinuria induced by high dose of paracetamol [21]. The present study revealed that propolis extract maintained almost normal serum protein and albumin, which is most likely due to preservation of liver function as well as the reduction of albuminuria. This is in agreement with our previous study that showed the ability of propolis to prevent proteinuria in ethylene glycol toxicity [10].
It has been shown that treatment of rats with paracetamol, for 42 days with 7.5 mg/kg.B.WT., caused a significant decrease in RBC [22]. However, paracetamol caused non-significant changes in total WBC, neutrophil, eosinophil, monocyte, lymphocyte and platelet counts [23]. In the present study, paracetamol induced significant changes of hematological parameters in rats. Similar to other reports, paracetamol overdose caused a significant decrease in the number of RBC, Hb and Ht value, and increased the number of WBC [24,25]. It was found that, the main reason for cellular red blood cell damage are the lipid peroxidation, membrane protein cross linking and fragmentation caused by free radicals [26,27]. However, the co-administration propolis effectively protected against paracetamol-induced hematotoxicity by restoring almost normal counts of the hematological parameters. This might be due to the antioxidative and anti-inflammatory effects of propolis.
In the present study, the high dose of paracetamol induced renal damage was evident by an elevation of blood urea, serum creatinine and urine protein and albumin excretion. However, paracetamol overdose decreased uric acid that might be related to its toxic effects on the nephron, which reduces its reabsorption and promotes its excretion [27]. Renal damage is common in paracetamol toxicity. Data from 522 patients showed that renal damage was present in 48.8% of patients with liver injury at the time of first presentation and creatinine was a predictor of outcome in paracetamol toxicity [28]. However, the use of propolis significantly prevented the kidney damage caused by paracetamol overdose.
Paracetamol did not cause a significant change in the urine volume on day 7 after the ingestion. However, urine volume was significantly increased on day 14 after the ingestion. Paracetamol overdose induced acute tubular necrosis [29,30]. Acute tubular necrosis is characterized by a reduction of urine volume during the first 1–2 weeks followed by increased urine volume [31]. Therefore, the high urine volume recorded on day 14 after paracetamol ingestion might be due to nonoliguric phase of acute tubular necrosis.
The ability of propolis to prevent proteinuria makes propolis a favorable candidate to be used in cases of proteinuria and nephrotic syndrome. In earlier study, we have found that propolis extract alleviates urinary protein excretion and ameliorates the deterioration of liver and kidney function caused by ethylene glycol ingestion in rats [10]. Furthermore, propolis has a potential to treat and prevent urinary calculus, crystaluria and proteinuria. In another study, it was found that propolis contains polyphenols including flavonoids, and it demonstrates higher antioxidant activities than honey [12].
The body weight loss caused by paracetamol overdose might be attributed to the liver and kidney dysfunction that result in a loss of appetite. However, the use of propolis with paracetamol significantly prevented the weight loss, which is most likely due to the favorable effect of propolis in kidney and liver function.
In conclusion, the data presented showed that propolis significantly prevents renal, hepatic and hematological toxicity caused by paracetamol overdose. Furthermore, propolis might be useful in the management of liver and renal diseases particularly proteinuria. The mode of action might be related to its anti-inflammatory and anti-oxidant effect.
ACKNOWLEDGEMENT
The authors would like to thank New York Medical Care for Nephrology (N Al-Waili MD, PhD), NY, USA, for paying the publication fee.
Footnotes
CONFLICT OF INTEREST: The authors declare no conflicts of interest.
References
- 1.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James LP, McCullough SS, Knight TR, Jaeschke H, Hinson JA. Acetaminophen toxicity in mice lacking NADPH oxidase activity: role of peroxynitrite formation and mitochondrial oxidant stress. Free Radic Res. 2003;37:1289–1297. doi: 10.1080/10715760310001617776. [DOI] [PubMed] [Google Scholar]
- 3.Larsson R, Ross D, Berlin T, Olsson LI, Moldéus P. Prostaglandin synthase catalyzed metabolic activation of p-phenetidine and acetaminophen by microsomes isolated from rabbit and human kidney. J Pharmacol Exp Ther. 1985;235:475–480. [PubMed] [Google Scholar]
- 4.Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- 5.Ojo OO, Kabutu FR, Bello M, Babayo U. Inhibition of paracetamol induced oxidative stress in rats by extracts of lemongrass (Cymbopogon citratus) and green tea (Camellia sinensis) in rats. Afr J Biotechnol. 2006;5:1227–1232. [Google Scholar]
- 6.Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- 7.Miettinen TP, Björklund M. NQO2 is a reactive oxygen species generating off-target for acetaminophen. Mol Pharm. 2014;11:4395–4404. doi: 10.1021/mp5004866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh J, Das J, Manna P, Sil PC. Acetaminophen induced renal injury via oxidative stress and TNF-alpha production: therapeutic potential of arjunolic acid. Toxicology. 2010;268:8–18. doi: 10.1016/j.tox.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Satav JG, Bhattacharya RK. Respiratory functions in kidney mitochondria following paracetamol administration to young-adult & old rats. Indian J Med Res. 1997;105:131–135. [PubMed] [Google Scholar]
- 10.El Menyiy N, Al Waili N, Bakour M, Al-Waili H, Lyoussi B. Protective effect of propolis in proteinuria, crystaluria, nephrotoxicity and hepatotoxicity induced by ethylene glycol ingestion. Arch Med Res. 2016;47:526–534. doi: 10.1016/j.arcmed.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Al-Waili N, Al-Ghamdi A, Ansari MJ, Al-Attal Y, Salom K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int J Med Sci. 2012;9:793–800. doi: 10.7150/ijms.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Guendouz S, Al-Waili N, Aazza S, Elamine Y, Zizi S, Al-Waili T, Al-Waili A, Lyoussi B. Antioxidant and diuretic activity of co-administration of Capparis spinosa honey and propolis in comparison to furosemide. Asian Pac J Trop Med. 2017;10:974–980. doi: 10.1016/j.apjtm.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Mouse H, Tilaoui M, Jaafari A, Aboufatima L, Chait A, Zyad A. Evaluation of the in vitro and in vivo anticancer properties of Moroccan propolis extracts. Rev Bras Farmacogn. 2012;22:558–567. [Google Scholar]
- 14.Aldahmash BA, El-Nagar DM, Ibrahim KE. Reno-protective effects of propolis on gentamicin-induced acute renal toxicity in swiss albino mice. Nefrologia. 2016;36:643–652. doi: 10.1016/j.nefro.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 15.da Costa MF, Libório AB, Teles F, Martins CS, Soares PM, Meneses GC, Rodrigues FA, Leal LK, Miron D, Silva AH, Martins AM. Red propolis ameliorates ischemic-reperfusion acute kidney injury. Phytomedicine. 2015;22:787–795. doi: 10.1016/j.phymed.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Mounieb F, Ramadan L, Akool ES, Balah A. Propolis alleviates concanavalin A-induced hepatitis by modulating cytokine secretion and inhibition of reactive oxygen species. Naunyn Schmiedebergs Arch Pharmacol. 2017;390:1105–1115. doi: 10.1007/s00210-017-1410-3. [DOI] [PubMed] [Google Scholar]
- 17.Singla S, Kumar NR, Kaur J. In vivo studies on the protective effect of propolis on doxorubicin-induced toxicity in liver of male rats. Toxicol Int. 2014;21:191–195. doi: 10.4103/0971-6580.139808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naramoto K, Kato M, Ichihara K. Effects of an ethanol extract of Brazilian green propolis on human cytochrome P450 enzyme activities in vitro. J Agric Food Chem. 2014;62:11296–11302. doi: 10.1021/jf504034u. [DOI] [PubMed] [Google Scholar]
- 19.Ryu CS, Oh SJ, Oh JM, Lee JY, Lee SY, Chae JW, Kwon KI, Kim SK. Inhibition of Cytochrome P450 by propolis in human liver microsomes. Toxicol Res. 2016;32:207–213. doi: 10.5487/TR.2016.32.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang WP, Huang SS, Matsuda Y, Saito H, Uramaru N, Ho HY, Wu JB, Huang GJ. Protective effects of tormentic acid, a major component of suspension cultures of Eriobotrya japonica cells, on acetaminophen-induced hepatotoxicity in mice. Molecules. 2017;22:E830. doi: 10.3390/molecules22050830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugimoto K, Sakamoto S, Nakakawa K, Hayashi S, Harda N, Yamaji R, et al. Suppression of inducible nitric oxide synthase expression and amelioration of lipopolysaccharide-induced liver injury by polyphenolic compounds in Eucalyptus globulus leaf extract. Food Chem. 2011;125:442–446. [Google Scholar]
- 22.Oyedeji KO, Bolarinwa A, Ojeniran SS. Effect of paracetamol (acetaminophen) on haematological and reproductive parameters in male albino rats. IOSR J Pharm Biol Sci. 2013;4:65–70. [Google Scholar]
- 23.Biu AA, Yusufu SD, Rabo JS. Studies on the effects of aqueous leaf extracts of Neem (Azadirachta indica A. juss) on haematological parameters in chicken. Afr Sci. 2009;10:189–192. [Google Scholar]
- 24.Yousef MI, Omar SA, El-Guendi MI, Abdelmegid LA. Potential protective effects of quercetin and curcumin on paracetamol-induced histological changes, oxidative stress, impaired liver and kidney functions and haematotoxicity in rat. Food Chem Toxicol. 2010;48:3246–3261. doi: 10.1016/j.fct.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Oyedeji O, Bolarinwa F, Ojeniran S. Effect of paracetamol (acetaminophen) on haematological and reproductive parameters in male albino rats. Res J Pharmacol. 2013;7:21–25. [Google Scholar]
- 26.Vives Corrons JL, Miguel-García A, Pujades MA, Miguel-Sosa A, Cambiazzo S, Linares M, Dibarrart MT, Calvo MA. Increased susceptibility of microcytic red blood cells to in vitro oxidative stress. Eur J Haematol. 1995;55:327–331. doi: 10.1111/j.1600-0609.1995.tb00705.x. [DOI] [PubMed] [Google Scholar]
- 27.Masaaki K, Masatoshi S, Koichi H, Keisuke T. Relationship between erythrocyte deformability and glutathione under oxidative stress. Comp Biochem Physiol A Mol Integr Physiol. 1994;107:7–12. [Google Scholar]
- 28.Pakravan N, Simpson KJ, Waring WS, Bates CM, Bateman DN. Renal injury at first presentation as a predictor for poor outcome in severe paracetamol poisoning referred to a liver transplant unit. Eur J Clin Pharmacol. 2009;65:163–168. doi: 10.1007/s00228-008-0580-9. [DOI] [PubMed] [Google Scholar]
- 29.Pakravan N, Shokrzadeh M, Akbari F, Shadbooresta A. Effect of a toxic dose of acetaminophen on electrolytes and histopathological changes in the kidney. Int J Clin Toxicol. 2014;2:64–70. [Google Scholar]
- 30.Blantz RC. Acetaminophen: acute and chronic effects on renal function. Am J Kidney Dis. 1996;28(Suppl 1):S3–S6. doi: 10.1016/s0272-6386(96)90561-2. [DOI] [PubMed] [Google Scholar]
- 31.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]