Abstract
Introduction
Mechanical heart valves (MHV) are extremely durable, but they require permanent use of anticoagulation to prevent thromboembolic events. The only approved therapeutic options are vitamin K antagonists (VKAs), such as warfarin. As a drug class, clinical management is difficult, therefore new alternatives need to be evaluated.
Methods
RIWA is a phase II/III, prospective, open-label, randomized, pilot study designed to investigate oral rivaroxaban 15 mg twice daily compared with dose-adjusted warfarin for the prevention of stroke (ischemic or hemorrhagic) and systemic embolism in patients with MHV, from August 2018 to December 2019. Patients will undergo transesophageal echocardiography at the beginning and the end of the study (follow-up time 90 days). On an explanatory basis, all events will be analyzed, including stroke, peripheral systemic embolism, valve thrombosis, significant bleeding and death.
Discussion
Warfarin and similar VKAs are standard therapy for patients with an MHV. Even with the appropriate use of therapy, the incidence of thromboembolic events is high at 1–4% per year. Furthermore, bleeding risk is significant, ranging from 2 to 9% per year. The new frontier to be overcome in relation to use of the new oral anticoagulants is undoubtedly in patients with MHV. A significant portion of people with MHV worldwide will benefit if noninferiority of these new agents is confirmed.
Trial Registration
ClinicalTrials.gov identifier: NCT03566303. Recruitment Status: Recruiting. First Posted: 25 June 2018. Last Update Posted: 25 June 2018.
Key Points
| Thromboembolic complications and anticoagulation-related bleeding are by far the most prevalent contributors to morbidity and mortality after surgery for mechanical heart valve (MHV). |
| The mainstay of treatment in guidelines remains indefinite anticoagulation with a vitamin K antagonist (VKA). The benefits of VKA therapy after MHV placement are marked. |
| Given the narrow therapeutic index, interactions, genetic variants, and need for blood monitoring of patients taking VKAs, it is necessary to evaluate new alternatives such as Factor Xa inhibitors. |
Introduction
For patients with severe and symptomatic valvular heart disease (VHD), valve replacement surgery improves morbidity and mortality outcomes. It is estimated that 4 million valve-replacement procedures have been performed over the last 50 years, and it remains the only definitive treatment for most patients with advanced VHD [1].
Patients who received mechanical heart valves (MHVs) have significantly lower mortality, higher cumulative incidence of bleeding and, in some age groups, stroke than recipients of a biologic prosthesis [2]. Despite this benefit, MHV demands lifelong anticoagulation with vitamin K antagonists (VKAs), most commonly warfarin, because of the high thrombogenicity of the prosthesis.
Even with the appropriate use of therapy, there is a high incidence of thromboembolic events: 1–4% per year. Furthermore, bleeding risk is significant, ranging from 2 to 9% per year [3]. Given the narrow therapeutic index, interactions, genetic variants, and need for blood monitoring of patients taking VKAs, alternatives to warfarin have now been made available, specifically, inhibitors that directly target Factor IIa (dabigatran) or Xa (rivaroxaban, apixaban, edoxaban) [4].
The only randomized controlled trial using a novel oral anticoagulant (NOAC) in patients with MHV (RE-ALIGN trial) was stopped early because of the higher incidence of negative outcomes, including thromboembolic events and bleeding [5]. The RE-ALIGN investigators proposed several reasons for the poor outcomes associated with dabigatran, including inadequate plasma levels of the drug and mechanisms of action. Although the results were disappointing, the trial was subject to several limitations. We recently published a series of cases with patients with MHV who had used rivaroxaban for 3 months without clinical complications [6]. Based on this, we believe the search for new antithrombotic alternatives in this scenario must continue.
The aim of the current study is to compare the incidence of thromboembolic events (stroke, systemic embolism, valvular thrombosis) and bleeding (intracerebral bleeding, digestive bleeding) in patients with MHV using rivaroxaban versus warfarin.
Methods
Study Objectives
The RIWA study (ClinicalTrials.gov NCT03566303) is a randomized, open-label, event-driven, pilot study designed to assess the incidence of thromboembolic (stroke, systemic embolic events, thrombosis of valve prosthesis—indicating efficacy) and bleeding (hemorrhagic stroke, gastrointestinal bleeding—indicating safety) events with the rivaroxaban-based strategy in comparison with those with the guideline-recommended warfarin dose-adjusted strategy after MHV replacement (with ≥ 3 months postoperatively). Although there is no predetermined specific outcome because it is an explanatory study, the incidence of ischemic stroke, peripheral embolic events, thrombosis of valve prosthesis, and major bleeding will be compared in both groups. The inclusion and exclusion criteria are outlined in Tables 1 and 2.
Table 1.
RIWA study inclusion criteria
| Age 18–74 years at entry |
| Patients with MHV in mitral and/or aortic position, for at least 3 months postoperatively |
| Brain CT scan without hemorrhage or findings of acute cerebral infarction on the last 2 days of screening |
| Exclusion of atrial thrombus or valve prosthesis thrombosis by TEE on the last 2 days of screening |
| Written, informed consent |
CT computed tomography, MHV mechanical heart valves, TEE transesophageal echocardiogram
Table 2.
RIWA study exclusion criteria
| Previous hemorrhagic stroke |
| Ischemic stroke in the last 3 months |
| Severe renal impairment (CrCl rates < 30 ml/min) |
| Active liver disease (any etiology) |
| Concomitant use of any antiplatelet (aspirin, clopidogrel, prasugrel, ticagrelor, ticlopidine, etc.) |
| Increased risk of bleeding (congenital or acquired) |
| Uncontrolled SAH |
| Gastrointestinal hemorrhage within the past year |
| Anemia (Hb level < 10 g/dl) or thrombocytopenia (platelet count < 100 × 109/l) |
| Active infective endocarditis |
| Pregnant or lactating women |
ASA acetylsalicylic acid, CrCl creatinine clearance, Hb hemoglobin, SAH systemic arterial hypertension
Study Population, Randomization and Follow-Up
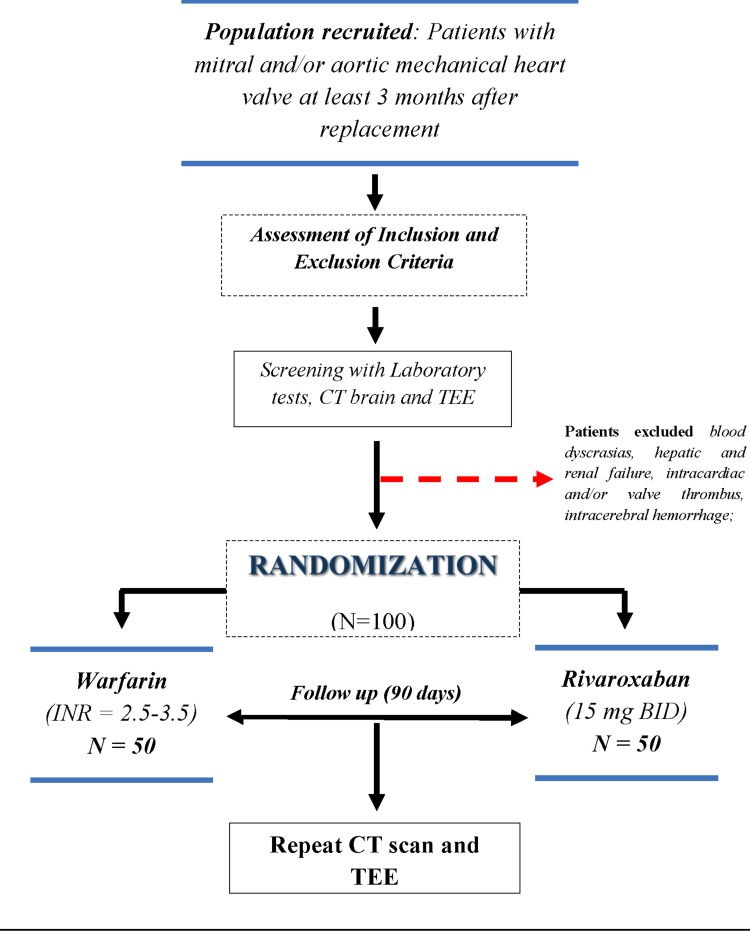
From September 2018 to December 2019, patients with MHV (n = 50) aged between 18 and 75 years who are at least 3 months postoperative, in accordance with the eligibility criteria, will be recruited in the General Hospital Roberto Santos in Salvador-Brazil. A total of 100 subjects who provide informed consent will be randomized in a 1:1 ratio to 15 mg rivaroxaban twice daily or dose-adjusted warfarin. Figure 1 shows the study flow. Randomization will be performed using a random number table generated by a computerized electronic system. Patients randomized to an even number will be allocated to the rivaroxaban group, and those with an odd number will receive warfarin. Sealed, opaque and sequentially numbered envelopes containing the treatment allocation will be opened by the recruiting clinician on participant enrolment.
Fig. 1.
RIWA study design. ECG electrocardiogram, CT computed tomography, TEE transesophageal echocardiography, BID twice daily
To participate in the study, subjects will undergo a computed tomography (CT) brain scan (without contrast) and a transesophageal echocardiogram (TEE) with the aim of increasing accuracy in the detection of subclinical events such as cerebral micro-infarcts or asymptomatic valve thrombosis. After 90 days of follow-up, even without events or symptoms, a repeat CT brain scan and TEE will be conducted. Transthoracic echocardiogram (TTE) will be performed at 30 and 60 days to check the gradient and mobility of the prostheses. On suspicion of valve thrombosis, the patient will be hospitalized and the TEE will be repeated. On-site visits are planned at days 30, 60, and 90. Telephone contact will be conducted weekly, and patients themselves will be able to contact the study at any time for clarification, questions and guidance.
Drug Administration Protocol
Rivaroxaban will be supplied as 15-mg tablets taken twice daily without international normalized ratio (INR) monitoring or food restriction. All patients randomized to rivaroxaban will continue to receive that drug for the duration of the study. After the end of follow-up, all subjects will return to warfarin use. Patients with previous use of warfarin will do washout with immediate introduction of rivaroxaban once the INR < 3.0.
Patients assigned to warfarin will require close coagulation monitoring to achieve the target INR (3.0, range 2.5–3.5 for mitral position, or 2.5, range 2.0–3.0 for isolated aortic position). Therefore, a protocol of monitoring and dosage adjustment for this drug is in place to ensure the safety and optimization of its use, as each patient may respond differently to the same dose. A warfarin dose-adjustment algorithm will be provided according to evidence-based guidelines [7, 8]. A modified Rosendaal et al. [9] method of linear interpolation will be used between each pair of measured INR values. For individuals with INR values outside the therapeutic range, INR will be repeated every 7 days for at least 3 months for improved time in therapeutic range accuracy [10]; only after this will they become candidates for study participation.
Definition of Outcome Events
Prosthetic valve thrombus is defined as soft and homogeneous, mobile or fixed echo density, similar to myocardium, located at the valve occluder, hinges, and/or valve struts. The largest diameter of the thrombus, as well as the length of the mobile portion, if present, will be measured. A diagnosis of pannus formation will be made when fixed, bright echo-dense structures, sometimes containing focal calcific deposits, are present primarily along the valve ring, with extension into the valve orifice [11]. In patients with inconclusive echocardiography, multidetector CT will be conducted to provide an accurate evaluation of the prosthetic valve structure and functional status [12].
Thromboembolic events involving the central nervous system are defined as a sudden, focal neurological deficit of presumed vascular origin lasting 24 h to 7 days (reversible ischemic neurological deficit) or enduring more than 7 days (stroke), confirmed by CT or magnetic resonance imaging and evaluated by a radiologist. Peripheral embolism will be diagnosed when there is sudden onset of arterial occlusion in the extremities (with or without cyanosis) and with reported absence of pulse, or sudden abdominal pain requiring urgent intervention (confirming acute intestinal ischemia during the surgical procedure).
The bleeding risk is based on the criteria of the Control of Anticoagulation Subcommittee of the International Society on Thrombosis and Haemostasis [13] (Table 3). Minor bleeds are defined as clinical bleeds that do not fulfill the criteria for major bleeds.
Table 3.
RIWA study major bleeding criteria
| Fatal bleeding and/or |
| Symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome, and/or |
| Bleeding causing a fall in hemoglobin level of 20 g L−1 (1.24 mmol L−1) or more, or leading to transfusion of two or more units of whole blood or red cells |
Adapted from Schulman et al. [13]
Echocardiography
A standardized TTE and TEE examination, including the standard imaging planes, will be performed according to the recommendations of the American Society of Echocardiography [14]. Left ventricular ejection fraction will be calculated using the Simpson method, and the left atrial diameter will be measured in the M mode. Mitral valve E-wave peak velocity will also be measured and analyzed.
For TTE, a 1.7/3.4-MHz harmonic transducer will be used. TEE will be performed with a 6.7-MHz multiplane V5Ms transducer. The left atrium and its appendage will be closely inspected for the presence of spontaneous echo contrast (SEC) and thrombi by adjustment of the gain settings to optimal, to be able to distinguish SEC from electronic background noise. The degree of SEC will be categorized as being either absent (0), mild (1 +), mild to moderate (2 +), moderate (3 +), or severe (4 +) [15]. Prosthetic valve function and investigation of thrombus will follow the criteria of Zoghbi et al. [16].
Computed Tomography Brain Scan
Patients will routinely undergo only nonenhanced CT. The images may first be analyzed with a standard window width and level setting of approximately 40/20 HU, with a second narrower setting of 20/32 HU used to demonstrate subtle abnormalities that suggest ischemia. Perfusion CT and angiography will be used only in selected cases: if there is an acute stroke and if intracranial bleeding has been excluded in the first stage. The main expected change in suspected ischemic stroke is a cortical-subcortical hypoattenuating area within a vascular territory.
Safety Monitoring, Data Monitoring
The trial is externally monitored (ClinicalTrials Unit, Bern, Switzerland) in accordance with good clinical practice standards.
Monitoring Harm
All patients will have weekly follow-up by a dedicated and trained team to detect complications such as bleeding, poor adherence to therapy, embolic events or valve thrombosis. In addition, patients will routinely have face-to-face consultations every 30 days. Any event of valve thrombosis or embolism will be immediately reported to the ethics committee for consideration and decision about the suspension of the study.
Statistical Considerations
The primary efficacy and safety analyses will be conducted on the full analysis set of all randomized patients according to the intention-to-treat principle using endpoints and will be blindly adjudicated by an independent clinical event committee. SPSS 17.0 (SPSS Inc.; Chicago, IL, USA) will be used to perform statistical analysis of the collected data. Quantitative variables will be described as mean and standard deviation. The mean comparison will be performed using the Student’s t test for independent populations or related populations, as appropriate. The qualitative and categorical variables will be presented as percentages, and their comparisons will be made with the Chi-squared or Fisher’s exact test when indicated.
Use of Concomitant Drugs
The use of drugs such as acetylsalicylic acid, clopidogrel and other antiplatelet agents will not be allowed during the study period. The same applies to antiarrhythmic drugs that interact with rivaroxaban such as quinidine.
Discussion
Each year 300,000 prosthetic valves are replaced worldwide, of which 100,000 are in North America alone; this is projected to rise to 850,000 by the year 2050. In addition, cardiovascular diseases are responsible for 10–20% of all cardiac surgical procedures in the USA [1, 17].
The most plausible hypothesis for the failure of dabigatran (direct thrombin inhibitor) in prior studies is that clotting on MHV is triggered via activation of the contact system, resulting in the local generation of thrombin in concentrations that overwhelm those of dabigatran plasma levels [18]. This would be completely different with Factor Xa inhibitors, where one molecule of Factor Xa triggers the generation of 1000 molecules of thrombin, suggesting a greater theoretical effectiveness with inhibition of this level of the coagulation cascade [19]. Another hypothesis for the failure of dabigatran previously was use in patients with newly implanted valve prosthesis, where epithelization has not yet occurred [18].
On the other hand, in vitro MHV studies and animal models of rivaroxaban in terms of efficacy of thromboprophylaxis have shown promising results [20, 21]. As mentioned, our group’s study was the first experience with rivaroxaban in MHV, and results were encouraging [6].
Funding
This work will be supported by the General Hospital Roberto Santos—HGRS.
Conflict of interest
André R. Durães, MD, PhD, Yasmin de S. L. Bitar, Bachelor in Health, José Admirço L. Filho, MD, PhD, Igor S. Schonhofen, MD, Edmundo J. N. Camara, MD, PhD, Leonardo Roever, MD, MHS, Hugo E. D. P. Cardoso, and Kevan M. Akrami, MD have no conflicts of interest that are directly relevant to the content of this study.
References
- 1.Sun JC, Davidson MJ, Lamy A, Eikelboom JW. Antithrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet. 2009;374(9689):565–576. doi: 10.1016/S0140-6736(09)60780-7. [DOI] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 3.Cortelazzo S, Finazzi G, Viero P, Galli M, Remuzzi A, Parenzan L, et al. Thrombotic and hemorrhagic complications in patients with mechanical heart valve prosthesis attending an anticoagulation clinic. Thromb Haemost. 1993;69(4):316–320. doi: 10.1055/s-0038-1651604. [DOI] [PubMed] [Google Scholar]
- 4.O’Dell KM, Igawa D, Hsin J. New oral anticoagulants for atrial fibrillation: a review of clinical trials. Clin Ther. 2012;34(4):894–901. doi: 10.1016/j.clinthera.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Durães AR, Bitar YSL, Lima MLG, Santos CC, Schonhofen IS, Filho JAL, et al. Usefulness and safety of rivaroxaban in patients following isolated mitral valve replacement with a mechanical prosthesis. Am J Cardiol. 2018 doi: 10.1016/j.amjcard.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 9.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. doi: 10.1055/s-0038-1651587. [DOI] [PubMed] [Google Scholar]
- 10.Reiffel JA. Time in the therapeutic range for patients taking warfarin in clinical trials: useful, but also misleading, misused, and overinterpreted. Circulation. 2017;135(16):1475–1477. doi: 10.1161/CIRCULATIONAHA.116.026854. [DOI] [PubMed] [Google Scholar]
- 11.Ozkan M, Kaymaz C, Kirma C, Sonmez K, Ozdemir N, Balkanay M, et al. Intravenous thrombolytic treatment of mechanical prosthetic valve thrombosis: a study using serial transesophageal echocardiography. J Am Coll Cardiol. 2000;35(7):1881–1889. doi: 10.1016/S0735-1097(00)00654-9. [DOI] [PubMed] [Google Scholar]
- 12.Dangas GD, Weitz JI, Giustino G, Makkar R, Mehran R. Prosthetic heart valve thrombosis. J Am Coll Cardiol. 2016;68(24):2670–2689. doi: 10.1016/j.jacc.2016.09.958. [DOI] [PubMed] [Google Scholar]
- 13.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost JTH. 2005;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 14.Henry WL, DeMaria A, Gramiak R, King DL, Kisslo JA, Popp RL, et al. Report of the American Society of Echocardiography Committee on nomenclature and standards in two-dimensional echocardiography. Circulation. 1980;62(2):212–217. doi: 10.1161/01.CIR.62.2.212. [DOI] [PubMed] [Google Scholar]
- 15.Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23(4):961–969. doi: 10.1016/0735-1097(94)90644-0. [DOI] [PubMed] [Google Scholar]
- 16.Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report From the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22(9):975–1014. doi: 10.1016/j.echo.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 18.Shazly A, Afifi A. RE-ALIGN: first trial of novel oral anticoagulant in patients with mechanical heart valves—the search continues. Glob Cardiol Sci Pract. 2014;2014(1):88–89. doi: 10.5339/gcsp.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan NC, Weitz JI, Eikelboom JW. Anticoagulation for mechanical heart valves: will oral factor Xa inhibitors be effective? Arterioscler Thromb Vasc Biol. 2017;37(5):743–745. doi: 10.1161/ATVBAHA.117.309223. [DOI] [PubMed] [Google Scholar]
- 20.Kaeberich A, Reindl I, Raaz U, Maegdefessel L, Vogt A, Linde T, et al. Comparison of unfractionated heparin, low-molecular-weight heparin, low-dose and high-dose rivaroxaban in preventing thrombus formation on mechanical heart valves: results of an in vitro study. J Thromb Thrombolysis. 2011;32(4):417–425. doi: 10.1007/s11239-011-0621-6. [DOI] [PubMed] [Google Scholar]
- 21.Greiten LE, McKellar SH, Rysavy J, Schaff HV. Effectiveness of rivaroxaban for thromboprophylaxis of prosthetic heart valves in a porcine heterotopic valve model. Eur J Cardio Thorac Surg Off J Eur Assoc Cardio Thorac Surg. 2014;45(5):914–919. doi: 10.1093/ejcts/ezt545. [DOI] [PubMed] [Google Scholar]