Abstract
Purpose
To evaluate dosimetric and clinical findings of MRI-guided HDR brachytherapy (HDR-B) for cervical carcinoma.
Material and methods
All patients had a CT, MRI and pelvic-paraaortic lymphadenectomy. Treatment: pelvic (+/−)para-aortic3D/IMRT radiotherapy (45 Gy), weekly cisplatin and HDR-B and pelvic node/parametrial boost 60 Gy until interstitial brachytherapy was done. Two implants: 2008–2011: 5 fractions of 6 Gy, 2011: 2016, 4 fractions of 7 Gy. MRI/TAC were done in each implant. The following were defined: GTV, CTH-HR, CTV-IR; OAR: rectum, bladder and sigmoid.
Results
From 2007 to 2016: 57 patients. Patients: T1b2-T2a: 4p, T2b 41p, T3a: 2p; T3B 8p T4a: 2p; N0: 32p, N1 21p, no lymphadenectomy: 4p. Median follow up: 74.6 m (16–122 m), recurrence: 5p local, 6p node, 9p metastasis and 37p without recurrence.
Local control 5 years: 90.1%; Ib2-IIB: 94.8%, III-IVa: 72.2%. (p:0.01). RDFS 5y was 92.5%; IB2-IIB: 93%, III: 85% (p:0.024); for pN0: 100%; pN+ iliac-paraaortic: 71.4% (p: 0.007). MFS 5y was 84.1%. Overall survival (OS) at 5y: 66.6% and the cancer specific survival (CEOS) was 74%. Univariate analysis survival: stage Ib2-II 83% vs. III-IVa 41% (p = 0.001); histology: squamous 78%, adenocarcinoma 59.7% (p: ns); lymph node: N0 85% vs. PA+P− 72%, and PA+P+ 35% (p = 0.010). In relation with: HR-CTV dose > 85 Gy, CEOS: 82.5% vs. 77%, and volume CTV-HR < 30 cc: 81.8% and >30 cc: 67%; p: ns. Acute grade 2–3 toxicity: rectal 15.7%, intestinal 15.7% and vesical 15.5%.
Conclusion
Use of interstitial HDR-BQ guided by RM increased CTV-HR dose and local control, like EMBRACE results. Nodal boost improves RDFS and perhaps OS.
Keywords: Cervix, Cancer, Brachytherapy, IG-HDR, MRI, IMRT
1. Background
Cervical cancer is the second most frequent gynaecological tumour worldwide. Its development is closely related to human papilloma virus infection. Surgical treatment is reserved for early tumour stages IA, IBA and IIA1. Treatment is based on the combination of external beam radiotherapy, brachytherapy and chemotherapy in the remaining stages.
In the last 15 years the main change improving local control and survival in patients with locally advanced tumours is image-guided brachytherapy.1 This approach is based on the use of 3D images, mainly magnetic resonance images (MRI), for better delimiting tumour volumes and organs at risk (OAR) as well as improving treatment planning.2, 3 In addition, in parallel, applicators have been developed which allow the combination of intracavitary and interstitial treatments, improving tumour coverage and results related to disease control in patients with poor tumour response and residual parametrial disease.
The above clinical results were demonstrated by several studies and later on the 2008 GEC-ESTRO initiated a study on image guided intensity modulated external beam radiochemotherapy and MRI-based adaptive brachytherapy in locally advanced cervical cancer (EMBRACE study) with the aim of collecting data from this multicentre experience with the use of MRI-guided brachytherapy (MRI-GBT). This study finished in December 2015. In 2012 the RetroEMBRACE study was opened and was aimed at retrospectively collecting the data of the patients treated in the hospitals participating in the EMBRACE study before the initiation of this study. In 2017 the EMBRACEII study was opened, including patients with advanced cervical cancer treated with intensity modulated radiation therapy (IMRT), chemotherapy and MRI-GBT. The aim of this article is to review the experience of the patients from our hospital who were included in the above mentioned studies.
2. Aim
To evaluate dosimetric and clinical findings of MRI-guided HDR brachytherapy (HDR-B) for cervical carcinoma.
3. Material and methods
The Brachytherapy Unit of our hospital was opened in April 2008. Since then, all patients have been treated with MRI-GBT.
All cases with locally advanced cervical tumours are evaluated by the Committee of Gynaecological Tumours. Patients undergo computerized tomography (CT), MRI and, in some cases, positron emission tomography-CT (PET-CT) studies, and pelvic-paraaortic lymphadenectomy is performed for staging.
External beam radiotherapy (EBRT) was initially 3D but since 2011 IMRT is performed. All the cases received 45 Gy over 5 weeks. In cases with lymph node remnants following lymphadenectomy or findings of extracapsular extension in some lymph nodes, a lymph node boost was carried out after brachytherapy. Patients included in the EMBRACE II study received an integrated boost at a dose of 55–57 Gy according to the pelvic or paraaortic localization, respectively. Before the availability of combined intracavitary-interstitial applicators, patients with macroscopic residual parametrial disease received a parametrial dose to achieve 60 Gy after brachytherapy, taking into account the parametrial doses of the brachytherapy.
Chemotherapy involved the use of cisplatin 40 mg/m2, 1 day/week during 5 weeks.
At week 5 of EBRT, all patients underwent MRI to evaluate response and to choose the applicator for brachytherapy. Brachytherapy was then done at weeks 6 and 7; first with 5 fractions of 6 Gy in 2 implants, and then with 4 fractions of 7 Gy after the entry in the EMBRACE study in 2011. In both protocols MRI was performed after each implant for treatment planning. The objective was to carry out all the treatment in less than 55 days.
The GEC-ESTRO guidelines were followed for the delimitation of volumes in MRI-GBT. The gross tumour volume b (GTVb) was delimited with the residual tumour area visible in T2 in MR. The high-risk clinical target volume (HR-CTV) includes the GTVb, all the cervix, the residual area of microscopic disease in parametrial tissue, and the vagina or uterine cavity visible as grey areas in T2 in MR or palpable on clinical examination. The intermediate risk CTV (IR-CTV) was delimited taking into account the initial involvement, with extension of 5–10 mm of the HR-CTV limited by the OAR. An empty bladder, rectum from 2 cm under the vaginal applicator to the peritoneal reflection and sigma from the reflection to 1 cm above the uterine fundus were also delimited as OARs. We also registered the doses at points A and B and the International Commission of Radiation Units points at the bladder, rectum and vagina and also at the vagina surface and at 0.5 cm of depth.
Taking into account the dose of EBRT, the dosimetric objectives were to achieve D90 HR-CTV > 100% of the prescription dose, D90 IR-CTV > 60 Gy EQD2, D2cc rectum and sigma < 75 Gy EQD2 and D2cc < 85 Gy EQD2.
The patients were followed every 3 months during the first two years. The follow-up study always included physical examination, cytology every 6 months, MR every 6 months and CT annually. From years 3 to 5 the consultations were made every 6 months, with MR every year. After year 5 the follow-ups were carried out annually. The visits were alternated between the Gynaecology Department and the Brachytherapy Unit.
General digestive, urinary and rectal toxicity were reported according to the Common Terminology Criteria for Adverse Events (CTCAE) v4 scale.
3.1. Statistical analysis
Treatment failures were classified according to the site of the first tumoral relapse: local (cervix, uterus, vagina, parametria), regional (pelvic or paraaortic nodes) or distant metastases. Time intervals for local control, regional failure or overall survival were calculated from the date of biopsy to the date of event or last follow-up appointment.
Toxicity was graded according to site and severity using the National Cancer Institute CTCAE v4.03 (Common Terminology Criteria for Adverse Events) guidelines. T de Student was used to compare median of doses between groups of toxicity grade 0–1 versus grade 2–3.
Kaplan–Meier test was used to calculate survival curves. Log rank test was used to compare prediction factors in survival.
4. Results
4.1. Patient characteristics
From November 2007 to July 2016 a total of 57 patients with a mean age of 52 years (range: 27–85 years) were included in the study.
Table 1 shows the characteristics of the patients included in the study. Most had FIGO stage IIB (72.4%) and III (17.2%), with 79.3% having squamous carcinoma and 20.7% adenocarcinoma. There was no case of small-cell carcinoma. Following the radiological study with CT or PET, 29.8% showed lymph node involvement. Lymphadenectomy was done in 53 patients and showed 32 p with negative nodes, 19 p with iliac lymph node involvement, 9 p with paraaortic involvement and 9 both.
Table 1.
Characteristics of the series.
| N | % | ||
|---|---|---|---|
| Age | Median | 51.2 | 27–85 |
| Stage T | T1b2 | 3 | 5.3 |
| T2a2 | 1 | 1.8 | |
| T2b | 41 | 71.9 | |
| T3a | 2 | 3.5 | |
| T3b | 8 | 14 | |
| T4a | 2 | 3.5 | |
| Stage pN | N0 | 32 | 56.1 |
| N1 | 21 | 36.8 | |
| No lymphadenectomy | 4 | 7 | |
| Histologic type | Squamous | 45 | 78.9 |
| Adenocarcinoma | 12 | 21.1 | |
| Grade | Grade I | 9 | 15.8 |
| Grade II | 29 | 59.9 | |
| Grade III | 10 | 17.5 | |
| nr | 9 | 15.8 | |
The treatment administered is shown in Table 2. All the women received a EBRT dose of 45 Gy in 25 fractions with 3D in 27 patients and IMRT in 30 patients. A parametrial boost was performed in 22% while 35% received a lymph node boost. A total of 51 patients received concomitant chemotherapy with cisplatin weekly for a median of 5 cycles.
Table 2.
Description of the treatment: external radiotherapy (ERT) and RMI guide-brachytherapy (IGBT).
| N | % | ||
|---|---|---|---|
| Radiotherapy | 3D | 27 | 47.4 |
| IMRT | 30 | 52.6 | |
| Brachytherapy | Intracavitary | 42 | 73.7 |
| Interstitial | 13 | 22.8 | |
| Intraoperative | 2 | 3.5 | |
| Median | Range | ||
|---|---|---|---|
| Doses EQD2 | GTV D100 | 103.7 | 53–345 |
| CTV-HR D100 | 77.7 | 46–103 | |
| CTV-HR D90 | 94.4 | 50–131 | |
| D2cc bladder | 83.5 | 55–103 | |
| D2cc rectum | 69.1 | 54–92 | |
| D2cc sigmoid | 64.7 | 48.7–81.7 |
MR-IGBT was carried out in 53 patients using high dose rate brachytherapy. In 5 patients this was not performed; 2 were undergoing radiotherapy due to disease progression and 3 presented bad response to radiochemotherapy and required rescue surgery. Interstitial brachytherapy was performed in 30.1% of the patients.
The mean HR-CTV in the first implant was 19.6 cc (5.2–79.6 cc), Median GTV 1.3 cc (0–14.3 cc): the mean D90 at HR-CTV was 94.5 Gy (50.8–131 Gy), D100 GTV was 115 Gy (59–138 Gy) D2cc to the rectum was 69.1 Gy (54–92 Gy) bladder 83.5 Gy (55–103 Gy) and sigma 66.7 Gy (48.73–81 Gy).
4.2. Local control
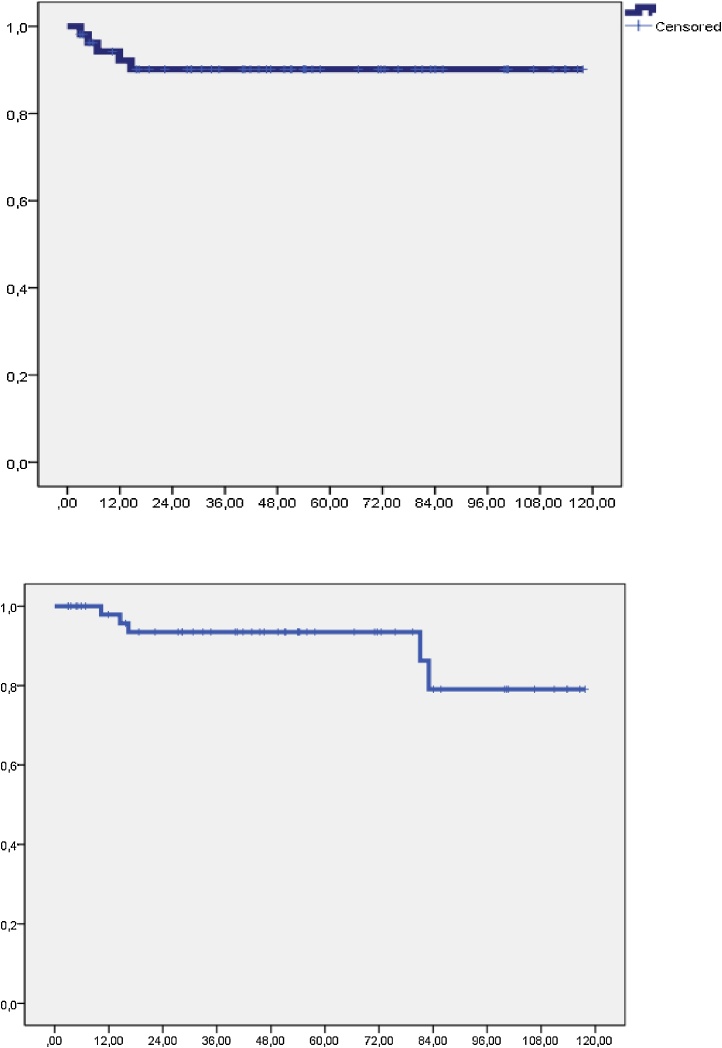
At a median follow up of 74.6 months (range: 16–122 m), a total of 5 local recurrences were observed, leading to a 5-year local control of 90.1% (Fig. 1). There were 2 patients with local progression and synchronous metastases during the radiotherapy and 3 patients had only local failure, who underwent exenteration and 1 is free of local disease.
Fig. 1.
Local control and regional control.
On univariate analysis it was found that according to the stage local control was achieved in 94.8% of patients in stages Ib2-II and 70.1% in stages III-IVa (p = 0.032). According to histological type, 94.9% had squamous carcinoma and 72.2% adenocarcinoma (p = 0.01). With regard to lymph node involvement following staging with lymphadenectomy, 100% of N0 patients achieved local control which was also obtained in 72.2% of N+ patients (p = 0.02).
There were no differences in local control between patients who received a HR-CTV dose > 85 Gy (83% vs 84.4%) or CTV-HR volume > 30 cc (92% vs. 93%). There were, however, differences according to the implant used: 100% of the patients receiving intracavitary brachytherapy achieved local control versus 66% (p = 0.01) of those receiving interstitial brachytherapy.
4.3. Regional control and survival
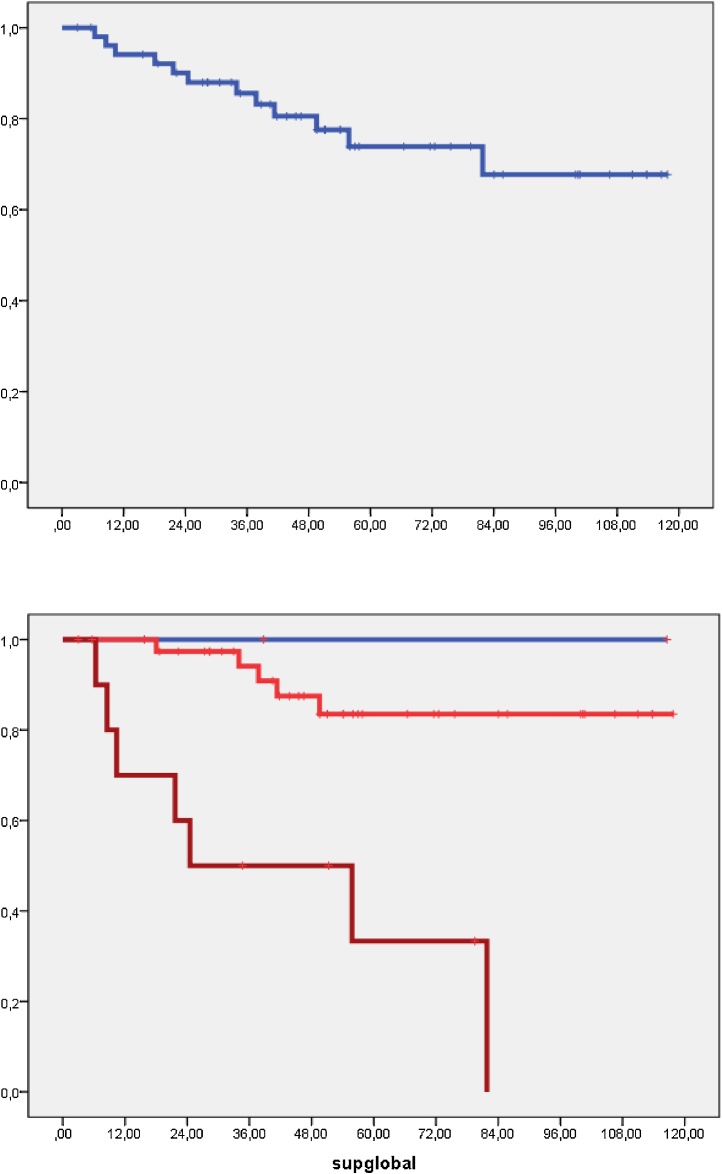
Six patients developed lymph node recurrence, regional control at 5 years was 93.5%, being 92.1% in patients with stage Ib2-II and 85.1% in stage III-IVa (p = 0.024). Regional control according to disease staging after lymphadenectomy was achieved in 100% of patients with N0, in 87% of patients with positive paraaortic and negative iliac lymph nodes and in 71.4% of those with positive paraaortic and iliac lymph nodes (p = 0.007) (Fig. 2).
Fig. 2.
Cancer specific overall survival. Stages: Blue: Stage Ib-IIA1, Red: IIB, Brown: III-IVa.
Metastatic disease was developed by 9 patients with or without regional or local disease. The metastasis-free survival at 5 years was 84.1%
At the time of the analysis 13 patients had died due to disease progression. The 5-year overall survival was 66.6%, and the cancer specific overall survival was 74% (Fig. 2). Univariate analysis showed the following results in relation to patient survival: stage Ib2-II 83% vs. III-IVa 41% (p = 0.001); histology: squamous carcinoma 78%, adenocarcinoma 59.7% (p: ns); lymph node involvement following lymphadenectomy: none 85% vs. paraaortic but not pelvic 72%, and paraaortic and pelvic 35% (p = 0.010).
There were no differences in survival between the patients who received 3D EBRT (66.3%) versus those receiving IMRT (76.5%). On analysing patients who received a HR-CTV dose > 85 Gy, the CEOS was 82.5% vs. 77%, and the volume of CTV-HR < 30 cc: 81.8% and > 30 cc: 67%; but were not significant.
4.4. Toxicity
Acute grade 2–3 toxicity was 5.2% in the present study: rectal 15.7%, intestinal 15.7% and vesical 15.5%.
No patient presented grade 4 toxicity, but late grade 2–3 toxicity was observed: rectal 8.6%, intestinal 8.6%, and vesical 15.5%. The latter type of toxicity was due to an increase in frequency in 6 cases; 2 for hydronephrosis and 1 vesicovaginal fistula in 1 patient after rescue surgery for suspicion of tumour persistence.
We analyzed late rectal and urinary morbidity in relation to the dosimetric parameters. Patients presenting toxicity grade 0–1 vs. grade 2.3 have a mean D2cc of: rectal 67.7 Gy Vs 72.4 Gy (p: ns) and bladder: 80.4 Gy vs. 83.2 Gy (p: ns): Patients with D2cc to the rectum < 75 Gy showed rectal toxicity in 10%, including 14% with a dose > 75 Gy (p: ns Chi-square). Patients receiving D2cc to the bladder < 85 Gy had a risk of grade 2–3 urinary toxicity of 13.7% versus 26.7% in patients with D2cc > 85 Gy (p: ns Chi-square).
We also analyzed the appearance of late toxicity grade 2–3 according to the EBRT received with the following results: rectal: 15% 3D vs. 10.5% IMRT (p: ns X2 Pearson), urinary: 22% 3D, 21% IMRT (p: ns).
5. Discussion
The objective of this study was to describe the experience of a centre treating locally advanced cervical cancer. All the patients underwent MRI-GBT. The treatment included previous 3D EBRT or IMRT when the treatment was performed after 2011. Weekly chemotherapy with cisplatin was administered to 88% of the patients with the mean of 5 cycles. In addition, all the patients also underwent radiological staging and surgical lymph node staging by lymphadenectomy.
Since the initiation of the use of brachytherapy in our hospital in 2008, we have used MRI-GBT. At that time we followed the GEC-ESTRO guidelines which described how to delineate the GTV, HR-CTV and IR-CTV tumour volumes in addition to the OAR, including the bladder, rectum and sigma. We also followed the recommendations on what dose should be given to the OAR in order to obtain adequate local control with little toxicity. These data were then based on the reports of individual hospitals. In 2007 Potter et al.4 published the results of a series of 145 patients treated with MRI-GBT. Local control at 3 years was 88% with a cancer-specific survival of 68%. In their experience women achieving D90 HR-CTV > 87 Gy presented local control of 95%. In addition, grade 3–4 toxicity was only 2%. In 2013 Lindegaard et al.5 described their results in 140 patients. Local control at 3 years was achieved in 91% with a survival of 79%. In a study including 163 patients treated with pulse dose rate brachytherapy carried out in 2013 Mazeron et al.6 achieved local control at 3 years of 92% and a survival of 76%.
In 2008 recruitment for the EMBRACE study was begun, finishing in December 2015. Our group participated in the study after 2011. In 2013 the centres which had participated in the EMBRACE study were requested to retrospectively collect the data of the patients treated with MRI-GBT prior to the entry in the study. This was for the RetroEMBRACE study.
The results of our centre are similar to those published by the previous institutions in addition to the data already reported by EMBRACE.
In our study local control and cancer-specific survival were 90.6% and 72.4%, respectively, at 5 years, being 89% and 73%, respectively, in the RetroEMBRACE study including 731 patients.7 The data of local control in the EMBRACE study have not been published yet, and only the preliminary results with a short follow-up of 25 months were reported in the ESTRO 2017 meeting.8 Local recurrence was presented in 80 out of 1230 patients with a median time to recurrence of 11.5 months.
In our study patients with stages III-IVa and those with a histology of adenocarcinoma had worse local control, being 95% in patients with stage Ib2-II and 72.9% in stages III-IVa (p = 0.016). According to the histological type, local control was achieved in 94.3% of squamous carcinomas and 79% of the adenocarcinomas (p: ns). It is also important to note how staging with regional lymphadenectomy affected local control; 100% of patients with N0 achieved local control vs. 72.2% of those who were N+ (p: 0.02).
We did not find any differences in local control in patients receiving a HR-CTV dose > 85 Gy (84%) compared to those receiving a lower dose (83%); nor did we find any differences between patients with CTV-HR volume > 30 cc (92% vs. 93%). Perhaps, this is in relation to the low number of local recurrences: 2 patients who did not complete the treatment and only 3 patients who finished all the treatment. There were, however, differences according to the implant used: patients with intracavitary brachytherapy achieved local control of 100% vs. 66% of those receiving interstitial brachytherapy (p = 0.01). The factors correlated with local control in the RetroEMBRACE study7 were: HR-CTV (HR: 1.017 per cm3, p: 0.004), the dose evaluated with D90 (HR: 0.967 per Gy, P: 0.022) and the total treatment time (HR: 1.023 per day, p: 0.004). A dose < 85 Gy in the HR-CTV achieved local control in 94% of the patients with a small HR-CTV (20 cc), 93% with an intermediate CTV and 86% with large CTV (70 cc).
Regional control at 5 years was 93.5%: 92.1% in stages Ib2-II and 85.1% in stage III-IVa (p: 0.024). We found that the location of lymph node involvement by pelvic-paraaortic lymphadenectomy had an important prognostic value. Regional control in N0 patients was 100%, including 87% in patients with positive paraaortic but negative iliac lymph nodes and 71.4% in patients with positive paraaortic and iliac lymph nodes (p:0.007) (Fig. 2). This is likely due to the use of a lymph node boost in residual disease or with a high tumour load defined by the lymphadenectomy. In the review by Smits et al.9 in 2014 comparing the value of lymphadenectomy with PET/CT, 4.15% of the patients with a negative study had positive adenopathies in the lymphadenectomy. Several studies have described the value of lymph node boost in cervical cancer. In 2014 Vargo et al.10 described their results with RT with IMRT extended to paraaortic nodes with a lymph node boost in patients with positive adenopathies, reporting a regional failure at 3 years in 5% and a survival of 73%, with a scarce grade 3 toxicity of 4%.
The 5-year overall survival was 66.6%, and the specific cervical carcinoma survival was 74%. In the univariate analysis the prognostic factors were: stage Ib2-II: 83% vs. III-IVa: 41% (p:0.001), histological type: squamous carcinoma: 78%, adenocarcinoma: 59.7% (p: ns) and also lymph node involvement after lymphadenectomy: N0: 85% vs. Paraaortic but not pelvic: 72%, paraaortic and pelvic: 35% (p = 0.010).
There were no differences between patients receiving 3D EBRT and those receiving IMRT (66.3% vs. 76.5%, respectively). On analysing the patients who received a HR-CTV dose > 85 Gy, the differences in survival were 85.7% vs. 74%, but these values were not significant.
With regard to toxicity, acute grade 2–3 toxicity in our study was rectal in 5.2%, intestinal in 15.7% and vesical in 15.5%. Late grade 2–3 toxicity was presented as rectal in 6%, intestinal in 8.6% and vesical in 15.5%, with no grade 4 toxicity being observed. In our study the use of IMRT reduced rectal toxicity from 15% with 3D EBRT to 10.5% with IMRT. Rectal toxicity was greater with D2cc > 75 Gy and vesical toxicity was greater with D2cc > 85 Gy, although the differences were not significant, likely due to the size of the study population.
The results of the RetroEMBRACE study showed late grade 3–5 morbidity11: 5% vesical and 7% gastrointestinal, being similar to the results in our centre. In the EMBRACE I study grade 2–3 urinary toxicity with incontinence was 16.4%, 16.2% in changes in urinary frequency and 11% with cystitis.12 Ureteral stenosis was observed at 5 years in 3.4%. With regard to rectal toxicity,13 7.7% of the patients presented grade 2–4 toxicity. On analysis of the correlation of toxicity with parameters of dosimetry, the patients with D2cc < 65 Gy had a two-fold lower risk of presenting proctitis, and patients with D2cc > 75 Gy had a 12.5% risk of presenting fistula at 3 years versus 0–2.7% in patients receiving lower doses. None of the patients in our study presented rectal fistula. The correlation between dose and volume has been demonstrated in other studies.14 In a series of the Hospital La Fe, they had similar results in toxicity: with a median follow-up of 19 months (3–35 m). Urinary and gastrointestinal G ≥ 3 toxicities occurred in 4.8% and 2.4% patients, respectively. These data show that IGBT is sure, also with interstitial treatments.15
6. Conclusion
In the present study the results obtained with the use of MRI-GBT in all the women with cervical cancer are comparable to those of the most important multicentre studies reported to date, with scarce, albeit manageable, late toxicity.
The future is leading in the direction of the EMBRACE II study which is aimed at reducing toxicity in women showing good response after radiochemotherapy and intensifying brachytherapy treatment, especially with interstitial brachytherapy, in order to improve local control and survival in patients with advanced tumours and poor initial response.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Tanderup K., Lindegaard J.C., Kirisits C. Image Guided Adaptive Brachytherapy in cervix cancer: a new paradigm changing clinical practice and outcome. Radiother Oncol. 2016;120(3):365–369. doi: 10.1016/j.radonc.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Haie-Meder C., Pötter R., Van Limbergen E., Gynaecological (GYN) GEC-ESTRO Working Group Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74(3):235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Pötter R., Haie-Meder C., Van Limbergen E., GEC ESTRO Working Group Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78(1):67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Pötter R., Dimopoulos J., Georg P. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83(2):148–155. doi: 10.1016/j.radonc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Lindegaard J.C., Fokdal L.U., Nielsen S.K., Juul-Christensen J., Tanderup K. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol. 2013;52(7):1510–1519. doi: 10.3109/0284186X.2013.818253. [DOI] [PubMed] [Google Scholar]
- 6.Mazeron R., Gilmore J., Dumas I. Adaptive 3D image-guided brachytherapy: a strong argument in the debate on systematic radical hysterectomy for locally advanced cervical cancer. Oncologist. 2013;18(4):415–422. doi: 10.1634/theoncologist.2012-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturdza A., Pötter R., Fokdal L.U. Image guided brachytherapy in locally advanced cervical cancer: improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiather Oncol. 2016;120(3):428–433. doi: 10.1016/j.radonc.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Schmid M., Haie-Meder C., Mahanshetty U., Jürgenliemk-Schulz I.M., Segedin B., Hoskin P. Local failures after radiochemotherapy and MR-image-guided brachytherapy in cervical cancer patients. Radiother Oncol. 2017;123:S26. [Google Scholar]
- 9.Smits R.M., Zusterzeel P.L., Bekkers R.L. Pretreatment retroperitoneal para-aortic lymph node staging in advanced cervical cancer: a review. Int J Gynecol Cancer. 2014;24(6):973–983. doi: 10.1097/IGC.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 10.Vargo J.A., Kim H., Choi S. Extended field intensity modulated radiation therapy with concomitant boost for lymph node-positive cervical cancer: analysis of regional control and recurrence patterns in the positron emission tomography/computed tomography era. Int J Radiat Oncol Biol Phys. 2014;90(5):1091–1098. doi: 10.1016/j.ijrobp.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Fokdal L., Sturdza A., Mazeron R. Image guided adaptive brachytherapy with combined intracavitary and interstitial technique improves the therapeutic ratio in locally advanced cervical cancer: analysis from the retroEMBRACE study. Radiother Oncol. 2016;120(3):434–440. doi: 10.1016/j.radonc.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Fokdal L.U., Kirchheiner K., Kibsgaard Jensen N. Physician assessed and patient reported bladder morbidity after RCHT and IGABT for cervical cancer. Radiother Oncol. 2017;123(Suppl. 1):S23–S24. [Google Scholar]
- 13.Mazeron R., Fokdal L.U., Kirchheiner K., on behalf of the EMBRACE collaborative group Dose–volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: Results from the prospective multicenter EMBRACE study. Radiother Oncol. 2016;120(3):412–419. doi: 10.1016/j.radonc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Mazeron R., Castelnau-Marchand P., Dumas I. Impact of treatment time and dose escalation on local control in locally advanced cervical cancer treated by chemoradiation and image-guided pulsed-dose rate adaptive brachytherapy. Radiother Oncol. 2015;114(2):257–263. doi: 10.1016/j.radonc.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 15.Cuervo E., Celada F., Roldán S. Outcome and toxicity using interstitial-MRI Utrecht applicator in cervical brachytherapy. Rep Pract Oncol Radiother. 2013;18:S141–S163. [Google Scholar]