ABSTRACT
Background
DBS in the ventral intermediate nucleus (VIM) of the thalamus has been a revolutionary treatment for patients with essential tremor (ET) by reducing tremor. Unfortunately, some patients develop habituation to DBS and thus experience reduced efficacy and loss of tremor control. There are no standardized methods of addressing habituation to DBS. We propose alternating stimulation patterns as a way to reduce habituation.
Methods
This was a randomized, placebo‐controlled trial for patients with VIM DBS for ET. Patients were randomized to either experimental treatment arm of alternating stimulation patterns on a weekly basis or standard care arm of continuous stimulation settings for 12 weeks. Primary outcome was change in the performance subscale of The Essential Tremor Rating Assessment Scale (TETRAS), which was performed at initial visit and 12‐week follow‐up. Secondary outcome included change in the activities of daily living subscale of TETRAS.
Results
Twenty‐two patients were enrolled in the trial, and 16 were analyzed at follow‐up. Experimental treatment subjects displayed sustained tremor control compared to standard care, as measured by the change in TETRAS performance subscale (–0.6 vs. 6.7 point change, respectively) with a 7.3 difference between the arms (P = 0.006).
Conclusion
Alternating stimulation patterns on a weekly basis for ET patients with VIM DBS reduced habituation in this pilot study. This study suggests that exposure to different stimulation groups may maintain better tremor control compared to constant stimulation parameters.
Keywords: essential tremor, deep brain stimulation, VIM, habituation
Essential tremor (ET) is a fairly common neurological disorder, which can cause disabling intention and postural tremor primarily in the upper extremities, but can also affect head, voice, trunk, and legs.1 Patients with ET can suffer from debilitating tremor that limits function and can affect performance of even simple activities such as eating, drinking, and dressing. There are effective pharmacological treatments for ET, but up to 25% to 55% of patients have medication‐refractory tremor.1 DBS of the ventral intermediate nucleus in the thalamus (VIM) has been used to treat medically refractory ET for over 25 years with several studies showing both safety and efficacy.3, 4, 5
Although tremor control provided by VIM DBS has been shown to be effective initially after implantation, there is growing recognition of waning tremor control over time and may occur in up to 73% of patients with a mean follow‐up period of 56 months.6 This waning of effect of DBS on tremor control is thought to be attributed, at least in part, to development of tolerance or habituation to chronic brain stimulation.6, 7 Waning of tremor control necessitates stimulation parameter adjustments, which typically result in an increased area of tissue activation in order to regain tremor control. This can ultimately lead to side effects, such as dysarthria, ataxia, and paresthesias, which can limit further tremor optimization.8 There is currently no standardized way to approach habituation in VIM DBS; thus, there is a treatment gap in how to properly address tremor control in this group of ET patients.
Adjustable parameters of stimulation include electrode configuration, current amplitude, pulse width, and frequency, which determine the efficacy of the signal. After a patient's DBS is optimally programmed in the clinic, parameters typically remain constant, thus making DBS more susceptible to habituation. Clinicians can program different “groups” or patterns of settings, which can be accessed by the patient outside of a clinic visit, but this is underutilized.9
We propose a novel approach to the treatment paradigm for VIM DBS programming, which currently uses constant parameter settings. This new approach entails giving a patient two different, but still effective, groups for tremor control and alternating between the two groups on a scheduled basis. The hypothesis of the study is that by regularly alternating settings, there will be less habituation to stimulation and thus tremor benefit will be maintained as compared to the current treatment paradigm. Habituation has been shown to occur in as little as 10 weeks,10 thus this study protocol took place over 12 weeks. We hypothesize that tremor control will be better maintained in the alternating arm compared to the control arm.
Patients and Methods
Study Participants
Patients were recruited from Oregon Health and Science University (OHSU) movement disorders and DBS neurosurgical clinic. Patients with a Current Procedural Terminology (CPT) code of tremor who had undergone either unilateral or bilateral VIM DBS placement (Medtronic 3387; Medtronic Inc., Minneapolis, MN) or implantable pulse generator replacement at OHSU from January 2005 to August 2016 were invited to participate. Inclusion criteria included (1) diagnosis of ET by a movement disorders neurologist, (2) history of placement of VIM DBS, (3) initial benefit from VIM DBS as judged by patient or clinician report, (4) willingness to undergo a baseline programming visit and 12‐week follow‐up assessment, and (5) ability to change DBS groups on a weekly basis. Exclusion criteria included (1) VIM DBS placement less than 3 months from entry into study, (2) presence of an atypical tremor disorder including, but not limited to, tremor attributed to multiple sclerosis, medication‐induced tremor, ataxia, Parkinson's disease, or parkinsonian syndrome, (3) DBS placement complicated by infection, hemorrhage, or ischemic stroke, and (4) previous DBS surgery resulting in explantation and reimplantation, previous thalamotomy, or known incorrect or poor lead placement. If a patient had previous unilateral thalamotomy or unilateral poor lead placement, the contralateral limb was not included in the analysis.
Study Design
This was a double‐blinded, placebo‐controlled, randomized trial of 12 weeks’ duration. Patients were randomized in a 1:1 ratio with a block randomization scheme using de‐identified numbers devised by a statistical expert (C.M.) who was not involved with subjects in the study. The experimental treatment arm received weekly alternating stimulation parameters, and the standard care arm received standard, constant stimulation. Subjects and tremor raters were blinded to treatment allocation.
All subjects had current DBS settings evaluated and recorded at initial visit. Medications to treat ET, duration post‐DBS surgery, current DBS adverse effects, duration of ET, and other data were collected. Two different, but equally effective, stimulation parameters (“groups”) were determined for each subject (group A and group B) by two DBS‐trained clinicians (M.S. and S.A.) who were blinded to treatment allocation until after this initial programming was performed. Equally effective stimulation parameters were defined as groups that provided that same level of tremor control as judged by subject and clinician. Groups differed by at least two of the following parameters: electrode configuration, voltage, milliamps, frequency, or pulse width. Subjects in the experimental treatment arm received a group A and group B, but standard care subjects received only group A, which was programmed in duplicate in order to maintain blinding of subjects who were not aware of their allocation of treatment. All subjects were trained to use the patient programmer to change between group A and group B and were instructed to do this at a prescribed time once a week. Subjects were taught to change between the two groups with the stimulator turned off to maintain blind.
Patients alternated between the two programmed groups every week and returned for reassessment at the end of 12 weeks. Patients were instructed to shut the DBS off overnight, as is the standard of care. Adherence was assessed by diary and patient report, because the technology in the DBS system did not reliably store group usage in a majority of the patients. Subjects were not allowed to alter their ET medications or their DBS parameters (other than the above‐described weekly group switching) during the 12‐week study.
Outcome Assessments
The primary outcome measure was change in The Essential Tremor Rating Assessment Scale (TETRAS) performance subscale from initial visit to 12‐week follow‐up visit. TETRAS was designed for assessing clinical assessment of ET in clinical trials, has excellent validity, has sensitivity to detect change in ET comparable to wearable sensors, and is better suited than the Fahn–Tolosa–Marín scale to assess patients with more‐severe tremor.11 TETRAS was scored by a blinded movement disorder specialist not aware of the treatment allocation. DBS settings for all subjects during the TETRAS assessment was done in group A to maintain consistency between initial and 12‐week follow‐up visit. The secondary outcome measure was change in TETRAS activities of daily living (ADLs) subscale from initial visit to 12‐week follow‐up visit.
The TETRAS performance subscale includes some items that score both the left and right extremities independently; thus, when subjects were included that had unilateral DBS, only one side was assessed and counted toward the total score. In order to account for this difference in baseline reporting, scores were also converted to a percentage at the initial visit. The primary outcome was change in total score for each subject; thus, this difference did not affect the primary outcome results.
Standard Protocol Approval and Patient Consent
This study was registered on http://ClinicalTrials.gov (NCT02947841). The study protocol, informed consent, and other study documents were reviewed and approved by OHSU's institutional review board. All patients provided written informed consent.
Statistical Analysis
We calculated sample size based on the primary outcome of interest, change in TETRAS performance subscale at 12 weeks based on a number of previous studies. The expected 12‐week change in tremor scores for control subjects is estimated to be at least a 25% increase from baseline according to 10‐week longitudinal data on videotaped clinical tremor rating scale from a cohort of ET patients similar to the current study's standard care group, which was considered clinically impactful.10 Variance for subjects’ performance on tremor rating scales while on DBS is expected to be constant across time, with a calculated minimum standard deviation of change of 12.56 in the treatment arm.12 The improved precision of TETRAS compared to the Fahn–Tolosa–Marin scale also allows for better resolution to detect more‐subtle changes in tremor assessment than the short‐term changes in TETRAS‐measured tremor previously observed in the literature (Δ∼8.2).12 Thus, a 6‐point difference in the change of TETRAS score after 12 weeks was considered both a clinically important and detectable attenuation between a treatment and standard care group and, based on the above parameters, would correspond to a standardized effect size of Cohen's d = 1.2. While allowing for a 20% total dropout rate, a significant difference at α = 0.05 (two‐tailed) with 80% power, a significant difference in tremor assessment would be detectable between groups with 26 subjects enrolled.
Primary analysis of primary and secondary outcomes was performed by comparing mean group change between the two cohorts using Welch's two‐sample t test in order to detect attenuated waning across 12 weeks while evaluating underlying assumptions of distribution normality using standard procedures, including Shapiro–Wilks tests, Anderson–Darling tests, and graphical assessment of residual errors. Secondary analyses of study outcomes used analysis of covariance with 12‐week change in TETRAS scores as the response variable, group assignment as the dependent variable of interest, and baseline TETRAS scores as a covariate. Additional covariates considered for the secondary model included age, sex, disease duration, duration post‐DBS placement, and use of ET medications. These additional covariates were also investigated individually stepwise within the treatment models to avoid overfitting given the relatively small number of subjects. When considering unequal variance between strata, a weighted least‐squares design was evaluated using sample weights based on group variance of residual errors from the original analysis of covariance model. With only a single time point beyond baseline, the final completers analysis cohort included only those that followed up at 12 weeks and were without significant protocol deviations. Exclusion of participants from the analysis was done without considering the participant's treatment assignment.
Results
Study Participants
Forty‐three subjects were assessed for eligibility; 22 patients were enrolled and equally randomized into standard care or experimental treatment arm. Patients were enrolled and completed follow‐up from September 2016 through May 2017. One subject from each arm was lost to follow‐up (because of inability to make follow‐up appointment) and 1 subject from each arm discontinued the study because of side effects encountered as a result of DBS programming changes made at the initial visit. The patient in the experimental treatment arm developed imbalance and leg pain related to DBS settings at week 3. The patient in the standard care arm developed nausea related to DBS settings in the first week. Two subjects in the experimental treatment arm were excluded from the analysis because of significant protocol deviations. One subject had two different raters performing TETRAS, which significantly altered this subject's results. The other subject unblinded themselves shortly into the study. Because of technical limitations of the DBS patient programmers, it is not possible to mask the voltage setting on the programmer and still give both an A group and B group; thus, that portion of the patient programmer screen was obscured by medical tape. This particular participant removed the tape and was able to see changes in voltage settings when groups were switched at the end of every week. Thus, 16 subjects were included in the analysis (9 subjects in the standard care group and 7 in the experimental treatment group; Fig. 1).
Figure 1.
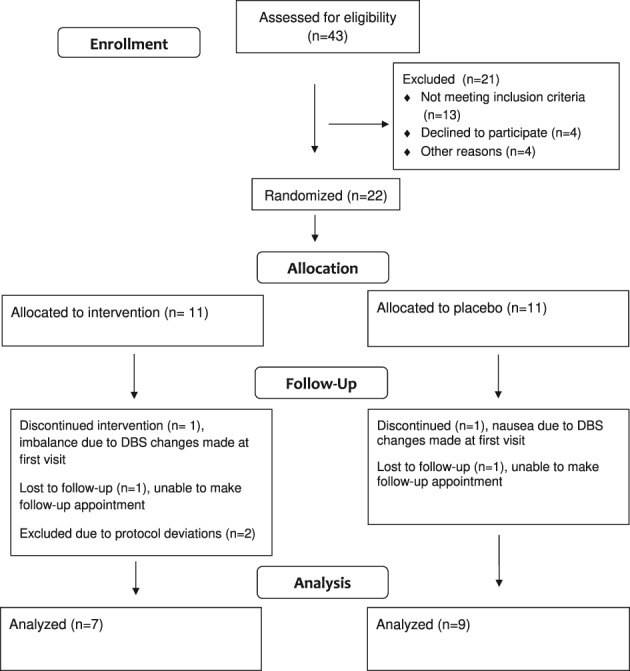
CONSORT diagram of patient allocation and follow‐up. Abbreviation: CONSORT = Consolidated Standards of Reporting Trials.
In the 16 subjects analyzed, 7 were female and average age was 71.1. Mean duration of ET was 32.5 years and average months post‐DBS placement was 61.8. Average baseline TETRAS ADL's subscales were 21 and 27.6, in the standard care and experimental treatment arms, respectively. Average baseline TETRAS performance subscale (measured while in group A) were also nonsignificantly different (20.4 and 27.2), in the standard care and in experimental treatment arms, respectively. TETRAS performance subscales were converted to a percentage to account for patients who had unilateral DBS or previous thalamotomy, as previously mentioned (Table 1). The supplemental table contains demographics for all subjects enrolled into the study (Supplemental Table S1).
Table 1.
Subject demographics
| All | Standard Care | Experimental Treatment | |
|---|---|---|---|
| Age, years | 71.1 ± 9.7 | 69.7 ± 9.2 | 73.0 ± 10.8 |
| Sex (F) | 7 / 16 | 3 / 9 | 4 / 7 |
| Tremor duration (years) | 32.5 ± 13.5 | 34.1 ± 13.5 | 30.4 ± 14.2 |
| Months since DBS placed | 61.8 ± 45.8 | 61.6 ± 37.9 | 62.0 ± 57.7 |
| Tremor meds | 6 / 16 | 4 / 9 | 2 / 7 |
| ET family history | 11 / 16 | 7 / 9 | 4 / 7 |
| EtOH response | 12 / 16 | 8 / 9 | 4 / 7 |
| Stimulation AEs | 8 / 16 | 5 / 9 | 3 / 7 |
| TETRAS: ADL | 23.9 ± 11.7 | 21.0 ± 13.4 | 27.6 ± 8.6 |
| TETRAS: Performance | 23.4 ± 9.7 | 20.4 ± 6.6 | 27.2 ± 12.1 |
| TETRAS: Performance % | 38.9 ± 15.3 | 33.9 ± 13.6 | 45.2 ± 15.9 |
Baseline demographics of subjects analyzed.
Abbreviations: EtOH, alcohol; AEs, adverse events.
Outcomes
Primary Outcome: TETRAS Performance Subscale
The primary outcome was change in TETRAS performance subscale over 12 weeks. The standard care group had a mean change in TETRAS of 6.7 ± 5.9 points compared to the experimental treatment group with a mean decrease of 0.6 ± 1.5 points. This was a difference between the arms of 7.3 points (t = 3.51; P = 0.006). Five subjects in the experimental treatment arm had stable or improved scores compared to only 1 subject in the standard care arm (Fig. 2). The secondary analysis of covariance model on 12‐week change in TETRAS performance found a significant difference between treatment groups (t = 2.78; P = 0.016), even while correcting for baseline score (t = 0.07; P = 0.95). During evaluation, the residual standard deviation was found to be 3.75 times higher in the placebo group (σresid = 5.93) compared to the treatment arm (σresid = 1.58). When using a weighted least‐squares design correcting for this heteroscedasticity, a significant difference between groups was still observed (t = 3.53; P = 0.004). Covariates assessed included age, sex, disease duration, duration post‐DBS, and use of ET medications, none of which were found to be associated with the change in outcomes. Percentage of total TETRAS performance subscales were also evaluated: The standard care arm had a mean increase of 10 ± 9%, and the experimental treatment arm had a decrease of 1 ± 3% in scores; this difference was also significant for the primary analysis (t = 3.55; P = 0.005), secondary analysis of covariance correcting for baseline (t = 2.70; P = 0.018), and weighted least‐squares assessment also accounting for unequal variance (t = 3.53; P = 0.004).
Figure 2.
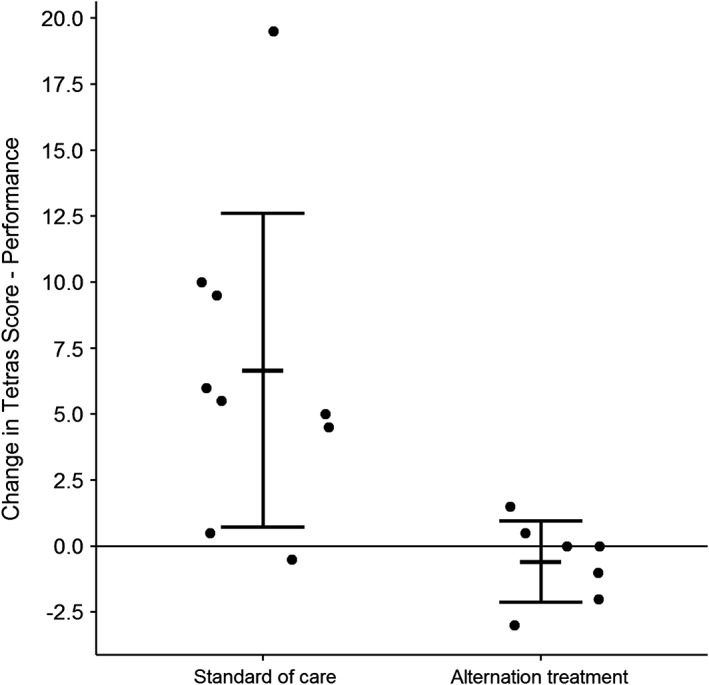
Change in TETRAS performance subscale over 12 weeks. Standard care arm had an increase by 6.7 points and experimental treatment arm decreased by 0.6 points, (t = 3.51; P = 0.006). N = 9 in standard care and n = 7 in experimental treatment.
Subanalyses of Primary Outcome: Sensitivity Assessment
Sensitivity assessment was performed on the final data set to assess for presence of outliers and leverage points that may be exerting undue influence on the results. Specifically, 1 subject in the standard care group was observed to be a significant outlier with an extensively large residual (rstd = 4.48; P = 0.009). This standard care subject had an observed increase in TETRAS performance subscale of 19.5 points, and this severe worsening was found to be placing extensive leverage on the model results, exaggerating the mean change in the experimental treatment group (standard deviation difference of β = –1.12; Cook's d = 0.59). Although there were no outstanding circumstances for this patient in the standard care arm to account for an increase in 19.5 points, this subject was removed to see whether the difference in TETRAS scores between the two groups was still significant. Removal of this subject resulted in a significantly improved model (ΔAIC, 100.1–80.6; ΔR2, 0.41–0.52). The mean increase in TETRAS performance subscale for the standard care arm was then observed to be 5.1 ± 3.7 points, which was still found to be significantly different from the mean change in the experimental treatment group in the primary two‐sample t test (t = 3.91; P = 0.003) and secondary analysis of covariance correcting for baseline (t = 3.25; P = 0.007). Removing this subject also greatly attenuated model heteroscedasticity by reducing the residual variance of the placebo arm (σresid = 3.67). Percentage of total TETRAS performance subscales showed similar results; mean increase in standard care arm was 8 ± 6%, which was still significantly different from the experimental treatment arm (t = 3.92; P = 0.002).
Secondary Outcome: TETRAS ADL Subscale
The change in TETRAS ADLS subscale over 12 weeks was not statistically different between the two groups. The standard care group had a mean increase of 5.7 ± 6.9 points compared to the experimental treatment group, with a mean increase of 1.7 ± 5.6 points (t = 1.27; P = 0.23). Sensitivity evaluation did not identify any significant outliers or leverage points.
Safety and Adverse Events
Thirteen of 22 subjects or 59.1% reported pre‐existing stimulation‐induced side effects preceding any changes in DBS programming at the initial visit. Most common effects were dysarthria and ataxia (Table 2). At the follow‐up visit, 10 of 18 subjects or 55.6% reported stimulation‐induced side effects. There was no difference between the experimental treatment or standard care arms. Serious adverse events occurred in 4 of the 22 subjects and resulted in 2 of the subjects discontinuing the study as previously discussed (Table 3).
Table 2.
Stimulation‐induced side effects (SEs)
| Initial Visit | 12‐Week Follow‐up | |||
|---|---|---|---|---|
| Standard Care (n = 11) | Experimental Treatment (n = 11) | Standard Care (n = 9) | Experimental Treatment (n = 9) | |
| Dysarthria | 4 | 7 | 4 | 5 |
| Ataxia | 3 | 5 | 3 | 4 |
| Dysphagia | 2 | 0 | 0 | 0 |
| Tingling in tongue | 1 | 0 | 1 | 0 |
| Transient dizziness | 1 | 0 | 1 | 0 |
| No. of patients with stim SEs | 6 | 7 | 5 | 5 |
| Percentage reporting stim SEs | 54.5 | 63.6 | 55.6 | 55.6 |
Table 3.
Adverse events
| Standard Care (n = 11) | Experimental Treatment (n = 11) | |
|---|---|---|
| Any adverse event | 3/11 (27.3%) | 1/11 (9.1%) |
| Fall | 2 (18.2%) | 0 (0%) |
| Nausea | 1 (9.1%) | 0 (0%) |
| Imbalance | 0 (0%) | 1 (9.1%) |
One subject, in the standard care arm, suffered a ground‐level fall shortly after the initial study visit, resulting in a fracture of the humerus and returned to the clinic for DBS adjustments less than a week after entering the study. This subject was able to continue with alternating settings, and despite this protocol deviation, his or her data were included in the final analysis because he or she had 12 weeks of exposure to alternating groups. Another subject, also in the standard care arm, had a fall near the end of the 12‐week course, which was not thought to be related to DBS changes, and was placed in a nursing facility. He or she was not able to follow‐up at 12 weeks.
Discussion
There is a treatment gap in how to properly address habituation and the waning of effect in VIM DBS for ET. Shutting off stimulation overnight does not seem to significantly reduce habituation given that 90% of patients in one large series developed significant habituation despite utilizing this measure.13 Others have suggested DBS holidays14 and on‐demand stimulation13, 15 in small patient series, but adherence is difficult for most patients and thus impractical for widespread application. On the horizon are closed‐loop or adaptive stimulation systems that could deliver stimulation on‐demand using feedback from physiological measures, but these are still in development and do not yet have widespread clinical use.16 There are also new electrode options with a larger number of contacts and ability to change the direction of stimulation, but these are not options for ET patients who have already been implanted and there is no evidence that they will prevent habituation.17
This study took a novel approach at addressing habituation to stimulation by exposing the brain to different stimulation patterns on a rotating schedule. Our hypothesis was that these patients would maintain benefit over time compared to those with constant settings. Our results showed that the standard care arm had worsening on the TETRAS performance subscale by an average of 7.3 points compared to the experimental treatment arm over the 12 weeks of the study. Even the sensitivity analysis showed a significant 5.7‐point difference between groups. This is not only statistically significant, but arguably also a significant clinical difference.18 Unfortunately, the tremor control observed in the experimental treatment arm did not translate into patient‐reported ADL functioning.
The significant worsening in the TETRAS performance subscale scores in the standard care arm is likely explained by habituation to stimulation, thus resulting in the loss of tremor control over the 12‐week period. This phenomenon has been described previously and can occur in as little as 10 weeks.10 It is likely that those with poor tremor control were motivated to participate in the study, possibly leading to a volunteer bias, but this would have been true for all subjects and thus does not fully account for the significant worsening observed in the standard care arm.
Alternating stimulation may allow for better preservation of tremor control over time and avoidance of stimulation side effects. Common stimulation side effects include dysarthria and ataxia and have been reported to occur in up to 69% of patients followed for 10 years or longer.8 In our study, 59% reported stimulation side effects at the initial visit and 55% at 12‐week follow‐up, with the most common being dysarthria and ataxia. Rates were similar in both the experimental treatment and standard care arms, thus alternating stimulation does not seem to reduce or alter these effects. One could argue that early application of this technique could be used shortly after DBS implantation in order to avoid these negative effects by preventing habituation from occurring. This study included subjects that had DBS implanted, on average, 5.1 years earlier; thus, one area of future study could include applying this technique on newly implanted patients.
Limitations of the study included inability to monitor compliance for the majority of patients. Most patients did fill out and return diaries tracking compliance with weekly group changes. Patients reported that once they were trained how to switch groups, it was easily performed. Half of all subjects who followed up at 12 weeks reported they would continue to alternate stimulation in the future: 4 in the treatment arm and 5 in the standard care arm. Subjects had difficulty predicting which arm they were assigned because only half of subjects (3 of 7 subjects in the experimental treatment arm and 5 of 9 in the standard care arm) identified correctly which arm they had been assigned, thus making it a blinded study.
Conclusions
Alternating stimulation on a weekly basis for ET patients with VIM DBS may be a way to address habituation that can occur in this population. This study suggests that exposure to different stimulation groups may maintain better tremor control compared to current treatment of constant stimulation parameters. This technique is easily utilized with the current technology and is well tolerated by patients. Future studies, with longer‐term outcomes, are needed before a wider application of this method can be routinely recommended.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
M.S.: 1A, 1B, 1C, 2A, 2B, 3A
A.H.: 1C 3B
J.Q.: 2C 3B
C.M.: 2A, 2B, 2C, 3B
M.B.: 1C, 3B
S.A.: 1A, 1B, 1C, 3B
Disclosures
Ethical Compliance Statement: This study was registered on http://ClinicalTrials.gov (NCT02947841). The study protocol, informed consent, and other study documents were reviewed and approved by OHSU's institutional review board. All patients provided written informed consent. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: M.B. received funding from Medtronic for an unrelated research project. M.S. received funding for this project from N. L. Tartar Trust Research Fellowship, Oregon Health and Sciences University, Department of Medicine.
Financial Disclosures for previous 12 months: M.S. is employed by the University of Nebraska Medical Center and has no other financial disclosures. A.H. is employed by the VA Portland Health Care System and OHSU and has funding from The Pacific Northwest Udall Center, Michael J. Fox Foundation, NeuroNEXT/Azevan Pharmaceuticals, Prothena Biosciences, and AbbVie. J.Q. is employed by the VA Portland Health Care System and OHSU and has funding from the NIH/NINDS and NIH/NCCIH. C.M. is currently employed by the University of Alabama at Birmingham and formally by OHSU and has funding from the NIH/NINDS, NIH/NCCIH, Allen Award, and the Department of Defense. M.B. is employed by OHSU and has funding from Medtronic and Roche. He has served on advisory board/honoraria for Abbot and Acadia. S.A. is employed by OHSU and has no other financial disclosures.
Supporting information
Supplemental Table S1. Patient demographics of all subjects enrolled
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Louis ED. Essential tremor. Lancet Neurol. 2005;4:100–110. [DOI] [PubMed] [Google Scholar]
- 2. Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. [DOI] [PubMed] [Google Scholar]
- 3. Kumar R, Lozano AM, Sime E, Lang AE. Long‐term follow‐up of thalamic deep brain stimulation for essential and parkinsonian tremor. Neurology. 2003;61:1601–1604. [DOI] [PubMed] [Google Scholar]
- 4. Rehncrona S, Johnels B, Widner H, Törnqvist AL, Hariz M, Sydow O. Long‐term efficacy of thalamic deep brain stimulation for tremor: double‐blind assessments. Mov Disord. 2003;18:163–170. [DOI] [PubMed] [Google Scholar]
- 5. Zhang K, Bhatia S, Oh MY, Cohen D, Angle C, Whiting D. Long‐term results of thalamic deep brain stimulation for essential tremor. J Neurosurg. 2010;112:1271–1276. [DOI] [PubMed] [Google Scholar]
- 6. Shih LC, LaFaver K, Lim C, Papavassiliou E, Tarsy D. Loss of benefit in VIM thalamic deep brain stimulation (DBS) for essential tremor(ET): how prevalent is it? Parkinsonism Relat Disord. 2013;19:676–679. [DOI] [PubMed] [Google Scholar]
- 7. Pilitsis JG, Metman LV, Toleikis JR, Hughes LE, Sani SB, Bakay RA. Factors involved in long‐term efficacy of deep brain stimulation of the thalamus for essential tremor. J Neurosurg. 2008;109:640–646. [DOI] [PubMed] [Google Scholar]
- 8. Baizabal‐Carvallo JF, Kagnoff MN, Jimenez‐Shahed J, Fekete R, Jankovic J. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J Neurol Neurosurg Psychiatry. 2014;85:567–572. [DOI] [PubMed] [Google Scholar]
- 9. Barbe MT, Pochmann J, Lewis CJ, et al. Utilization of predefined stimulation groups by essential tremor patients treated with VIM‐DBS. Parkinsonism Relat Disord. 2014;20:10–13. [DOI] [PubMed] [Google Scholar]
- 10. Barbe MT, Liebhart L, Runge M, et al. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: habituation of tremor suppression. J Neurol. 2011;258:434–439. [DOI] [PubMed] [Google Scholar]
- 11. Elble RJ. The Essential Tremor Rating Assessment Scale. J Neurol Neuromed. 2016;1:34–38. http://www.jneurology.com. Accessed May 21, 2018. [Google Scholar]
- 12. Limousin P, Speelman JD, Gielen F, Janssens M. Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry. 1999;66:289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kronenbuerger M, Fromm C, Block F, et al. On‐demand deep brain stimulation for essential tremor: a report on four cases. Mov Disord. 2006;21:401–405. [DOI] [PubMed] [Google Scholar]
- 14. Garcia Ruiz P, Muñiz de Igneson J, Lopez Ferro O, Martin C, Magariños Ascone C. Deep brain stimulation holidays in essential tremor. J Neurol. 2001;248:725–726. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto T, Katayama Y, Ushiba J, et al. On‐demand control system for deep brain stimulation for treatment of intention tremor. Neuromodulation Technol Neural Interface. 2013;16:230–235. [DOI] [PubMed] [Google Scholar]
- 16. Picillo M, Fasano A. Recent advances in essential tremor: surgical treatment. Parkinsonism Relat Disord. 2016;22(Suppl 1):S171–S175. [DOI] [PubMed] [Google Scholar]
- 17. Fasano A, Lozano AM. Deep brain stimulation for movement disorders. Curr Opin Neurol. 2015;28:423–436. [DOI] [PubMed] [Google Scholar]
- 18. Voller B, Lines E, McCrossin G, et al. Alcohol challenge and sensitivity to change of the Essential Tremor Rating Assessment Scale. Mov Disord. 2014;29:555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Patient demographics of all subjects enrolled


