ABSTRACT
Introduction
Neurogenic orthostatic hypotension (nOH) is associated with neurodegenerative conditions, may cause symptoms of end‐organ hypoperfusion, increases fall risk, and can negatively impact quality of life. Droxidopa is approved for the treatment of symptomatic nOH in adults. As the largest subpopulation of patients with nOH has a diagnosis of Parkinson disease (PD), the efficacy and tolerability of droxidopa in patients with PD and nOH were examined using integrated clinical trial data.
Methods
Post hoc analyses included data from the phase 3, randomized, placebo‐controlled clinical trials of droxidopa (two short‐term [1−2 weeks] trials and one medium‐term [8−10 weeks] trial) in the subset of participants with PD and symptomatic nOH. Efficacy was assessed using standing blood pressure (BP) measurements and the Orthostatic Hypotension Questionnaire (OHQ), a patient‐reported evaluation of nOH symptoms (Orthostatic Hypotension Symptom Assessment [OHSA]), and their impact (Orthostatic Hypotension Daily Activity Scale [OHDAS]).
Results
The analysis included 307 patients with PD (droxidopa, n = 150; placebo, n = 157). Compared with placebo, droxidopa significantly improved the OHQ composite score (P = 0.014), the OHSA composite score (P = 0.022), and the OHDAS composite score (P = 0.029) from baseline to end of study/week one. We found significant increases in standing mean systolic/diastolic BP for droxidopa versus placebo (P = 0.003/0.002). Adverse event (AE) rates were qualitatively similar between groups; the most frequently reported AEs in the droxidopa groups included headache, dizziness, nausea, and hypertension.
Conclusions
These post hoc analyses suggest that droxidopa provides meaningful clinical benefits and is well tolerated in the treatment of symptomatic nOH in patients with PD.
Keywords: droxidopa, norepinephrine, orthostatic hypotension, Parkinson disease, treatment
Introduction
Neurogenic orthostatic hypotension (nOH) is associated with a variety of neurodegenerative conditions, including Parkinson disease (PD), multiple system atrophy (MSA), and pure autonomic failure.1 It is characterized by a reduction of ≥ 20 mmHg in systolic blood pressure (BP) or ≥ 10 mmHg in diastolic BP, typically within three minutes of standing. It is the result of an inadequate sympathetic nervous system response to a gravitational challenge when moving from a lying or sitting position to a standing position.2 As a consequence of the drop in BP and resultant insufficient perfusion of the brain, patients commonly experience the cardinal symptoms of nOH, including dizziness, lightheadedness, presyncope, or syncope.3, 4, 5 nOH increases the risk of falls and injuries from falls,6 and may cause patients to limit their daily activities7 because of fear of falling.8 It has been estimated that 30%9 to 58%10 of patients with PD have a BP decrease on standing that meets the consensus definition of nOH2; approximately 20% of patients experience symptoms as a result of these hemodynamic changes.11
Droxidopa is approved by the US Food and Drug Administration for the treatment of orthostatic dizziness, lightheadedness, or the “feeling like you are about to black out” in adults with symptomatic nOH resulting from primary autonomic failure (i.e., PD, MSA, and pure autonomic failure), dopamine beta‐hydroxylase deficiency, or nondiabetic autonomic neuropathy. Approval was based on the results of three phase 3 randomized, controlled, double‐blind clinical trials.12, 13, 14, 15, 16 nOH is associated with a common set of symptoms regardless of the underlying diagnosis.5 Based on epidemiologic data,11, 17, 18 the largest subpopulation of patients with nOH has a diagnosis of PD. Thus, examination of the efficacy and tolerability of droxidopa exclusively in patients with PD may help further guide clinical practice.
Changes in dizziness/lightheadedness symptoms for droxidopa versus placebo in the subgroup of patients with PD were previously examined as part of integrated analyses in patients with nOH associated with various autonomic failure conditions (i.e., PD, MSA, or pure autonomic failure, dopamine beta‐hydroxylase deficiency, or nondiabetic autonomic neuropathy).19 However, because of the incidence of nOH in patients with PD, greater detail on the effects of droxidopa (i.e., additional efficacy outcomes and safety profile) in this population is of interest. Herein, we report the results of post hoc analyses on the pooled PD patient population from the initial phase 3 clinical trials to provide more extensive information on the use of droxidopa for the treatment of nOH in patients with PD.
Methods
We conducted a post hoc analysis using three previously described phase 3 clinical trials of droxidopa: Study NOH301 (NCT00782340)16 and Study NOH302 (NCT00633880),13 which were short‐term (1−2 weeks) trials, and Study NOH306 (NCT01176240),14, 15 which was a medium‐term (8−10 weeks) trial.
Study Patients
In all three clinical trials, patients eligible for enrollment were ≥ 18 years old with PD and had symptomatic nOH, defined as a documented decrease of ≥ 20 mmHg in systolic BP or ≥ 10 mmHg in diastolic BP within three minutes of standing. In Study NOH306, patients also were required to have a composite score of ≥ 3 on the Orthostatic Hypotension Questionnaire (OHQ)20 and an investigator‐rated score of ≥ 3 on the Clinical Global Impression‐Severity scale, indicating at least a moderate severity of symptoms. Key exclusion criteria included current use of antihypertensive agents other than short‐acting antihypertensive medications at nighttime; vasoconstrictive agents; preexisting sustained hypertension (i.e., ≥ 180/110 mmHg); current use of norepinephrine reuptake inhibitors (NRIs), including tricyclic antidepressants (NOH301 and NOH302 only); significant cardiac arrhythmia or a history of significant cardiac, hepatic, or renal disease; diabetes (NOH301 and NOH302 only); or diabetic neuropathy (NOH306 only).
Study Designs
The designs for each of the studies included in this post hoc analysis have previously been reported.13, 14, 15, 16 The key attributes of each study are briefly described below.
In Study NOH301, patients who met response criteria during an open‐label dose‐optimization period (≤ 14 days) were randomized to receive double‐blind droxidopa or matched placebo at the individually optimized dosage after a seven‐day washout period. In Study NOH302, patients who met response criteria during an open‐label dose‐optimization period (≤ 14 days) continued open‐label droxidopa for seven days and then were randomized to receive double‐blind droxidopa or matching placebo for 14 days. Response criteria for the short‐term studies included a change of ≥ 1 unit on Item 1 of the Orthostatic Hypotension Symptom Assessment (OHSA; dizziness/lightheadedness) and an improvement of ≥ 10 mmHg in systolic BP after three minutes of standing. In Study NOH306, patients were randomized to double‐blind titration with droxidopa or placebo (≤ 14 days), followed by an eight‐week treatment period.
In each of the studies, patients randomized to droxidopa received a dose (100–600 mg 3 times daily [TID]) at approximately four‐hour intervals during the day (i.e., 8 am, noon, and 4 pm) that had been individually optimized during the titration period.13, 14, 15, 16
Symptomatic Efficacy Assessments
In all three clinical trials, key efficacy assessments were based on the OHQ, which consists of two parts, the OHSA and the Orthostatic Hypotension Daily Activity Scale (OHDAS).20 The OHSA assesses six items: (1) dizziness, lightheadedness, feeling faint, or feeling like you might black out; (2) problems with vision (e.g., blurring, seeing spots, tunnel vision); (3) generalized weakness; (4) fatigue; (5) trouble concentrating; and (6) head/neck discomfort.20 The OHDAS assesses four items for the ability to conduct activities that require: (1) standing for a short time; (2) standing for a long time; (3) walking for a short time; and (4) walking for a long time.20 Each OHQ item is rated by the patient on an 11‐point scale, where zero = none or no interference and 10 = the worst possible or complete interference.
Hemodynamic Efficacy Assessments
Patients underwent an orthostatic standing test at all scheduled visits in each of the clinical trials to obtain measurements of BP at the end of a 10‐minute semi‐supine period (head and torso elevated approximately 30 degrees from horizontal), and after three minutes of standing (consistent with the orthostatic challenge method suggested to most closely replicate a real‐life scenario21).
Safety/Tolerability Assessments
In each of the clinical trials, safety parameters included treatment‐emergent adverse events (TEAEs), serious adverse events (AEs), AEs leading to study discontinuation, and vital signs.
Statistical Analyses
The OHQ composite score was the mean of the OHSA composite score, defined as the mean symptom rating score for items one to six with a score of ≥ 1 at baseline, and the OHDAS composite score, defined as the mean of OHDAS items one to four, excluding those with a value “cannot do for other reasons.” Missing data were imputed based on the last observation carried forward. Mean changes from baseline to end of study/week one in the droxidopa and placebo groups and mean changes in BP were assessed using Wilcoxon rank‐sum tests. Safety outcomes were summarized by system organ class and preferred term using descriptive statistics.
Ethics and Good Clinical Practice
The studies were approved by centralized or local institutional review boards, conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent before enrollment and initiation of study procedures.
Results
Study Participants
In Study NOH301, 111 patients with PD were enrolled in the open‐label titration phase of the study. Of those patients, 66 (59.5%) were classified as treatment responders and were subsequently enrolled in the randomization phase of the study. In Study NOH302, 82 patients with PD were enrolled in the open‐label titration phase of the study. Of those patients, 44 (53.7%) were classified as treatment responders and were subsequently enrolled in the randomization phase of the study. In Study NOH306, which enrolled only patients with PD, patients were randomized to either droxidopa or placebo. A total of 332 patients with PD were randomized in the original studies to receive double‐blind treatment with droxidopa (n = 171) or placebo (n = 162). However, 25 patients discontinued the study before the first efficacy measurements and therefore were included in the safety data set but not the efficacy analyses. Three‐hundred and seven patients with PD were included in the current efficacy analyses of droxidopa (n = 150) compared with placebo (n = 157; Table 1).
Table 1.
Baseline characteristics
| Variable | Droxidopa n=150 | Placebo n=157 |
|---|---|---|
| Men, n (%) | 96 (64.0) | 108 (68.8) |
| White race, n (%) | 146 (97.3) | 150 (95.5) |
| Mean (SD) age, y | 70.9 (8.8) | 71.0 (9.5) |
| Concomitant DDCI use, n (%) | 127 (84.7) | 146 (93.0) |
| Mean (SEM) OHQ composite scorea | 5.8 (0.1) | 5.7 (0.1) |
| Mean (SEM) OHSA scoresa | ||
| Composite score | 5.5 (0.1) | 5.4 (0.1) |
| Dizziness/lightheadedness | 5.6 (0.2) | 5.3 (0.2) |
| Visual disturbances | 4.2 (0.2) | 3.4 (0.2) |
| Weakness | 5.7 (0.2) | 5.3 (0.2) |
| Fatigue | 5.8 (0.2) | 5.8 (0.2) |
| Trouble concentrating | 4.7 (0.2) | 4.8 (0.2) |
| Head/neck discomfort | 3.7 (0.2) | 4.3 (0.3) |
| Mean (SEM) OHDAS scoresb | ||
| Composite score | 6.1 (0.2) | 6.0 (0.2) |
| Standing a short time | 5.0 (0.2) | 5.2 (0.2) |
| Standing a long time | 6.8 (0.2) | 6.8 (0.2) |
| Walking a short time | 4.7 (0.2) | 4.5 (0.2) |
| Walking a long time | 6.6 (0.2) | 6.5 (0.3) |
| Mean (SEM) systolic BP, mmHgc | 98.8 (1.7) | 100.0 (1.6) |
| Mean (SEM) diastolic BP, mmHgc | 86.2 (2.3) | 89.3 (2.1) |
| Hoehn and Yahr rating, n (%)d | ||
| Asymptomatic (0) | 20 (19.4) | 8 (8.8) |
| Unilateral involvement only (1) | 5 (4.8) | 6 (6.6) |
| Bilateral involvement (2) | 28 (27.2) | 46 (50.5) |
| Mild to moderate involvement (3) | 38 (36.9) | 26 (28.6) |
| Severe disability (4) | 12 (11.6) | 3 (3.3) |
| Wheelchair‐bound to bedridden (5) | − | 2 (2.2) |
Abbreviations: BP, blood pressure; DDCI, dopa decarboxylase inhibitor; OHDAS, Orthostatic Hypotension Daily Activity Scale; OHQ, Orthostatic Hypotension Questionnaire; OHSA, Orthostatic Hypotension Symptom Assessment.
n=156 for placebo.
n=144–150 for droxidopa; n=151–155 for placebo.
n=149 for droxidopa.
Data only collected in one study [Study NOH306]; n=91 for droxidopa; n=103 for placebo. Hoehn and Yahr data collected in “on” state relative to anti‐Parkinson therapy.
Demographic and Baseline Characteristics
A majority of patients were men (66%) and white (96%), and the mean age of patients was approximately 71 years. Concomitant use of dopa decarboxylase inhibitors (DDCIs) at baseline was reported by similar percentages of patients randomized to droxidopa (85%) or placebo (93%). There were no meaningful differences in baseline OHQ composite and item scores or BP measurements between patients randomized to droxidopa and those randomized to placebo (Table 1).
Dosing Three Times Daily
During double‐blind treatment, the doses of droxidopa that had been individually optimized during the double‐blind titration period ranged from 100 to 600 mg TID (Supporting Fig. 1). The largest percentage of patients (35%) received the maximally allowed droxidopa dose of 600 mg TID.
Symptomatic and Hemodynamic Efficacy
Droxidopa treatment was associated with a significant improvement in OHQ composite score compared with placebo (mean [SEM], –2.56 [0.18] units vs –1.91 [0.19] units; P = 0.014) from baseline to end of study/week one (Fig. 1A). Droxidopa treatment was associated with a significant improvement in symptom severity based on the OHSA composite score compared with placebo (mean [SEM], –2.50 [0.18] units vs –1.87 [0.20] units; P = 0.022) and in three of the six individual items. Notably, dizziness/lightheadedness, the most common symptom of nOH, was significantly improved (P = 0.001) in patients receiving droxidopa compared with those receiving placebo. Problems with vision (P = 0.022) and generalized weakness (P = 0.002) were also significantly improved in patients receiving droxidopa compared with those receiving placebo. Patients receiving droxidopa also exhibited numerical improvements in two other items (i.e., trouble concentrating and fatigue; Fig. 1B).
Figure 1.
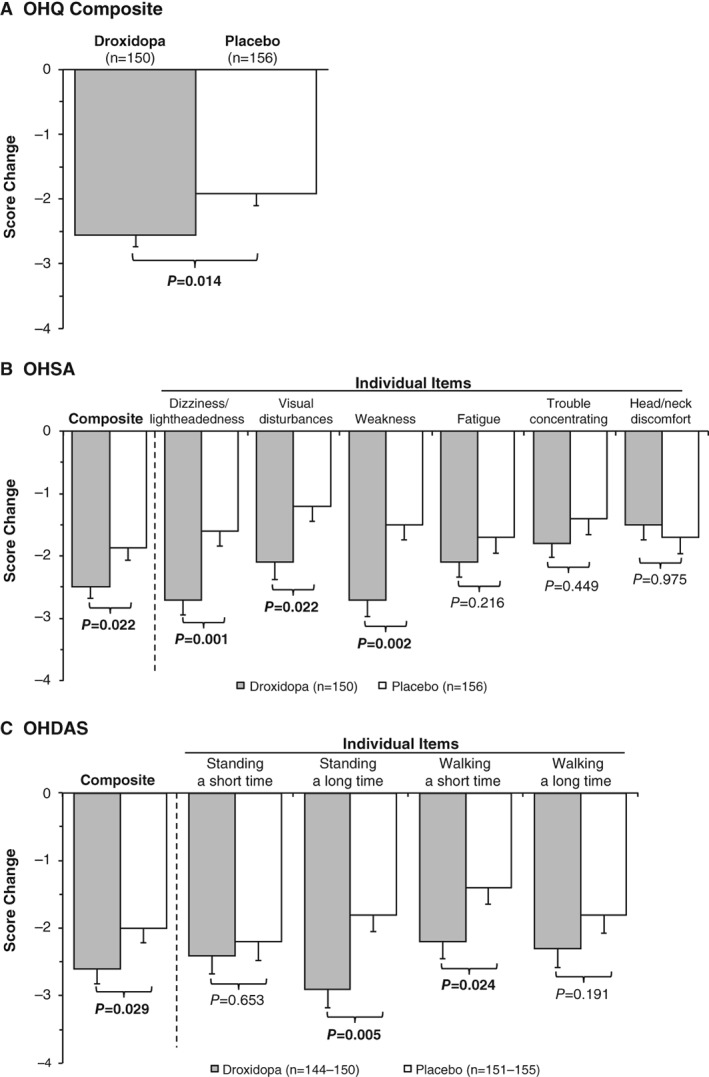
Mean changes from baseline to end of study/week one in (A) OHQ, (B) OHSA, and (C) OHDAS scores. Error bars represent SEMs. OHDAS=Orthostatic Hypotension Daily Activity Scale; OHQ=Orthostatic Hypotension Questionnaire; OHSA=Orthostatic Hypotension Symptom Assessment.
Treatment with droxidopa was also associated with significant improvements in the OHDAS composite score compared with placebo (mean [SEM], –2.62 [0.23] units vs –1.95 [0.21] units; P = 0.029) and in two of the four OHDAS individual items, including standing a long time (P = 0.005) and walking a short time (P = 0.024). Patients exhibited numerical improvement in standing a short time and walking a long time (Fig. 1C).
In addition to improvements in symptom severity and impact on daily life compared with placebo, treatment with droxidopa was associated with significant improvements from baseline to end of study/week one in standing systolic BP (P = 0.003) and diastolic BP (P = 0.002; Fig. 2).
Figure 2.
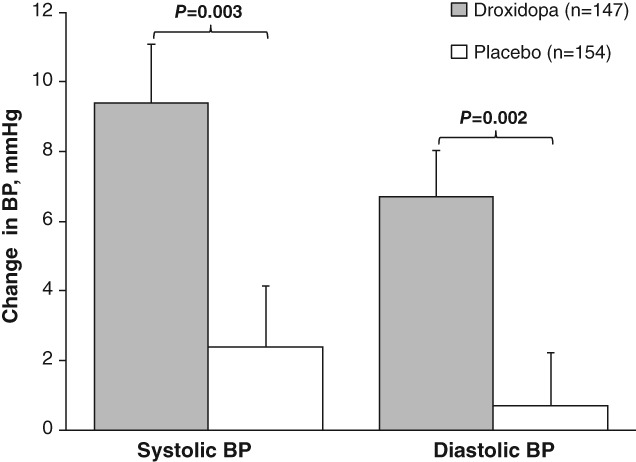
Mean BP changes from baseline to end of study/week one during the orthostatic standing test. Error bars represent SEMs. BP=blood pressure.
Safety
Treatment‐emergent adverse events for Studies NOH301 and NOH302 were pooled, whereas TEAEs in Study NOH306 were reported separately because of differences in study design and a greater duration of exposure to droxidopa (Table 2). Overall, the rates of AEs were similar in both treatment groups during short‐term (droxidopa, 17.9%; placebo, 25.9%) or longer‐term studies (droxidopa, 79.8%; placebo, 80.6%). Rates of moderate and severe AEs were also comparable between treatment groups during the short‐term studies (moderate AEs: droxidopa, 8.9%; placebo, 13.0%; severe AEs: none in either group) as well as in the longer‐term study (moderate AEs: droxidopa, 31.6%; placebo, 33.3%; severe AEs: droxidopa, 8.8%; placebo, 8.3%). There were no deaths reported during any of the studies.
Table 2.
AEs during double‐blind treatment
| Pooled studies NOH301 and NOH302 | Study NOH306 | |||
|---|---|---|---|---|
| Variable | Droxidopa n=56 | Placebo n=54 | Droxidopa n=114 | Placebo n=108 |
| Duration, wk | 1–2 | 8–10 | ||
| Any AE, n (%) | 10 (17.9) | 14 (25.9) | 91 (79.8) | 87 (80.6) |
| Any serious AE, n (%) | 0 | 1 (1.9) | 5 (4.4) | 4 (3.7) |
| Any AE leading to study drug discontinuation, n (%) | 0 | 1 (1.9) | 12 (10.5) | 5 (4.6) |
| AE severity | ||||
| Mild | 7 (12.5) | 9 (16.7) | 45 (39.5) | 42 (38.9) |
| Moderate | 5 (8.9) | 7 (13.0) | 36 (31.6) | 36 (33.3) |
| Severe | 0 | 0 | 10 (8.8) | 9 (8.3) |
| AE type, n (%) | ||||
| Headache | 2 (3.6) | 1 (1.9) | 15 (13.2) | 8 (7.4) |
| Dizziness | 2 (3.6) | 2 (3.7) | 11 (9.6) | 5 (4.6) |
| Nausea | 1 (1.8) | 1 (1.9) | 10 (8.8) | 5 (4.6) |
| Hypertension | 1 (1.8) | 0 | 8 (7.0) | 1 (0.9) |
| Excoriation | 0 | 1 (1.9) | 6 (5.3) | 8 (7.4) |
| Fatigue | 0 | 1 (1.9) | 8 (7.0) | 6 (5.6) |
| Contusion | 0 | 0 | 6 (5.3) | 12 (11.1) |
Abbreviations: AE, adverse event.
Classified by Medical Dictionary for Regulatory Activities preferred term; > 5% of patients receiving droxidopa.
In the droxidopa groups, no serious AEs or discontinuations due to an AE were reported with short‐term drug exposure, whereas these events occurred in 4.4% and 10.5% of patients, respectively, with longer drug exposure in Study NOH306. The most frequently reported AEs in the droxidopa groups included headache (≤ 13.2%), dizziness (≤ 9.6%), nausea (≤ 8.8%), and hypertension (≤ 7.0%). The rates of supine (i.e., defined as the patient in a semi‐recumbent position with their head and torso elevated approximately 30 degrees from horizontal) systolic BP > 180 mmHg were low but nominally increased with droxidopa treatment compared with placebo in the pooled short‐term studies NOH301 and NOH302 (5.4% vs 0%, respectively) and the longer‐term NOH306 study (7.9% vs 4.6%). In studies NOH301 and NOH302 (pooled data), the rates of supine systolic BP > 160 mmHg were 12.5% for droxidopa treatment and 9.3% for placebo. In Study NOH306, the rates of supine systolic BP > 160 mmHg were 28.9% for droxidopa and 24.1% for placebo.
In studies NOH301 and NOH302, falls were recorded as an AE. Pooled data from these studies show the percentage of patients who experienced ≥ 1 fall was 13.0% in the placebo group and 1.8% in the droxidopa treatment group. In Study NOH306, fall data was collected prospectively as a secondary efficacy outcome. The aggregate rate of falls per patient‐week was 0.4 in the droxidopa treatment group and 1.05 in the placebo group (P = 0.014, Poisson‐inverse Gaussian test on the mean).22 In total, patients in the droxidopa group reported 66% fewer falls than patients in the placebo group over the course of the 10‐week study (308 falls vs 908 falls, respectively).22
Discussion
In this pooled analysis evaluating droxidopa compared with placebo in patients with nOH associated with PD, droxidopa was observed to provide significant clinical benefit as assessed by the OHQ and its two components, the OHSA (which focuses on nOH symptoms) and the OHDAS (which focuses on daily activities related to nOH). Droxidopa significantly improved dizziness/lightheadedness, the most common symptom of nOH, as well as vision disturbance and weakness, two additional symptoms of nOH that typically occur when patients with nOH change from a sitting to a standing position. These findings suggest that patients with PD receiving droxidopa may exhibit improvements in a variety of key symptoms associated with symptomatic nOH. Moreover, the patient‐reported OHDAS responses reflect a beneficial impact of droxidopa on activities of daily living such as standing and walking.
Patients with nOH are at risk for falls and serious injury due to BP dysregulation that may result in presyncope/syncope.6, 23 Earlier analyses suggested that droxidopa treatment of PD patients with nOH may reduce falls, and this deserves further investigation.22 However, the patient burden of nOH may also extend beyond risk for falls and associated morbidity. A study in patients with PD showed that orthostatic dizziness significantly impaired activities of daily living that included mobility, independence, and other activities.7 The fear of falls and the inability to perform daily activities of living may cause patients to become more sedentary, leading to social isolation and diminished quality of life.1 In the present analyses, patients with PD who received droxidopa reported improvement in the impact of symptoms on a number of daily activities; outcomes that could be clinically relevant by leading to improved well‐being in patients with nOH.
The findings of the current analyses support the benefit of droxidopa for symptomatic improvement of nOH in patients with PD and are consistent with the effects of droxidopa observed in the individual studies of patients with nOH caused by a variety of conditions.13, 15, 16 Of particular note, the efficacy of droxidopa treatment was demonstrated in this PD patient cohort despite the high rate of DDCI use (85% in the treatment group and 93% in the placebo group). These results are consistent with previously reported data on the effect of concomitant DDCI use on the efficacy of droxidopa from a broader population of patients with nOH related to various underlying diagnoses.19 Understanding the influence of DDCIs on the effects of droxidopa is clinically relevant because droxidopa is metabolized to norepinephrine by dopa decarboxylase, and the conversion of droxidopa to norepinephrine has been shown to be blocked with DDCIs (albeit at a DDCI dose much greater than those used as standard of care in clinical practice).24 The current analyses demonstrate that improvement of nOH symptoms and their impact on daily activities can be achieved with droxidopa treatment despite concomitant use of DDCIs at clinically relevant dosages. However, the range of optimized doses suggests the importance of individualizing the dosage to achieve the desired outcomes.
There are limited data regarding the use of droxidopa in patients also receiving NRIs. Concomitant NRI use was only allowed in one trial included in these analyses, and relatively few patients used NRIs overall (n = 28). No subset analysis was conducted due to the small sample size, but there were no observed differences in the safety (including cardiovascular safety)19 or efficacy profile of droxidopa in patients using NRIs. However, clinicians should be aware of potential interactive effects from concomitant use of droxidopa and NRIs (i.e., inhibiting the reuptake of norepinephrine and enhancement of the effects of droxidopa), especially when initiating NRI use in a patient currently on a stable droxidopa regimen.19
Droxidopa was well tolerated in patients with PD with symptomatic nOH. There were no important differences in AE rates in the short‐term studies (1–2 weeks’ exposure to droxidopa). In the study with a longer duration of drug exposure (8–10 weeks), there were more frequent reports of headaches, dizziness, nausea, and hypertension in patients receiving droxidopa compared with those receiving placebo. The overall safety profile observed in these subanalyses of patients with PD is consistent with that observed in the wider clinical trial populations.13, 14, 15, 16
Potential limitations of this study include the evaluation of treatment effects using a subjective, patient‐reported scale (the OHQ) and the relatively large placebo effects observed. A further limitation of this study is that individual adherence to nonpharmacologic measures was not tracked. It is possible that differences in the adherence to nonpharmacologic treatment recommendations contributed to the strong placebo effect. It should be noted that symptomatic nOH is an orphan indication, and the establishment of statistical significance is challenging in this patient population because of the limited number of patients eligible for enrollment into clinical trials. Also, the present efficacy and tolerability analyses examined a limited duration of treatment (1 to ≤ 10 weeks) with the main pooled efficacy outcomes evaluated after one week of treatment; however, longer‐term safety and efficacy outcomes with droxidopa in the broader population of patients with nOH have been reported.25, 26
Conclusions
The most prevalent underlying cause of nOH observed in clinical practice is PD; thus, understanding nOH and the response to droxidopa in patients with this disease is important. These post hoc analyses suggest that droxidopa provides meaningful clinical benefits in the treatment of symptomatic nOH in patients with PD. Droxidopa was well tolerated by patients with PD participating in these clinical trials. Similar results were noted in a pooled analysis of droxidopa in patients with symptomatic nOH caused by various underlying autonomic failure conditions.19
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
R.A.H.: 1A, 1C, 2C, 3A, 3B
I.B.: 1A, 1B, 3B
L.A.H.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
S.V.: 2C, 3A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The data analyzed in the current study were collected during three previously reported clinical trials and were used with the permission of Lundbeck (sponsor of the three clinical trials). No data (personal or clinical) from any individual participant are reported in this manuscript. During the original clinical trials, all individual data contributing to the analyses of the group data reported herein were collected in accordance with local or centralized institutional review board approval. The original trials were conducted according to the Declaration of Helsinki and its amendments, the International Conference on Harmonisation Good Clinical Practice guidelines, and applicable laws and regulations for each research site. Patients provided written informed consent before original study participation.
Funding Sources and Conflict of Interest: The data reported were derived from clinical trials funded by Chelsea Therapeutics (now Lundbeck). The authors received editorial assistance from CHC Group (North Wales, PA), which was supported by Lundbeck. R.A.H. reports consulting fees from Lundbeck. I.B. is a consultant for Lundbeck. L.A.H. is an employee of Lundbeck. S.V. has received personal compensation as an advisory board member and speakers bureau member for Lundbeck.
Financial Disclosures for the previous 12 months: R.A.H. reports consulting fees from Guidepoint Global, Gerson Lehrman Group (GLG), LCN Consulting, Putnam Associates, National Parkinson Foundation, eResearch Technology, Inc., Cynapsus Therapeutics, Sarepta Therapeutics, Adamas Pharmaceuticals, Neurocrine Biosciences, Back Bay Life Science, US WorldMeds, Biotie Therapies, Michael J. Fox Foundation, Neuropore Therapies, National Institutes of Health, Projects in Knowledge, Prexton Therapeutics, Acorda Therapeutics, Vista Research, LifeMax, Peerview Press, ClinicalMind Medical and Therapeutic Communications, Sunovion Pharmaceuticals, Inc., Academy for Continued Healthcare Learning, Outcomes Insights, Expert Connect, HealthLogix, Teva Pharmaceutical Industries, Cowen and Company, Pharma Two B, Ltd, Pfizer Inc, RMEI Medical Education for Better Outcomes, ClearView Healthcare Partners, Health Advances, Kyowa Kirin Pharmaceutical Development, Ltd., Impax Laboratories, Quintiles, AbbVie Inc, AstraZeneca, and Eli Lilly & Company.
I.B. is a consultant for Theravance Biopharma and receives National Institutes of Health funding for research unrelated to this project.
L.A.H. declares that there are no additional disclosures to report.
S.V. has received personal compensation as a Consultant for Athena Diagnostics (Quest), and as an Associate Editor of JAMA Neurology.
Supporting information
Supplemental Fig. 1. Distribution of the individualized optimized doses of droxidopa used during randomized treatment (n=171). TID=3 times daily.
Acknowledgments
The authors appreciate the contributions of Katya Cherny (statistical analyses), Steven Kymes (critical review), and Annika Lindsten (statistical support), all employees of Lundbeck, during manuscript preparation and revision. The authors received editorial assistance from CHC Group (North Wales, PA).
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Low PA. Neurogenic orthostatic hypotension: pathophysiology and diagnosis. Am J Manag Care. 2015;21(13 suppl):s248–257. [PubMed] [Google Scholar]
- 2. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. [DOI] [PubMed] [Google Scholar]
- 3. Maule S, Papotti G, Naso D, Magnino C, Testa E, Veglio F. Orthostatic hypotension: evaluation and treatment. Cardiovasc Hematol Disord Drug Targets. 2007;7(1):63–70. [DOI] [PubMed] [Google Scholar]
- 4. Sclater A, Alagiakrishnan K. Orthostatic hypotension. A primary care primer for assessment and treatment. Geriatrics. 2004;59(8):22–27. [PubMed] [Google Scholar]
- 5. Gibbons CH, Schmidt P, Biaggioni I, et al. The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264(8):1567–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonell KE, Shibao CA, Claassen DO. Clinical relevance of orthostatic hypotension in neurodegenerative disease. Curr Neurol Neurosci Rep. 2015;15(12):78. [DOI] [PubMed] [Google Scholar]
- 7. Magerkurth C, Schnitzer R, Braune S. Symptoms of autonomic failure in Parkinson's disease: prevalence and impact on daily life. Clin Auton Res. 2005;15(2):76–82. [DOI] [PubMed] [Google Scholar]
- 8. Vellas BJ, Wayne SJ, Romero LJ, Baumgartner RN, Garry PJ. Fear of falling and restriction of mobility in elderly fallers. Age Ageing. 1997;26(3):189–193. [DOI] [PubMed] [Google Scholar]
- 9. Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis. 2012;46(3):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Senard JM, Rai S, Lapeyre‐Mestre M, et al. Prevalence of orthostatic hypotension in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;63(5):584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ha AD, Brown CH, York MK, Jankovic J. The prevalence of symptomatic orthostatic hypotension in patients with Parkinson's disease and atypical parkinsonism. Parkinsonism Relat Disord. 2011;17(8):625–628. [DOI] [PubMed] [Google Scholar]
- 12. NORTHERA® (droxidopa) . Full Prescribing Information, Lundbeck NA Ltd, Deerfield, IL, 2017. [Google Scholar]
- 13. Biaggioni I, Freeman R, Mathias CJ, et al. Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa. Hypertension. 2015;65(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hauser RA, Hewitt LA, Isaacson S. Droxidopa in patients with neurogenic orthostatic hypotension associated with Parkinson's disease (NOH306A). J Parkinsons Dis. 2014;4(1):57–65. [DOI] [PubMed] [Google Scholar]
- 15. Hauser RA, Isaacson S, Lisk JP, Hewitt LA, Rowse G. Droxidopa for the short‐term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson's disease (NOH306B). Mov Disord. 2015;30(5):646–654. [DOI] [PubMed] [Google Scholar]
- 16. Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo‐controlled, phase 3 trial. Neurology. 2014;83(4):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pure autonomic failure. Available at: http://www.orpha.net. Accessed April 4, 2017.
- 18. Hauser RA. Parkinson disease. Medscape Reference Website. Available at: http://emedicine.medscape.com/article/1831191-overview#showall. Accessed April 7, 2017.
- 19. Biaggioni I, Arthur Hewitt L, Rowse GJ, Kaufmann H. Integrated analysis of droxidopa trials for neurogenic orthostatic hypotension. BMC Neurol. 2017;17(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaufmann H, Malamut R, Norcliffe‐Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res. 2012;22(2):79–90. [DOI] [PubMed] [Google Scholar]
- 21. Naschitz JE, Rosner I. Orthostatic hypotension: framework of the syndrome. Postgrad Med J 2007; 83(983): 568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hauser RA, Heritier S, Rowse GJ, Hewitt LA, Isaacson SH. Droxidopa and reduced falls in a trial of Parkinson patients with neurogenic orthostatic hypotension. Clin Neuropharmacol. 2016;39(5):220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palma JA, Gomez‐Esteban JC, Norcliffe‐Kaufmann L, et al. Orthostatic hypotension in Parkinson disease: how much you fall or how low you go? Mov Disord. 2015;30(5):639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaufmann H, Saadia D, Voustianiouk A, et al. Norepinephrine precursor therapy in neurogenic orthostatic hypotension. Circulation. 2003;108(6):724–728. [DOI] [PubMed] [Google Scholar]
- 25. Isaacson S, Shill HA, Vernino S, Ziemann A, Rowse GJ. Safety and durability of effect with long‐term, open‐label droxidopa treatment in patients with symptomatic neurogenic orthostatic hypotension (NOH303). J Parkinsons Dis. 2016;6(4):751–759. [DOI] [PubMed] [Google Scholar]
- 26. Isaacson S, Vernino S, Ziemann A, Rowse GJ, Kalu U, White WB. Long‐term safety of droxidopa in patients with symptomatic neurogenic orthostatic hypotension. J Am Soc Hypertens. 2016;10(10):755–762. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Distribution of the individualized optimized doses of droxidopa used during randomized treatment (n=171). TID=3 times daily.


