Abstract
Acute myeloid leukemia (AML) is a heterogeneous hematological malignancy in which the only curative approach is allogeneic stem cell transplant (Allo-HSCT). The recognition and elimination of leukemic clones by donor T-cells contribute significantly to Allo-HSCT success. FLT3-ITD, a common mutation in AML, is associated with poor prognosis. Recently, midostaurin became the first FDA approved FLT3-inhibitor for pre-transplant patients with FLT3-ITD in combination with standard therapy. In addition to their multikinase activity which may affect T-cell signaling, FLT3-inhibitors induce apoptosis of malignant cells which may also enhance antigen presentation to activate T-cells. Considering the increased clinical use of these inhibitors in patients with AML, and the limited clinical benefit derived from their use as single agents, understanding how FLT3-inhibitors affect T cell population and function is needed to improve their clinical benefit. We examined the effect of four different FLT3 inhibitors (midostaurin, sorafenib, tandutinib, and quizartenib) on T cell populations in peripheral blood mononuclear cells (PBMC) obtained from healthy donors and from patients with AML. Midostaurin exhibited a significant decrease in CD4 + CD25 + FOXP3+ T cell population and FOXP3 mRNA expression in healthy and AML PBMCs. Similarly, samples collected from patients with AML treated with midostaurin showed a reduction in Tregs markers. Interferon-γ(IFN-γ), tumor necrosis factor-α(TNF-α), and IL-10 levels were also reduced following midostaurin treatment. Considering the FDA approval of midostaurin for use in patients with AML in the pre-transplant setting, our finding will have important clinical implication as it provides the rationale for functional investigation of the use of midostaurin in post-transplant patients.
Introduction
Acute Myeloid Leukemia (AML) accounts for the highest mortality rate of all leukemias, and is the most common form of acute leukemia for adults in the United States1. AML is a heterogeneous hematologic malignancy characterized by clonal expansion of myeloid blasts in the blood and bone marrow2. The current therapies available for patients diagnosed with AML can vary according to the patient’s age and their disease risk status, however, the typical treatment options are induction therapy, consolidation therapy, and allogeneic hematopoietic stem cell transplant (Allo-HSCT)3. Of the treatments available, post-remission Allo-HSCT therapy is the only option which provides curative potential largely due to an immunological process called the graft-vs-leukemia (GvL) effect, in which donor cytotoxic T cells eradicate residual malignant cells4. However, Allo-HSCT may also result in a challenging condition called graft versus host disease (GvHD), wherein T cells target normal healthy cells, presenting a major toxicity to overcome5. Therefore, understanding the role of T cells in GvL is pivotal to optimizing patient’s treatment for AML.
Regulatory T cells (Tregs) are a subset of T cells that function in Allo-HSCT to maintain immune self-tolerance through suppression of aberrant or excessive immune responses that can be harmful to the patient6. One study demonstrated that the cotransfer of CD4 + CD25+ Tregs and CD4 + CD25− effector T cells into MHC-mismatched mice with leukemia prevented GvHD while preserving the beneficial GvL effect7. In addition, there is evidence that patients with AML receiving peripheral blood stem cell grafts with higher proportions of Tregs had a better 3-year survival rate compared with those receiving grafts with lower proportions of Treg populations8. Conversely, there is evidence for Treg inhibition of cytotoxic T lymphocytes and creation of an immunosuppressive or anti-apoptotic microenvironment that favors the survival of malignant hematopoietic cells9. Additionally, AML cells can influence the conversion of CD4+ CD25− cells into Tregs via tryptophan catabolism10. Tregs have been shown to suppress the T cell-mediated immune response against the leukemia cells by secretion of cytokines such as transforming growth factor β(TGFβ) or IL-10, and inhibiting dendritic cell maturation11.
One of the most common mutations in patients with AML is the FMS-like Tyrosine Kinase 3 receptor Internal Tandem Duplication (FLT3-ITD). The FLT3 receptor is expressed by immature hematopoietic progenitor cells and functions to induce proliferation and promote survival12. The ITD mutation alters the structure in the juxtamembrane domain of the FLT3 receptor, which leads to constitutive activation and continued proliferation of the AML cells. The FLT3-ITD mutation occurs in 30% of normal karyotype patients with AML and is associated with poor outcomes as well as increased incidence of relapse13,14. Although Allo-HSCT has significantly improved outcome of patients with AML, those positive for the FLT3-ITD mutation have a 50% chance of relapse within two years.
Due to the prevalence of the FLT3-ITD mutation in AML patients, targeting the FLT3 pathway through tyrosine kinase inhibitors has become a major focus in clinical efforts for developing novel therapeutic agents. Currently, a class of drugs called receptor tyrosine kinase inhibitors (TKIs) have been heavily investigated in patients with FLT3-ITD positive AML, and have demonstrated promising results in clinical trials. Very recently, the first FLT3 inhibitor midostaurin received FDA approval for treating patients with FLT3-ITD in combination with standard chemotherapy prior to Allo-HSCT15,16. However, one caveat of this novel therapeutic approach is that the inhibitors are not specific to the FLT3 receptor and may affect other signaling pathways, including those involved in T cell activation. In fact, previous in vitro studies have indicated that another TKI, sorafenib, may inhibit proper T cell function17. Therefore, if a T cell signaling pathway is affected in a way that can enhance the GvL effect, it is possible that TKIs can be used post-Allo-HSCT for therapeutic benefit. In this study, we examined the effect of four different TKIs sorafenib, midostaurin, tandutinib, and quizartenib on T cell populations in blood samples obtained from both healthy donors and patients with AML. Assessment of T cell populations, expression markers and cytokine levels showed that only midostaurin treatment significantly reduced the regulatory T cells population in the healthy and leukemic samples. These results indicate that further functional investigations are needed to establish whether midostaurin may have potential benefit or drawback if used in post-transplant setting.
Materials and Methods
Patient Samples
Blood samples were obtained from healthy donors or from patients with AML at diagnosis from Norris Comprehensive Cancer Center at USC. All samples were collected after obtaining written informed consent. The use of human materials was approved by the University of Southern California Health Sciences Campus Institutional Review Board in accordance with the Helsinki Declaration.
Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs from Healthy donors were drawn and collected in sterile EDTA tubes (BD Vacutainer, Franklin Lakes, NJ). PBMCs were isolated by centrifugation over Ficoll-Paque PLUS density gradients (GE Healthcare, Uppsala, Sweden). Blood was diluted 1:2 in PBS, overlaid on Ficoll lymphocyte separation medium, and centrifuged at 400 × g for 30 minutes at room temperature. PBMCs were collected and washed twice with phosphate buffered saline (Sigma, St. Louis, MO). The final pellet was resuspended in 20% FBS RPMI, and used in the proceeding experiments.
Cell Culture
PBMCs were isolated as previously stated. Immediately after isolation, PBMCs were cultured in Roswell Park Memorial Institute 1640 1x (RPMI) medium supplemented with 20% fetal bovine serum (FBS) (Gibco, Gaithersburg, MD). In addition, PBMCs were treated with IL-2 (5 ng/mL) and IL-7 (10 ng/mL) (Life Technologies, Carlsbad, CA), as well as 1 µM of either Sorafenib, Midostaurin, Tandutinib, or Quizartenib. PBMCs were seeded at 2 million cells per well in a 6 well plate (Genesee Scientific, San Diego, CA), and allowed to incubate at 5% CO2, 37 °C for 72 hours before analysis via flow cytometry or quantitative polymerase chain reaction.
RNA Extraction
Cells were centrifuged at 1300 rpm for 5 minutes, supernatant was removed, and pellet was resuspended in 500 μl of TRIzol reagent and allowed to incubate at room temperature for 2.5 minutes. Subsequently, 200 μl of Chloroform was added to each sample and shaken vigorously for 15 seconds and allowed to incubate on ice for 15 minutes. Samples were then centrifuged at 15000 × g for 15 minutes at 4 °C. The upper aqueous phase was then transferred to an RNAse-free 1.5 mL Eppendorf tubes. 200 μl of isopropanol was added to the samples, and incubated on ice for 10 minutes. Then, samples were centrifuged at 15000 × g for 15 minutes at 4 °C. Once complete, the pellet was washed with 75% ethanol, and centrifuged at 15000 × g for 15 minutes at 4 °C. The supernatant was discarded. The pellet was air-dried for 5 minutes, and resuspended in 20 μl of DEPC-treated ddH20.
Quantitative Polymerase Chain Reaction (qPCR)
cDNA synthesis was performed with random hexamer primers using the Superscript first strand synthesis system for qPCR (Invitrogen, Carlsbad, CA). qPCR was carried out using the 7500 Real Time PCR system and Taqman assays from Applied Biosystems for analysis of B2M, FOXP3, and Granzyme B. Expression of genes of interest were normalized to B2M, the house-keeping gene. Subsequently, expression was normalized to that of the negative control sample.
Flow Cytometry Analysis
The T cell population from PBMCs were analyzed via FACS after 72 hours of treatment with tyrosine kinase inhibitors and IL-2/IL-7. T cell populations were identified through PerCP-labelled anti-CD3 (eBioscience, San Diego, CA); specific T cell subpopulations were identified with PE-labelled anti-CD4 (eBioscience, San Diego, CA), PE-Cyanine7-labelled anti-CD8 (eBioscience, San Diego, CA), APC-labelled anti-CD25 (eBioscience, San Diego, CA), and FITC-labelled anti-Foxp3 (eBioscience, San Diego, CA). Data was acquired using the BD LSRII Flow Cytometer (BD Biosciences, San Jose, CA), and analyzed using FACS Diva software (BD Biosciences, San Jose, CA). Cells were gated for CD3+, then CD4+, then the percentage of CD4+ CD25+, and finally the percentage of CD4+CD25+Foxp3+ populations were quantified.
Cytokine Measurement via Meso Scale Discovery Assay
Supernatants from PBMCs treated with tyrosine kinase inhibitors were stored at −80 °C for analysis. Cytokine ELISAs were performed using electrochemiluminescent multiplex assays to determine the p levels of four cytokines (IFN-γ, TNF-α, IL-10, TGFβ). Calibration curves were prepared in the supplied assay diluents, with a range of 17500 to 0.93 pg/ml. Cytokine concentrations were determined with MSD Workbench 3.0 software (Meso Scale Discovery, Gaithersburg, MD, USA), using curve fit models (log- log or four-parameter log-logistic).
Cell Viability
Cell viability was determined by Alamar Blue–based metabolic assay according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). At 0 and 72 hours, 10 μl of Alamar Blue reagent was added to each well containing 90 μl of resuspended cells; and absorbance (ΔOD570 nm–600 nm) was measured on an automated 96-well spectrophotometer after color development. In addition, cell viability was also determined by Trypan Blue stain.
Statistics
The data are presented as mean ± standard error (SE). The Student t test was used to determine if the difference in mean between samples was statistically significant: p < 0.05 was considered significant.
Ethics approval and consent to participate
This study was conducted according to the approved IRB protocol.
Results
Midostaurin Reduces CD4 + CD25 + FOXP3 + T cell Population in healthy PBMC
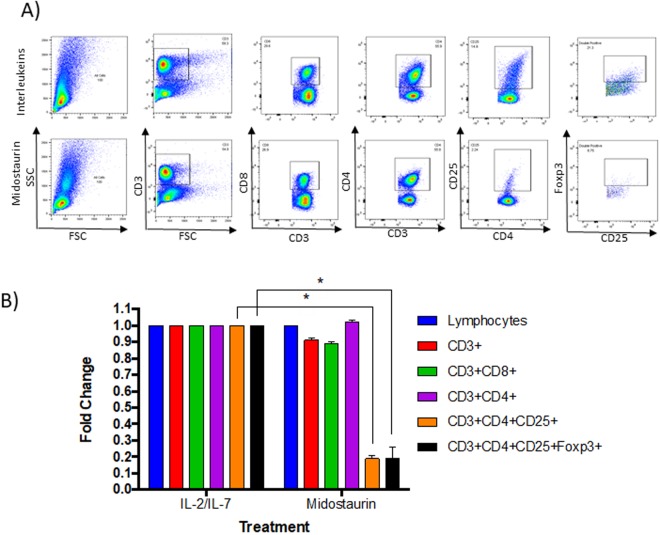
We isolated PBMCs obtained from healthy volunteers and from patients with AML. We treated cells for 72 hours with either sorafenib, midostaurin, tandutinib, or quizartenib, each at concentration of 1μM, combined with IL-2 (5 ng/ml) and IL-7 (10 ng/ml). We used 1μM dose, which is significantly higher than the IC50 calculated for each inhibitor in FLT3-ITD positive AML cells (MV4-11), but not effective in T cells (T ALL cell lines: MOLT4 and RPMI8402) (Fig. S1), and is still achievable in blood of patients with AML. T cell populations (CD3+, CD4+, CD8+ and CD4 + CD25+ cells) were evaluated by flow cytometry analysis (Fig. 1A). We found that PBMCs from Healthy donors treated with midostaurin had a statistically significant decrease in mean CD4+ CD25+ T cell population when compared with other treatment groups (N = 3, 80% decrease, P < 0.001, Fig. 1B). Sorafenib showed a modest decrease in mean CD4 + CD25+ T cells. On the other hand, treatment with tandutinib and quizartenib did not affect CD4 + CD25+ population. No effect was observed on total lymphocytes, CD3+, CD8+, and CD4+ T cells, when cells were treated with FLT3 inhibitors (Fig. 1B). We also treated healthy PBMC from three different donors with increasing concentrations of midostaurin (0.5 μM, 1 μM, and 2 μM), and observed a dose response effect of midostaurin on CD4 + CD25 + FOXP3+ cells (Fig. 1C). Additionally, intracellular staining of FOXP3 demonstrated a significant decrease of T cells in the midostaurin-treated group compared with the control group (n = 3, 70–90% decrease, P < 0.001, Fig. 2).
Figure 1.
Midostaurin reduces CD4 + CD25+ population in healthy PBMCs. (A) Representative contour plots of T cell populations from Healthy donors treated with inhibitors. (B) In vitro PBMCs were treated with 4 kinase inhibitors and normalized to IL-2/IL-7 control population percentages (N = 3, p < 0.05). (C) Combined quantification of multiple samples of PBMCs treated with 0.5, 1 and 2uM of midostaurin and normalized to IL-2/IL-7 control population percentages (N = 3, p < 0.001).
Figure 2.
Midostaurin reduces CD4 + CD25 + FOXP3+ population in healthy PBMCs. In vitro PBMCs were treated with 1μM midostaurin, T cell populations were analyzed by flow cytometry (A) a representative figure. (B) Normalized to IL-2/IL-7 control population percentages and averaged (N = 3, p < 0.001).
Midostaurin Reduces FOXP3 mRNA expression and Modulates T cell Cytokine Activity in healthy PBMC
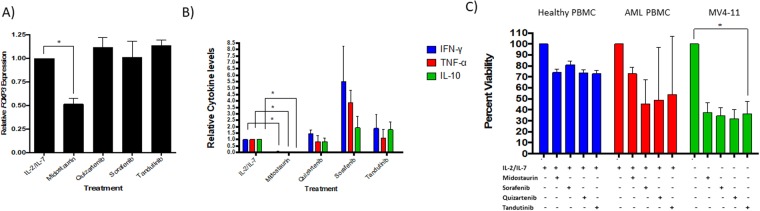
Treatment with midostaurin resulted in a statistically significant decrease in relative FOXP3 mRNA expression compared with other treatment groups of the healthy PBMCs (N = 5, 2-fold decrease, P = 0.02, Fig. 3A). On the other hand, sorafenib, tandutinib, and quizartenib did not affect FOXP3 mRNA levels. Then, we measured the levels of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and IL-10 in the supernatants of treated cells and compared it with untreated cells. We found that midostaurin treatment resulted in significant decrease in IFN-γ, TNF-α, and IL-10 (N = 3. 95–98% decrease, P < 0.001 levels, Fig. 3B). Sorafenib showed an increase in IFN-γ (N = 3, 5-fold increase, P = 0.04), TNF-α (N = 3, 4-fold increase, P = 0.02), and IL-10 (N = 3. 2-fold increase, P = 0.17) levels (Fig. 3B). None of the kinase inhibitors exhibited significant effect on the TGFb levels compared with that of the control groups (Fig. S3A). The effects of different treatments on cell viability are shown in Fig. 3C. T cells express very low level of FLT3 compared with myeloid leukemic cells (Fig. S2, n = 5 for T cells, n = 3 MV4-11 controls, p < 0.001). Thus, the effect of midostaurin on T cell markers is likely a FLT3-independent effect.
Figure 3.
Midostaurin Reduces FOXP3 Expression and T cell cytokines. (A) Effect of various inhibitors on FOXP3 mRNA expression in healthy PBMCs (N = 5, p < 0.05). (B) Cytokine levels in picograms/ml were measured in cell treated supernatants and normalized to IL-2/IL-7 treated group (n = 3 p < 0.05). (C) PBMCs were treated with four different kinase inhibitors for 72 hours and showed a 20% reduction in viability for both healthy (N = 3) and AML (N = 5) groups. MV4-11 cells were used as a positive control for kinase inhibitors and showed over 50% reduction in viability (N = 8).
Midostaurin-treated PBMCs from Patients with AML Display a Decrease in Regulatory T cells
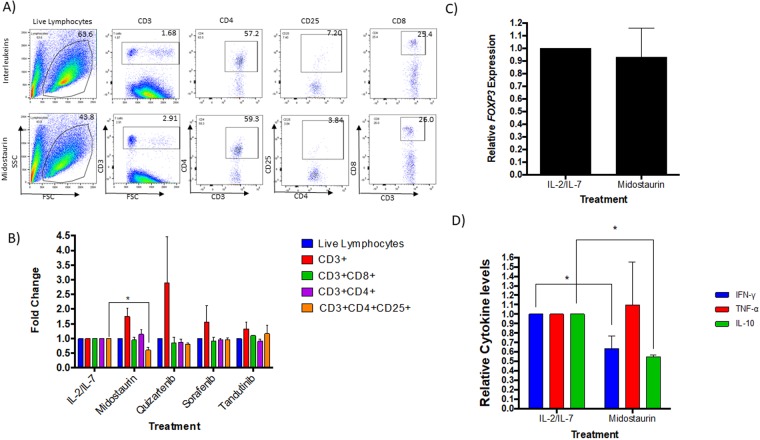
To validate these findings in AML; PBMCs isolated from blood of patients with AML were treated with midostaurin for 72 hours in the presence of IL-2 and IL-7, and compared with untreated cells. Our initial results, obtained via flow cytometry (Fig. 4A)-found a significant decrease in the CD4 + CD25+ T cell population (N = 6, 30% decrease, P = 0.001, Fig. 4B) in midostaurin treated samples compared with their respective controls. PBMCs extracted from patients with AML and treated with midostaurin also displayed a slight decrease in FOXP3 mRNA expression compared to the untreated PBMCs; however, the difference was not statistically significant (N = 6, 4–55% decrease, P = 0.30, Fig. 4C). In addition, cytokine analysis of supernatants of AML-PBMCs treated with midostaurin showed a change in IFN-γ (N = 5, 25% decrease, P = 0.002), TNF-α (N = 3, 10% increase, P = 0.42), and IL-10 (N = 2, 50% decrease, P = 0.001) when compared to the control group (Fig. 4D). IL-10 levels were below the detection level of our assay in three patient’s samples. Also, similar to the healthy PBMCs, TGFβ levels were not significantly changed following midostaurin treatment of AML samples (Fig. S3B). Viability assay confirmed that treatment with FLT3 inhibitors decreased cell viability in AML PBMCs by about 17–33%, however, treatment with FLT3 inhibitors decreased cell viability of MV4-11 cells (an AML cell line that carries the FLT3-ITD mutation) by more than 50% (Fig. 3C). Although the percentage of T cells was very low in the blood obtained from the diagnostic samples obtained from patients with AML, the observed trend was similar to that found in healthy PBMC.
Figure 4.
Midostaurin reduces Tregs in AML cells. (A) Representative contour plots of T cell populations from patients with AML treated with midostaurin. (B) In vitro PBMCs were treated with one of the four kinase inhibitors and normalized to IL-2/IL-7 control population percentages (n = 4, p < 0.05). (C) FOXP3 mRNA expression in AML PBMCs (n = 4) treated ex vivo with 1uM of midostaurin. (D) Cytokine levels in picograms/ml were measured in AML cells treated supernatants and normalized to IL-2/IL-7 treated group (N = 5).
Midostaurin reduces Tregs markers in patients with AML
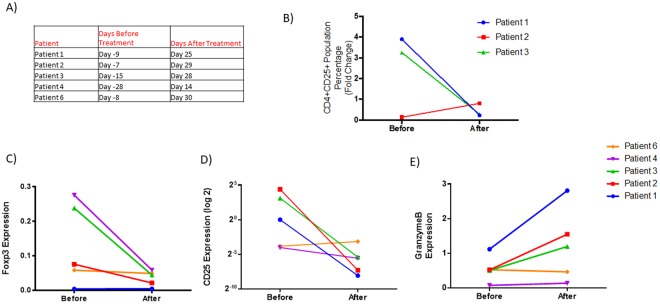
Because midostaurin is currently FDA approved for treatment of AML in combination with chemotherapy, we validated our findings in patients who received midostaurin. We obtained samples from three patients with AML at sequential times before and after midostaurin treatment. CD4 + CD25+ cells population decreased approximately four weeks after the initiation of midostaurin treatment compared with time points before the treatment in two patients (Fig. 5B). In one patient, CD4 + CD25+ cells were already low at base line and no change was observed following midostaurin treatment. We also assessed FOXP3, CD25 and GranzymeB mRNA levels in five patients. We found a consistent decrease in FOXP3 and CD25 levels and increase in GranzymeB levels in three out of five patients, the two patients where no significant change observed also had very low base level of FOXP3 and CD25 mRNA before treatment (Fig. 5C–E).
Figure 5.
Midostaurin alters CD4 + CD25 + T cell population and T cell gene expression markers in patients with AML. (A) Table indicating time points before and after midostaurin treatment for patient samples. (B) Levels of CD4 + CD25+ population before and after midostaurin treatment. (C) FOXP3 and (D) CD25 mRNA normalized to CD3 and CD4 expression before and after midostaurin treatment. (E) Expression change of GZMB mRNA normalized to CD3 and CD8 mRNA expression before and after midostaurin treatment.
Discussion
FLT3 and FLT3 ligand (FLT3L) signaling has been shown to indirectly expand Treg through increasing dendritic cell number18. Therefore, it is plausible that mechanisms responsible for activating FLT3 signaling pathways may also cause an expansion in regulatory T cells and thus induce a repressive immune response and immune evasion in leukemic cells. As a result, we hypothesized that inhibiting FLT3 signaling pathways with FLT3 inhibitors would affect T cell populations and particularly regulatory T cells. Unlike the second generation FLT3 inhibitors, sorafenib and quizartinib, the first generation FLT3 inhibitors midostaurin and tandutinib are less specific and inhibit a wide range of tyrosine kinases. Unexpectedly, only midostaurin but not every FLT3 inhibitor resulted in the significant decrease in Tregs both in healthy PBMCs and AML PBMCs. This suggests that mechanisms other than those mediated by FLT3 signaling pathways are responsible for this reduction in the Treg. Previous studies have demonstrated that IL-2 and IL-7 are important for maintaining homeostasis of Treg cells19,20. IL-7 induces the expression of CD25 on the surface of T cells and expand Treg21,22. Also, IL-2 has been shown to increase the CD4 + CD25+ T cell population in cancer patients by 4-fold in vivo, as well as increase their suppressive capabilities in vitro23. In addition, there is evidence that induced Tregs (iTregs) require a constant supply of IL-2 for proper development and function20. Whether midostaurin interferes with IL-2 and IL-7 signaling pathways and downstream targets is unclear. Midostaurin inhibitory effects on JAK/STAT and PI3K/AKT signaling pathways which are also downstream of IL-2 and IL-7 may potentially be a plausible mechanism. Previous studies have demonstrated that PI3K/AKT inhibitors can decrease Treg populations without affecting other T cell populations24. STAT5 is crucial for IL-2 response, Treg cell development, and for the expression of FOXP325–27. In fact, STAT5 is a transcription factor that also functions downstream of FLT3-ITD, making it a potential target for midostaurin28. Recent studies have reported that tyrosine kinase inhibitors increase the cell surface localization of FLT3-ITD29. Although the study did not address the effect of FLT3 inhibition on regulatory T cells, this suggests a dual effect of midostaurin in enhancing FLT3-directed immunotherapy of AML. Although the mechanism by which midostaurin reduces Tregs is not clear, our data indicates possible off-target effects of this multi-kinase inhibitor on T cell signaling pathways that is differentially specific to Treg cells. A limitation of the study is the lack of comprehensive profiling of T cell markers, in order to exclude potential effects of midostaurin on other T cell populations. We are currently exploring the effect of midostaurin on Tregs function and repertoire, as this may provide valuable information on improving GvL effect in patients with AML.
We conclude that midostaurin demonstrated a decrease in Treg populations both in vitro and in some patients with AML. In light of the recent FDA approval of midostaurin combined with chemotherapy in patients with AML16, these results highlight a novel therapeutic advantage of this drug that may be beneficial particularly in the post-transplant setting as well as in combination with immunotherapy.
Electronic supplementary material
Acknowledgements
We would like to acknowledge Sharon Wu and Vijaya Pooja Vaikari for proofreading the manuscript. We would like to acknowledge Rachel Reyes for acquiring blood samples for our experiments, as well as the flow cytometry core department in the Eli and Edythe Broad CIRM Center for Regenerative Medicine and Stem Cell Research at USC for their assistance. We like to acknowledge the University of Southern California School Of Pharmacy Seed Fund. This work was also supported by the STOP Cancer Funding Award, the American Cancer Society Institutional research Grant IRG-16-181-57 (ACS-IRG), The Ming Hsieh Institute research fund, and by grant UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
H.A. and L.G. conceived the project. H.A. and L.G. designed the research. H.A. supervised the experiments conducted in the laboratory. L.G. conducted most of the experiments and performed the data analyses. M.J. and T.Z. contributed to flow cytometry and drug treatment experiments. M.A. provided clinical input and patients samples.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35978-0.
References
- 1.Lowenberg, B., Downing, J. R. & Burnett, A. Acute Myeloid Leukemia, 10.1056/NEJM199909303411407, doi:NJ199909303411407 (2008).
- 2.Estey E, Döhner H. Acute myeloid leukaemia. The Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen YB, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20:2042–2048. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta V, Hari P, Hoffman R. Allogeneic hematopoietic cell transplantation for myelofibrosis in the era of JAK inhibitors. Blood. 2012;120:1367–1379. doi: 10.1182/blood-2012-05-399048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 6.Wing JB, Sakaguchi S. Multiple treg suppressive modules and their adaptability. Front Immunol. 2012;3:178. doi: 10.3389/fimmu.2012.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edinger M, et al. CD4 + CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 8.Danby RD, et al. High proportions of regulatory T cells in PBSC grafts predict improved survival after allogeneic haematopoietic SCT. Bone Marrow Transplant. 2016;51:110–118. doi: 10.1038/bmt.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buggins AG, et al. Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-kappaB, c-Myc, and pRb pathways. J Immunol. 2001;167:6021–6030. doi: 10.4049/jimmunol.167.10.6021. [DOI] [PubMed] [Google Scholar]
- 10.Curti A, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 11.Jin Yan, Wi Hae Joo, Choi Min-Ho, Hong Sung-Tae, Bae Young Mee. Regulation of anti-inflammatory cytokines IL-10 and TGF-β in mouse dendritic cells through treatment with Clonorchis sinensis crude antigen. Experimental & Molecular Medicine. 2014;46(1):e74–e74. doi: 10.1038/emm.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drexler HG, Quentmeier H. FLT3: receptor and ligand. Growth Factors. 2004;22:71–73. doi: 10.1080/08977190410001700989. [DOI] [PubMed] [Google Scholar]
- 13.Kottaridis P. D. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98(6):1752–1759. doi: 10.1182/blood.V98.6.1752. [DOI] [PubMed] [Google Scholar]
- 14.Khwaja, A. et al. Acute myeloid leukaemia. Nature Reviews Disease Primers2, 16010 (2016). [DOI] [PubMed]
- 15.Fischer Thomas, Stone Richard M., DeAngelo Daniel J., Galinsky Ilene, Estey Elihu, Lanza Carlo, Fox Edward, Ehninger Gerhard, Feldman Eric J., Schiller Gary J., Klimek Virginia M., Nimer Stephen D., Gilliland D. Gary, Dutreix Catherine, Huntsman-Labed Alice, Virkus Jodi, Giles Francis J. Phase IIB Trial of Oral Midostaurin (PKC412), the FMS-Like Tyrosine Kinase 3 Receptor (FLT3) and Multi-Targeted Kinase Inhibitor, in Patients With Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome With Either Wild-Type or Mutated FLT3. Journal of Clinical Oncology. 2010;28(28):4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone, R. M. et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation, 10.1056/NEJMoa1614359, doi:NJ201708033770508 (2017). [DOI] [PMC free article] [PubMed]
- 17.Cabrera R, et al. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2013;62:737–746. doi: 10.1007/s00262-012-1380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swee LK, Bosco N, Malissen B, Ceredig R, Rolink A. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 2009;113:6277–6287. doi: 10.1182/blood-2008-06-161026. [DOI] [PubMed] [Google Scholar]
- 19.Simonetta Federico, Gestermann Nicolas, Bloquet Stéphane, Bourgeois Christine. Interleukin-7 Optimizes FOXP3+CD4+ Regulatory T Cells Reactivity to Interleukin-2 by Modulating CD25 Expression. PLoS ONE. 2014;9(12):e113314. doi: 10.1371/journal.pone.0113314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 21.Simonetta F, et al. Interleukin-7 influences FOXP3 + CD4+ regulatory T cells peripheral homeostasis. PLoS One. 2012;7:e36596. doi: 10.1371/journal.pone.0036596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Caro V, et al. Interleukin-7 matures suppressive CD127+ forkhead box P3 (FoxP3)+ T cells into CD127− CD25high FoxP3+ regulatory T cells. Clin Exp Immunol. 2011;165:60–76. doi: 10.1111/j.1365-2249.2011.04334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmadzadeh, M. & Rosenberg, S. A. In Blood Vol. 107, 2409–2414 (2006). [DOI] [PMC free article] [PubMed]
- 24.Abu-Eid R, et al. Selective inhibition of regulatory T cells by targeting PI3K-Akt pathway. Cancer Immunol Res. 2014;2:1080–1089. doi: 10.1158/2326-6066.cir-14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passerini L, et al. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4 + CD25+ regulatory T cells and CD4 + CD25− effector T cells. Int Immunol. 2008;20:421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 26.Antov A., Yang L., Vig M., Baltimore D., Van Parijs L. Essential Role for STAT5 Signaling in CD25+CD4+ Regulatory T Cell Homeostasis and the Maintenance of Self-Tolerance. The Journal of Immunology. 2003;171(7):3435–3441. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- 27.Burchill M. A., Yang J., Vogtenhuber C., Blazar B. R., Farrar M. A. IL-2 Receptor -Dependent STAT5 Activation Is Required for the Development of Foxp3+ Regulatory T Cells. The Journal of Immunology. 2006;178(1):280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 28.Okutani Y, et al. Src directly tyrosine-phosphorylates STAT5 on its activation site and is involved in erythropoietin-induced signaling pathway. Oncogene. 2001;20:6643–6650. doi: 10.1038/sj.onc.1204807. [DOI] [PubMed] [Google Scholar]
- 29.Reiter K, Polzer H, Krupka C, Maiser A, Vick B, Rothenberg-Thurley M, Metzeler K H, Dörfel D, Salih H R, Jung G, Nößner E, Jeremias I, Hiddemann W, Leonhardt H, Spiekermann K, Subklewe M, Greif P A. Tyrosine kinase inhibition increases the cell surface localization of FLT3-ITD and enhances FLT3-directed immunotherapy of acute myeloid leukemia. Leukemia. 2017;32(2):313–322. doi: 10.1038/leu.2017.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.