Abstract
Second-generation (2G) chimeric antigen receptors (CARs) targeting CD19 are highly active against B cell malignancies, but it is unknown whether any of the costimulatory domains incorporated in the CAR have superior activity to others. Because CD28 and 4-1BB signaling activate different pathways, combining them in a single third-generation (3G) CAR may overcome the limitations of each individual costimulatory domain. We designed a clinical trial in which two autologous CD19-specific CAR-transduced T cell products (CD19.CARTs), 2G (with CD28 only) and 3G (CD28 and 4-1BB), were infused simultaneously in 16 patients with relapsed or refractory non-Hodgkin’s lymphoma. 3G CD19.CARTs had superior expansion and longer persistence than 2G CD19.CARTs. This difference was most striking in the five patients with low disease burden and few circulating normal B cells, in whom 2G CD19.CARTs had limited expansion and persistence and correspondingly reduced area under the curve. Of the 11 patients with measurable disease, three achieved complete responses and three had partial responses. Cytokine release syndrome occurred in six patients but was mild, and no patient required anti-IL-6 therapy. Hence, 3G CD19.CARTs combining 4-1BB with CD28 produce superior CART expansion and may be of particular value when treating low disease burden in patients whose normal B cells are depleted by prior therapy.
Keywords: CAR-T cells, CD19, immunotherapy, second-generation CAR, third-generation CAR, chimeric antigen receptor
CD19.CAR-T cells are highly active against B cell malignancies, but the optimal CAR structure is controversial. Ramos et al. show that combining 4-1BB and CD28 endodomains produces superior CD19-CAR-T cell expansion and persistence compared to CD28 alone and that these cells are clinically effective and safe in aggressive lymphomas.
Introduction
A chimeric antigen receptor (CAR) combines the antigen binding portion of a monoclonal antibody with components of the signaling machinery of a T cell, most commonly the ζ (zeta) chain of the T cell receptor (TCR) complex. Genetic transfer of this construct into T cells endows them with the ability to bind a surface antigen in tumor cells, leading to T cell activation and target cell killing.1 Although CARs containing the ζ-chain alone have cytotoxic activity in vitro, early clinical studies demonstrated that full activation of these modified T cells in vivo required the CAR to incorporate additional elements derived from costimulatory domains such as CD28 or 4-1BB (CD137).2 When these so-called second-generation (2G) CARs target CD19, they have proved to be highly active against B cell malignancies.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 It is, however, still unknown whether some costimulatory domains have superior activity to others. For instance, it is asserted that CD28 may lead to quicker T cell expansion and faster tumor eradication, and 4-1BB may be associated with longer persistence and protection from relapse,20 but simultaneous comparisons in single individuals have not been reported. Because CD28 and 4-1BB signaling activate different pathways in T cells, combining them in a single third-generation (3G) CAR may provide added benefits and overcome the limitations of each individual costimulatory domain. It is, however, unknown whether such a combination of two costimulatory endomains in a 3G vector will produce more rapid, greater, or more persistent CART cell expansion in humans with CD19+ malignancies than the single costimulatory signals embedded within 2G CD19-specific CARs. The potential benefits of 3G CARs may be particularly important in the context of a low burden of disease, since the antigenic stimuli for expansion and persistence of CAR-T cells will be more limited, and additional costimulation may be required to exceed the threshold of CAR-T cell activation.
We designed a clinical trial in which two CD19-specific CAR-transduced T cell products (CD19.CARTs) were prepared in parallel from autologous peripheral blood mononuclear cells (PBMCs). The first product was retrovirally transduced with a 2G CAR containing the CD28 costimulatory sequences alone, and the second was transduced with a 3G CAR containing both CD28 and 4-1BB. After ex vivo expansion, these two products were infused simultaneously in the same patient. Specific qPCR assays then allowed us to track each population independently in vivo. This strategy enabled us to avoid interpatient variability as a confounder and thus to directly measure the impact of changing costimulatory domains on CAR-T cell fate in vivo. In this phase 1 study, we treated patients with CD19+ non-Hodgkin’s lymphoma (NHL), assessed treatment toxicity and outcome, and compared the fate of each CD19.CART population in every patient.
We show that simultaneous adoptive transfer of 2G and 3G CD19.CARTs is feasible. These cells produced clinical responses and readily manageable toxicities. Cells containing the 3G vector had superior expansion and longer persistence than the 2G vector, and this difference was most striking in patients with low disease burden whose normal CD19+ B cells are depleted by prior therapy, a group in whom the expansion and persistence of 2G CARTs may be insufficient for antitumor activity.
Results
Patient Characteristics
We administered CD19.CARTs to 16 NHL patients, 11 with active disease and five in remission after high-dose therapy and autologous stem cell transplantation (HDT/ASCT). The characteristics of the T cell products are summarized in Table 1. Patient characteristics are summarized in Tables 2 and 3. All patients with active disease at the time of CD19.CART infusion had relapsed or persistent disease after three or more lines of chemotherapy. All of these patients received lymphodepleting doses of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) daily for 3 days, finished 24 to 72 hr before administration of CD19.CARTs. Patients in remission after ASCT did not receive any additional chemotherapy immediately prior to CART infusion; all these patients were engrafted and were treated within 60 days after ASCT.
Table 1.
Characteristics of the Generated CD19.CART Products
| Second Generation (n = 28) | Third Generation (n = 28) | p Value | |
|---|---|---|---|
| Days in culture | 9.9 ± 0.7 | 10.2 ± 1.2 | 0.2350 |
| Transduction (% CAR+ cells) | 35.0 ± 7.6 | 71.9 ± 4.8 | <0.0001 |
| CD3+ cells (%) | 99.3 ± 0.7 | 98.9 ± 1.1 | 0.0009 |
| CD3+ CD8+ cells (%) | 47.5 ± 17.8 | 47.6 ± 17.6 | 0.9289 |
| CD3+ CD45RO+ cells (%) | 82.3 ± 19.0 | 86.4 ± 14.6 | 0.0276 |
| CD3+ CD127+ (%) | 4.0 ± 11.3 | 2.8 ± 9.2 | 0.0964 |
| CD4+ CD45RO+ CD62L+ (%) | 25.4 ± 12.7 | 24.1 ± 13.2 | 0.4477 |
| CD4+ CD45RO+ CCR7+ cells (%) | 10.5 ± 10.1 | 16.4 ± 11.6 | <0.0001 |
| CD8+ CD45RO+ CCR7+ cells (%) | 6.7 ± 7.8 | 12.0 ± 9.1 | <0.0001 |
| CD3− CD56+ cells (%) | 0.4 ± 0.7 | 0.6 ± 1.1 | 0.0562 |
| CD3+ CXCR3+ cells (%) | 71.9 ± 19.8 | 66.4 ± 25.7 | 0.2595 |
| CD3+ CXCR4+ cells (%) | 19.3 ± 22.4 | 14.3 ± 15.2 | 0.1572 |
Table 2.
Active Disease Patient Group Characteristics
| UPIN | Age | Sex | Diagnosis | Previous Therapies | DL | CAR+ Cells in 2G Product (%) | CAR+ Cells in 3G Product (%) | Total CAR+ T Cells/m2 Administered per Infusion (×106) | Total CAR+ T Cells/kg Administered per Infusion (×106) | Number of Infusions | Best Response | Current Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | M | DLBCL | R-CHOP, R-EPOCH, R-BEAM and ASCT, R-ICE, lenalidomide | 1 | 25.6 | 75.0 | 2 | 0.05 | 2 | SD × 6 weeks | dead from progression |
| 2 | 67 | M | DLBCL-tFL | R-CHOP, R-lenalidomide, R-ICE | 1 | 37.1 | 72.8 | 2 | 0.05 | 2 | CR × 18+ months | alive without disease |
| 4 | 64 | F | DLBCL-tENMZL | XRT, R-CVP, R-CHOP, R-BEAM and ASCT | 2 | 27.8 | 65.4 | 10 | 0.27 | 1 | CR × 12+ months | alive without disease |
| 5 | 66 | M | DLBCL | R-CHOP, R-CAE, ESHAP, R-ICE, EPOCH, methotrexate, GemOx, MACE, R-lenalidomide, XRT | 2 | 29.1 | 73.0 | 10 | 0.21 | 1 | PR × 12 weeks | dead from sepsis |
| 7 | 46 | M | CLL or SLL and Richter | R-venetoclax, FCR, R-bendamustine, idelalisib, ibrutinib, R-ICE, R-hCVAD, R-CHOP | 3 | 34.2 | 70.7 | 40 | 0.84 | 1 | NR | alive with disease |
| 11 | 17 | M | LL or ALL | Per COG AALL1131 (arm A), per COG AALL1331 (blocks 1–2) without blinatumomab | 3 | 32.4 | 75.9 | 40 | 0.96 | 1 | PR × 4 weeks | dead from progression |
| 12 | 67 | F | DLBCL-tFL | R-bendamustine, obinutuzumab/lenalidomide, R-CHOP, GemOx | 3 | 45.3 | 77.6 | 40 | 1.01 | 2 | SD × 6 weeks | alive without disease |
| 13 | 73 | F | DLBCL-tFL | R, R-CHOP, R-ICE, R-lenalidomide | 3 | 29 | 73.2 | 40 | 1.25 | 2 | PR × 6 weeks | alive with disease |
| 14 | 34 | M | LL or ALL | hCVAD, HiDAC, XRT, allotransplant, blinatumumab | 3 | 41.9 | 63.5 | 40 | 0.93 | 1 | NR | alive without disease |
| 15 | 39 | M | DLBCL | R-CHOP, XRT, GDP, R-BEAM and ASCT | 3 | 47.9 | 70.4 | 40 | 0.76 | 1 | NR | dead from progression |
| 16 | 52 | M | DLBCL | R-CHOP, R-ICE, R-GemOx | 3 | 30 | 69.4 | 40 | 1.05 | 1 | CR × 6+ weeks | alive without disease |
Abbreviations: ASCT, autologous stem cell transplant; BEAM, carmustine, etoposide, cytarabine, melphalan; COG, children’s oncology group protocol; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CAE, cyclophosphamide, doxorubicin, etoposide; CVP, cyclophosphamide, vincristine, prednisone; DL, dose level; DLBCL, diffuse large B cell lymphoma; CR, complete response; EPOCH, etoposide, prednisolone, vincristine, cyclophosphamide, doxorubicin; ESHAP, etoposide, methylprednisolone, cytarabine, cisplatin; FCR, fludarabine, cyclophosphamide, rituximab; hCVAD, hyperfractionated cyclophosphamide, vincristine, cytarabine, doxorubicin, dexamethasone; GDP, gemcitabine, dexamethasone, cisplatin; GemOx, gemcitabine, oxaliplatin; HiDAC, high-dose cytarabine; ICE, ifosfamide, carboplatin, etoposide; MACE, methotrexate, doxorubicin, cyclophosphamide, etoposide; LL or ALL, lymphoblastic lymphoma/acute lymphoblastic leukemia; NR, no response; PR, partial response; R-, rituximab; SD, stable disease; tENMZL, transformed extra nodal marginal zone lymphoma; tFL, transformed follicular lymphoma; UPIN, universal patient identifier number; XRT, radiation therapy.
Table 3.
Post-ASCT Patient Group Characteristics
| UPIN | Age | Sex | Diagnosis | Previous Therapies | Time from ASCT (Days) | DL | CAR+ Cells in 2G Product (%) | CAR+ Cells in 3G Product (%) | Total CAR+ T Cells/m2 Administered per Infusion (×106) | Total CAR+ T Cells/kg Administered per Infusion (×106) | Number of Infusions | Best Response | Current Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 46 | M | BCLU | R-EPOCH, R-DHAP, R-IE, BuGemMel and vorinostat and ASCT | 19 | 3 | 32.6 | 66.2 | 40 | 0.97 | 1 | CCR × 10 months | alive without disease |
| 6 | 58 | F | DLBCL | Methotrexate, R-EPCH, R-BEAM and ASCT | 46 | 3 | 39.7 | 74.8 | 40 | 1.01 | 1 | CCR × 12+ months | alive without disease |
| 8 | 75 | M | DLBCL | R-CHOP, R-ICE, R-brentuximab, XRT, R-BEAM and ASCT | 56 | 3 | 52.7 | 81.1 | 40 | 0.96 | 1 | CCR × 9+ months | alive without disease |
| 9 | 16 | M | LL | COP, COPADM, R-ICE, R-BEAM and ASCT | 58 | 3 | 36.5 | 72.6 | 40 | 0.75 | 1 | CCR × 9+ months | alive without disease |
| 10 | 59 | F | DLBCL | R-EPOCH, R-DHAP. BuGemMel and vorinostat and ASCT | 40 | 3 | 45.2 | 71.5 | 40 | 1.18 | 1 | CCR × 9+ months | alive without disease |
Abbreviations: ASCT, autologous stem cell transplant; BCLU, B cell lymphoma unclassifiable with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma; BEAM, carmustine, etoposide, cytarabine, melphalan; BuGemMel, busulfan, gemcitabine, melphalan; COP, cyclophosphamide, vincristine, prednisolone; COPADM, cyclophosphamide, vincristine, prednisolone, cytarabine, doxorubicin, methotrexate; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; DL, dose level; DLBCL, diffuse large B cell lymphoma; CCR, continued complete response; DHAP, dexamethasone, high-dose cytarabine, cisplatin; EP(O)CH, etoposide, prednisolone, (vincristine), cyclophosphamide, doxorubicin; I(C)E, ifosfamide, (carboplatin), etoposide; R-, rituximab; LL, lymphoblastic lymphoma; UPIN, universal patient identifier number; XRT, radiation therapy.
Characteristics of Infused CD19.CARTs
We manufactured 56 CD19.CARTs from 28 patients. CAR transduction levels were above the release criterion of >20% in all manufactured T cell products, but the 2G CARTs had an average transduction level (35% ± 8%) that was approximately half of the 3G CARTs (72% ± 5%, p < 0.0001). Nevertheless, the same numbers of 2G and 3G CARTs were infused in each patient because total cell numbers were adjusted for transduction levels. All lines were composed of >98% CD3+ T cells and each contained a variable ratio of CD4+ and CD8+ T cells, with no overall CD4+ or CD8+ T cell predominance across the T cell products (Table 1). The majority of CD19.CARTs were CD45RO+ and lacked CCR7, but a small fraction expressed central memory-associated phenotypic markers such as CD62L (Table 1), CD27, and CD28. Notably, the proportion of central memory CD4+ and CD8+ T cells was increased in 3G compared to 2G CARTs (CD4+CD45RO+CCR7+, 16.4% ± 11.6% versus 10.5% ± 10.1%, p < 0.0001; CD8+CD45RO+CCR7+, 12.0% ± 9.1% versus 6.7% ± 7.8%, p < 0.0001). Natural killer cells (CD3− CD56+) were not detectable. All products were cytotoxic against CD19+ targets in vitro, as assessed by 51Cr-release assays, without significant differences between 2G and 3G CARTs. Cytotoxic activity against CD19− targets was negligible. In vitro experiments have previously shown that 3G CD19.CARTs have a higher degree of intracellular signaling activity than 2G CART, although this was not associated with significant differences in cytotoxic activity between 2G and 3G CARTs after repeated exposure to targets.21 Twelve patients did not receive their cell products because they were not eligible for treatment, pursued other treatments, or are awaiting treatment.
CD19.CART Expansion and Persistence
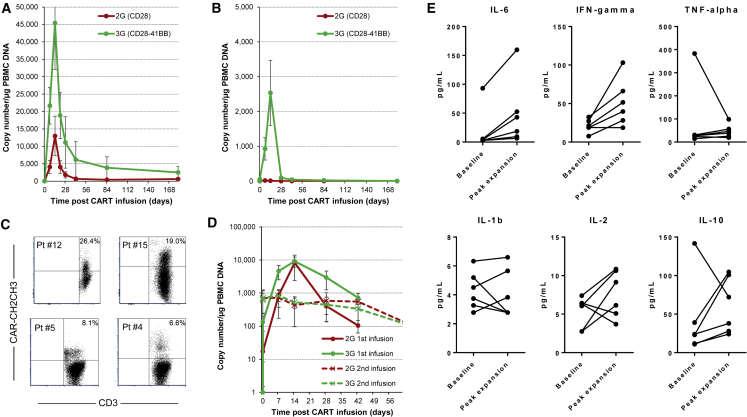
We consistently detected low level molecular signals for both 2G and 3G CD19.CARTs in the peripheral blood 3 hr after the first CART infusion, which increased to peak at 2 weeks post-infusion (Figures 1A and 1B). We observed the highest peak CART expansion in the patients with active disease (Figure 1A), in all but one of whom the 3G CARTs expanded (up to 40-fold) more than the 2G CARTs. At 2 weeks, we detected a mean of 45,383 ± 43,957 copies of the 3G vector/μg of genomic PBMC DNA (gDNA) versus 12,969 ± 18,801 copies of the 2G vector/μg gDNA (p = 0.002 for log area under the curve [AUC]). In samples with higher transgene levels, we were able to detect a distinct CAR+ T cell population by flow cytometry (Figure 1C). The transgene copy numbers then progressively declined to low but detectable levels by week 6, with the 3G product still being detected at a higher level than the 2G one. Four patients with active disease received a second infusion of CD19.CARTs that was not preceded by lymphodepleting chemotherapy. In these patients, we observed lower peak CART expansion levels compared to those seen after first infusion, with chemotherapy (Figure 1D), but the superiority of 3G over 2G vector was retained. In the surviving patients, molecular signals were still detectable 6 months after the last CD19.CART infusion, albeit at low levels compared to peak expansion. In these patients, the 3G CAR signal remained approximately one log higher than the signal from the 2G CAR (Figure 1A).
Figure 1.
In Vivo Expansion and Persistence of Infused CD19.CARTs in Peripheral Blood
Expansion was assessed by qPCR and is shown after one infusion of CD19.CARTs in patients with active disease (A), after lymphodepleting chemotherapy, and in remission post-autologous stem cell transplant (B), without lymphodepleting chemotherapy. (C) Representative flow cytometry data at peak (week 1 to 2) CD19.CART expansion in four patients (data were acquired in two instruments and thus axes’ scales differ slightly). The flow cytometry staining for the CD19.CARs cannot distinguish between 2G and 3G CARs, and thus the data represent the combined 2G and 3G circulating CARTs. (D) Summary of expansion data after the first (with lymphodepleting chemotherapy) and second (without preceding lymphodepleting chemotherapy) infusions for those patients who received two CD19.CART infusions. Data points represent critical post-infusion intervals after infusion of CD19.CARTs. The lines summarize the mean expansion and persistence (error bars, SEM). (E) Cytokine levels at baseline and during peak CART expansion in patients who had clinical evidence of possible CRS.
In patients who were in remission post-ASCT, we observed the same overall pattern of CART expansion (Figure 1B). Although peak levels were significantly lower than in patients with active disease (p < 0.0001 and p = 0.01 for log AUC, in 2G and 3G CARTs, respectively), the difference between the CD19.CART products was more striking in this setting: at 2 weeks, we detected an average of 2,534 ± 2,089 copies of the 3G vector/μg gDNA versus 13 ± 11 copies of the 2G vector/μg gDNA (p = 0.001 for log AUC).
Acute and Long-Term Toxicities
All infusions were well tolerated. The most frequent treatment emergent adverse events (Table 4) were cytopenias, which were attributable to the administration of lymphodepleting chemotherapy to patients who had previously been exposed to multiple cytotoxic agents. Other frequently reported adverse events included hypoalbuminemia, hyponatremia, fatigue, and nausea, all transient, which were likely related to the lymphodepleting chemotherapy, the underlying lymphoma, or other medications. Notable adverse events included three instances of grade 2 cytokine release syndrome (CRS),22 manifest by fever, mild tachypnea, and mild hypotension not requiring vasopressor support or tocilizumab use. Only one patient developed significant neurotoxicity (incontinence and aphasia), treated with tocilizumab and dexamethasone, with quick resolution of symptoms. Three other patients had possible grade 1 CRS. We generally observed modest increases in inflammatory cytokines at peak CART expansion compared to baseline levels even in patients with clinical evidence of CRS (Figure 1E). All patients had baseline B cell lymphopenia and polyclonal hypogammaglobulinemia resulting from prior treatment, which made assessment of new-onset B lymphopenia due to CD19.CARTs impractical. Long-term recovery of immunoglobulin levels continues to be studied in survivors.
Table 4.
Adverse Events Associated with Treatment Occurring in Two or More Patients
| Event | All Grades | Grades 1 and 2 | Grade ≥3 |
|---|---|---|---|
| Neutropenia | 12 | 2 | 10 |
| Leukopenia | 12 | 1 | 11 |
| Hypoalbuminemia | 11 | 9 | 2 |
| Anemia | 9 | 7 | 2 |
| Thrombocytopenia | 9 | 5 | 4 |
| Lymphopenia | 7 | 1 | 6 |
| Fatigue | 6 | 6 | – |
| Hypokalemia | 5 | 4 | 1 |
| Cough | 5 | 5 | – |
| Hypercalcemia | 5 | 5 | – |
| Constipation | 4 | 4 | – |
| Pain | 4 | 3 | 1 |
| Nausea | 4 | 4 | – |
| Elevated AST | 4 | 3 | 1 |
| Hyponatremia | 4 | 1 | 3 |
| Vomiting | 3 | 3 | – |
| Hyperkalemia | 3 | 2 | 1 |
| Diarrhea | 3 | 2 | 1 |
| Dyspnea | 3 | 3 | – |
| Hypernatremia | 3 | 3 | – |
| Anorexia | 3 | 3 | – |
| Muscle weakness | 3 | 2 | 1 |
| Febrile neutropenia | 2 | – | 2 |
| Extremity pain | 2 | 2 | – |
| Hypocalcemia | 2 | 1 | 1 |
| Increased creatinine | 2 | 2 | – |
| Limb edema | 2 | 2 | – |
| Nasal congestion | 2 | 2 | – |
| Headache | 2 | 2 | – |
| Peripheral neuropathy | 2 | 2 | – |
| Hematuria | 2 | 2 | – |
| Back pain | 2 | 2 | – |
| Skin hyperpigmentation | 2 | 2 | – |
Clinical Outcomes Post-CD19.CART Infusion
We observed clinical responses in 6 out of 11 patients with active disease: three complete responses (CRs) and three partial responses (PRs). Two of the patients (#2 and #4) who had a CR had history of indolent NHL transformed to diffuse large B cell lymphoma (DLBCL); the third (#16) had a de novo high-risk DLBCL. Patients #2 and #16 (Figures 2 and 3) developed grade 2 CRS but patient #4 (Figure 3) had no clinical evidence of CRS. Patients #2 and #4 have remained in CR for more than 12 months. All three PRs were short lived. Two patients (#1 and #12) had stable disease after CART infusion, one of whom (#12) is now in remission on a combination of rituximab and lenalidomide, to which she previously had an incomplete response. Four of the five patients treated in CR after HDT/ASCT have remained in continued CR for more than 9–12 months (Table 3).
Figure 2.
Patient #2 Achieved Durable CR after Infusion of CD19.CARTs
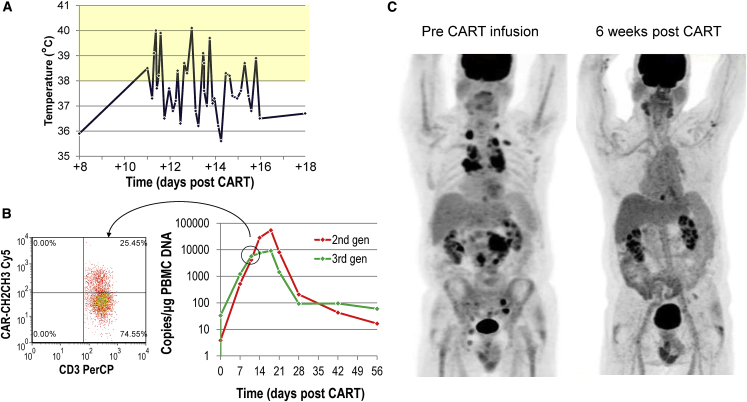
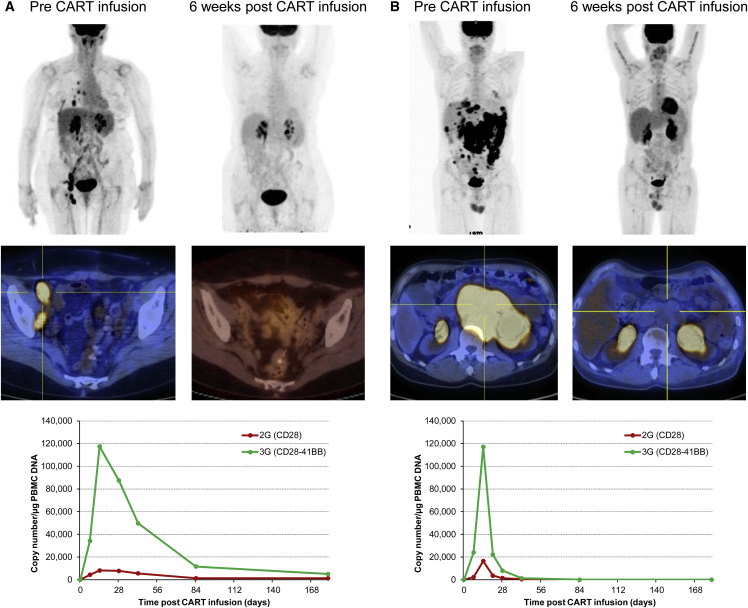
Patient #2 is a 67-year-old male who had primary refractory DLBCL arising in a background of follicular lymphoma and was unable to proceed to ASCT after two salvage regimens. He was given lymphodepletion followed by CART infusion and, 10 days later, he developed fever (A) and tachypnea and was admitted to the hospital, where all cultures remained negative. On admission, inflammatory markers, including CRP (peaking at 12.2 mg/dL on day 11) and IL-6 (peaking at 91.2 pg/mL on day 11 from 6.3 pg/mL at baseline) were elevated, consistent with mild CRS. At this point, we documented a 5-log expansion of his CART cells by genomic qPCR, corresponding to 25% of circulating T cells by flow cytometry (B). In contrast to all other patients, the 2G CAR-T cells ultimately expanded more. His symptoms resolved spontaneously in less than a week while receiving conservative treatment and a repeat PET scan obtained 6 weeks after CART infusion showed CR (C), which has been sustained for more than 18 months.
Figure 3.
Patients #4 and #16 Achieved CR after Infusion of CD19.CARTs
(A) Patient #4 is 64-year-old female with DLBCL transformed from ENMZL, which had relapsed a few months post-high-dose-therapy and ASCT. At this point, she received lymphodepleting chemotherapy followed by CART cells. Notably, she did not develop any clinically apparent CRS, but a repeat PET scan 6 weeks after infusion was consistent with CR, which has been maintained for more than 12 months. (B) Patient #16 is a 52-year-old male with de novo DLBCL, which relapsed 3 months after high-dose therapy and ASCT and was refractory to two second-line chemotherapy regimens and led to gastric outlet obstruction syndrome. The patient received lymphodepleting chemotherapy followed by CART cells, developed mild CRS within 1 week, and a repeat PET scan 6 weeks after infusion demonstrated a dramatic CR. Expansion data for each patient is shown on the bottom row.
We further analyzed antitumor immune responses in the recipients to seek evidence for newly emergent T cell-mediated immunity to additional tumor-associated antigens (TAAs). No evidence for such T cell “antigen spreading” was obtained, since we found no differences in the frequency of precursor T cells responding to known TAAs, such as NY-ESO, survivin, PRAME, or MAGE, in peripheral blood collected before and after CD19.CART infusion (data not shown).
Discussion
We report the first clinical trial directly comparing 2G with 3G CARTs targeting the same B cell marker (CD19) as a treatment for lymphoid malignancies. We found that adoptive transfer of 3G CARTs targeting CD19 is feasible and safe at all dose levels studied, which are lower than those used in most studies, a fact that may have contributed to limited toxicity. Additionally, we observed that the inclusion of 4-1BB in addition to CD28 is associated with superior expansion and persistence. The differences are particularly striking when these CD19.CARTs are administered to patients with no measurable disease but who are at high risk for relapse after autologous stem cell transplantation. These CD19.CARTs can lead to significant clinical responses, including sustained CRs, in patients with relapsed or resistant NHL.
The incorporation of costimulatory endodomains into CD19.CAR molecules is essential to provide proliferative and survival signals to the CARTs. Although B lymphocytes can express costimulatory ligands, we have previously demonstrated that only 2G CD19.CARTs containing the CD28 endodomain expanded in vivo in lymphoma patients receiving two CD19.CART products simultaneously, one with and the other without inclusion of CD28.2 It is now evident that adoptive transfer of T cells bearing 2G CD19-specific CARs containing either CD28 or 4-1BB costimulatory endodomains has remarkable clinical efficacy against B cell malignancies,8, 11, 13, 14, 15, 16, 17, 18, 19 but the optimal choice of costimulatory domains in these and other CARs remains controversial. CD28 and 4-1BB costimulation have distinct temporal and biochemical profiles, with CD28 signaling occurring immediately after TCR engagement, before subsequent activation of the 4-1BB pathway. While CD28 serves as a docking site for phosphoinositide-3 kinase (PI3K),23 4-1BB recruits tumor-necrosis-factor-receptor associated-factor (TRAF) adaptor proteins.24
Recently, Cheng and collaborators25 reported on the clinical responses and biological correlates of a phase 1 clinical trial treating seven acute lymphoblastic leukemia patients with the simultaneous infusion of two 2G products (with either a CD28 or a 4-1BB endodomain) targeting CD19. The in vivo expansion pattern of each CAR-T cell product was variable among patients and no consistent differences between products were observed. Nonetheless, preclinical models have demonstrated that inclusion of both CD28 and 4-1BB endodomains in so-called 3G CARs combine the effects of both signaling pathways and enhance effector function, expansion, and survival of CARTs. Indeed, preclinical assessment of these 3G CD19.CARTs showed higher degree of intracellular signaling activity compared to the 2G CD19.CARTs.21 In addition, while recent evidence suggests that single costimulation through either CD2826 or 4-1BB27 may have deleterious effects on CARTs by leading to ligand-independent tonic signaling, coexpression of both CD28 and 4-1BB may compensate for the negative effects of single signaling.26, 27 The clinical data reported here show that expansion and persistence of 3G CD19.CARTs are superior to 2G CD19.CARTs in vivo, without potential biases resulting from the comparison of distinct CARTs in different patients. This difference was particularly striking in patients with minimal residual disease and low numbers of circulating normal CD19+ B cells, a patient subset for whom the benefits of 2G CD19 CAR may be limited by their poor expansion and persistence, and the resulting small area under the curve.
It is generally accepted that CD28 costimulation promotes rapid expansion of CARTs upon antigen engagement, while 4-1BB costimulation is critical in promoting their long-term persistence.20 Our direct comparison of 2G versus 3G CD19.CARTs in lymphoma patients with active disease suggests that the inclusion of 4-1BB into 3G CD19.CARTs does not hamper the expansion kinetics observed for 2G CD28-containing CD19.CARTs, but rather increases the magnitude of their expansion. In fact, both 2G and 3G CD19.CARTs showed peak expansion 2 weeks after infusion, but CAR copy numbers were much higher for the 3G CD19.CARTs. On the other hand, the presence of the CD28 in 3G CD19.CARTs does not appear to compromise the ability of 4-1BB to support long-term persistence of CD19.CARTs, as reported in other clinical studies using only 4-1BB.28 Indeed, in our direct comparison, 3G CD19.CARTs remained detectable at higher levels up to day 160 post-infusion as compared to 2G CD19.CARTs. Remarkably, when 2G and 3G CD19.CARTs were infused in patients in remission after ASCT, a setting in which the cognate CD19-expressing cells are absent or minimally present, only 3G CD19.CARTs showed sizeable expansion at 2 weeks. This antigen-independent expansion of 3G CD19.CARTs may be attributed to the increased numbers of central memory CD4+ and CD8+ T cells in the 3G CD19.CARTs but can also be explained by the previously described antigen-independent proliferation in vitro of CD19.CARTs encoding the 4-1BB alone.27 Overall, combining CD28 and 4-1BB within the same CD19.CAR may preserve the physiological effects of each costimulatory molecules when used as a single domain while mitigating detrimental effects of either costimulation alone.
Despite high expansion and significant clinical responses, the rates of severe toxicities in the active disease cohort were acceptable, and tocilizumab was used in just one patient, to treat neurotoxicity. Patients had responses (including CR) even with no clinical evidence of CRS, showing that responses can occur even in the absence of significant CRS. Although we investigated whether responses correlated with CD19.CART dose infused, CART expansion, and blood cell counts at infusion (total white blood cell, absolute lymphocyte, and absolute neutrophil count), we could not demonstrate any significant association between these parameters and response (data not shown).
Even with these encouraging results, many potential barriers remain in the path to universal responses to CART therapy. First, loss or modulation of expression of the targeted antigen is a common mechanism of tumor escape, which has already been documented in patients treated with 2G CD19.CARTs.29 This escape mechanism may be particularly important with CARTs, since epitope spreading to recruit other cytotoxic T cells has not yet been clearly demonstrated, in contrast to the epitope spreading observed with native TCR-mediated tumor killing.30, 31 Certainly, we found no evidence of cytotoxic T cell epitope spreading in the patients treated in this study. The absence of epitope spreading to recruit additional cytotoxic responses may limit the generation of a sustained and broad enough immune response to prevent antigen editing, tumor escape, and incomplete eradication of tumors. Thus, the ability to obtain substantial expansion of 3G CD19.CARTs even in minimal residual disease may be of value for avoiding relapse by CD19-negative variant cells, since these will likely be more numerous in bulky compared to minimal disease.
In conclusion, our comparison study of autologous 2G with 3G CD19.CARTs shows that this strategy produces antitumor activity while causing manageable toxicities and that the superior expansion and persistence of the 3G vector may make it particularly suited to the eradication of minimal residual disease.
Materials and Methods
Clinical Study
We conducted a phase 1 study (NCT01853631) designed to assess the feasibility and safety of treating patients with relapsed or refractory aggressive or highly aggressive CD19-positive lymphoproliferative disorders with escalating doses of autologous, polyclonally activated, peripheral blood T cells that were genetically modified to express a CD19-specific CAR (CD19.CAR). We infused two cell products simultaneously in each patient, one transduced with a second-generation CD19-CAR (2G) containing one costimulatory domain (CD28) and another with a 3G CD19-CAR (3G) having both CD28 and CD137 (4-1BB) costimulatory domains. Both constructs included the CD3ζ endodomain. We treated patients in one of two settings: with measurable disease at the time of infusion (active disease cohort) or in radiological remission after high-dose therapy and autologous stem cell transplant (post-ASCT cohort) (Figure 1). We gave patients in the active disease cohort lymphodepleting doses of cyclophosphamide (500 mg/m2) and fludarabine (30 mg/m2) daily for 3 days, finished 24 to 72 hr before administration of CD19.CARTs. We administered three dose levels of CD19.CARTs, 1 × 106 (dose level 1), 5 × 106 (dose level 2), and 2 × 107 (dose level 3) CAR+ cells/m2 of each CART population, with total cell numbers adjusted for the percentage of CAR+ cells in the final products. We infused both cell populations intravenously over 2–5 min with a 5- to 10-min interval between them. We used an interpatient dose escalation that followed a continual reassessment method, which required safety to be demonstrated 6 weeks after infusion in two patients at each dose level before escalation. We allowed additional infusions at least 6 weeks apart of the same dose of CD19.CARTs without lymphodepleting chemotherapy as long as there was evidence of clinical benefit, defined as at least stable disease. We performed clinical and laboratory evaluation at weeks 1, 2, 4, and 6, months 3, 6, 9, and 12 post-CART infusion, and yearly thereafter. We graded adverse events during and after T cell infusions according to the NIH Common Terminology Criteria for Adverse Events (CTCAE), version 4 (https://ctep.cancer.gov/). We assessed responses by imaging and pathology studies, as applicable, at week 6 after CART infusion. For NHL, we defined complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to the revised response criteria for malignant lymphomas of the International Working Group.32 For patients who had exclusively bone marrow disease at the time of treatment, we defined CR as having no tumor cells detectable on bone marrow aspirate and biopsy, PR as at least halving the amount of tumor in the marrow compared to the level present at study entry, PD as doubling of the amount of tumor in the marrow, and SD as not meeting criteria for any of the other categories.
Generation of Retroviral Constructs
We have previously described the 2G vector.2 We cloned an identical single chain variable fragment (scFv) to generate the 3G CAR construct containing both CD28 and 4-1BB (CD137) endodomains, including a spacer region derived from the human immunoglobulin G1 (IgG1)-CH2CH3 domains in frame between the scFv and the signaling domains to facilitate detection by phenotypic analysis of the transgenic product. We then cloned this cassette into the SFG retroviral backbone. We generated the clinical grade packaging cell line using PG13 cells (gibbon ape leukemia virus pseudotyping packaging cell line; American Type Culture Collection CRL-10686).33 We used the highest-titer clones to establish master cell banks and released them for clinical use only after safety testing and vector sequencing. We stored the final viral supernatant at −80°C and tested it before clinical release.
Generation and Transduction of CD19.CARTs
To generate both CD19.CART products (2G and 3G), we transduced, independently but in parallel, PBMCs prepared by Ficoll density centrifugation of peripheral blood obtained by phlebotomy 1 to 3 months before CART administration. We activated PBMCs in 24-well plates coated with CD3 and CD28 antibodies (Miltenyi Biotec, Cambridge, MA) and containing interleukin-7 (IL-7) (10 ng/mL, R&D Systems, Minneapolis, MN) and IL-15 (5 ng/mL, R&D Systems, Minneapolis, MN), for 24 to 72 hr. At the time of transduction, we transferred the activated PBMCs to 24-well plates coated with a recombinant fibronectin fragment (FN CH-296, Retronectin Takara, Clontech, Mountain View, CA). After transduction, T cells were expanded ex vivo in the presence of IL-7 (10 ng/mL) and IL-15 (5 ng/mL), added twice a week.34 Generation of each of the 56 CD19.CART products required a median of 10 days (range 9–14 days) of culture.
Immunophenotyping
We used phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, peridinin chlorophyll protein (PerCP)-, or allophycocyanin (APC)-conjugated CD3, CD4, CD8, CD56, CD19, TCR-αβ, TCR-γδ, CD62L, CD27, CD28, CD45RA, CD45RO, CCR2, CCR4, CCR5, CCR7, CXCR3, CXCR4, CD162, CD54, CD38, CD106, CD11a, CD11c, and CD18 (Becton Dickinson-PharMingen, San Diego, CA) antibodies to stain the T cell products or tumor cells. We included control samples labeled with appropriate isotype-matched antibodies in each experiment. We detected the CD19.CAR with an Fc-specific Alexa Fluor 647-conjugated goat polyclonal F(ab’)2 fragment anti-human IgG (Jackson ImmunoResearch, West Grove, PA), which recognizes the IgG1-CH2CH3 component of the CAR. We analyzed the cells by FACScan or FACSCanto II (Becton Dickinson) equipped with a filter set for four fluorescence signals, using CellQuest software. Data analysis was performed using FlowJo (Treestar, Ashland, OR) or Kaluza (Beckman Coulter, Indianapolis, IN) software.
Cytotoxicity Assays
The cytotoxic specificity of each T cell line was measured in a standard 4-hr 51Cr release assay, using effector-to-target (E:T) ratios of 40:1, 20:1, 10:1, and 5:1. As targets, we used Daudi and Raji (CD19+ tumor cells), HDLM-2 (CD19− tumor cells), and K562 (natural killer [NK]-sensitive) cells. Target cells were labeled simultaneously for 1 hr with 51Cr. We calculated the percentage of specific lysis as ([experimental release − spontaneous release]/[maximum release − spontaneous release]) × 100.
Real-Time qPCR of CD19.CAR Transgene
We quantified the integrated genome of the retrovirus encoding the CD19.CAR by real time qPCR.2 After extracting DNA from peripheral blood samples with the QIAamp DNA Blood Mini Kit (QIAGEN, Hamburg, Germany), we amplified the DNA with specific primers and TaqMan probes (Applied Biosystems, Foster City, CA) complementary to specific sequences within the 2G and 3G retroviral vectors.35 We performed amplifications using the ABI7900HF (Applied Biosystems) cycler. The baseline range was set at cycles 6–15, with the threshold at 10 standard deviations above the baseline fluorescence. The standard curve was established using serial dilutions of the plasmid encoding the transgene. DNA integrity was assessed using a PCR assay for the β-actin gene.
Cytometric Bead Array and Multiplex Assays
We analyzed plasma or serum samples collected before and after CD19.CART infusion using a BD cytokine cytometric bead array (CBA) kit (Becton Dickinson-PharMingen) or a Milliplex kit (EMD Millipore, Billerica, MA). In parallel with the samples, we used the human cytokine standards provided with the kit to prepare standard curves. We ran the CBA assays using the FACS Calibur (Becton Dickinson-PharMingen) and analyzed data from the Milliplex kits using the Luminex 200 System and the Milliplex Analyst Software (EMD Millipore).
Statistics
We used descriptive statistics (means, median, ranges, and standard deviations, or standard errors) to summarize the data. We calculated the area under the curve (AUC) for CAR-T cell expansion using the trapezoidal rule. A log transformation was performed to achieve normality if appropriate. Student’s t test was used for between-group comparisons, while the paired t test was applied for within-subject comparisons. A p value less than 0.05 was considered statistically significant.
Study Approval
This study was approved by the United States Food and Drug Administration, the Recombinant DNA Advisory Committee, and the Institutional Review Board of Baylor College of Medicine and was conducted in accordance with the Declaration of Helsinki. All participants gave informed consent on enrollment.
Author Contributions
C.A.R., R.R., B.G., C.M.R., H.E.H., M.K.B., B.S., and G.D. conceptualized the overall strategy and developed its clinical translation and implementation. The clinical protocol was written by C.A.R. and M.K.B. C.A.R. was the principal investigator of the protocol, and B.G., M.K.B., and H.E.H. were the IND sponsors. Manufacturing of T cells, flow cytometry, and qPCR acquisition of clinical samples was performed by N.N., G.V., B.M, H.Z., and O.D. supervised by C.A.R. and B.S., and directed by A.P.G. and Z.M. The manuscript was written by C.A.R., M.K.B, B.S., and G.D., and all authors discussed and interpreted the results. M.-F.W. and H.L. performed statistical analyses. C.A.R., G.C., and R.T.K. enrolled patients to the protocol and/or managed the patients, and A.R. and C.S.R. were the study coordinators, assisting with enrollment, sample acquisition, and data safety monitoring of patients.
Conflicts of Interest
While this clinical trial was being conducted, the Center for Cell and Gene Therapy at BCM had a Collaborative Research Agreement with Celgene Corporation and Bluebird Bio.
Acknowledgments
We thank the following for financial support: the NIH National Cancer Institute (grant 3P50CA126752) and the Leukemia and Lymphoma Society Specialized Center of Research (grant 7018). The clinical trial also received support from shared resources of the Dan L. Duncan Comprehensive Cancer Center support grant 5P30CA125123 and the National Gene Vector Biorepository.
Contributor Information
Carlos A. Ramos, Email: caramos@bcm.edu.
Gianpietro Dotti, Email: gdotti@med.unc.edu.
References
- 1.Ramos C.A., Heslop H.E., Brenner M.K. CAR-T Cell Therapy for Lymphoma. Annu. Rev. Med. 2016;67:165–183. doi: 10.1146/annurev-med-051914-021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savoldo B., Ramos C.A., Liu E., Mims M.P., Keating M.J., Carrum G., Kamble R.T., Bollard C.M., Gee A.P., Mei Z. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kochenderfer J.N., Wilson W.H., Janik J.E., Dudley M.E., Stetler-Stevenson M., Feldman S.A., Maric I., Raffeld M., Nathan D.A., Lanier B.J. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I., Stetler-Stevenson M., Phan G.Q., Hughes M.S., Sherry R.M. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer J.N., Dudley M.E., Carpenter R.O., Kassim S.H., Rose J.J., Telford W.G., Hakim F.T., Halverson D.C., Fowler D.H., Hardy N.M. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P., Carpenter R.O., Stetler-Stevenson M., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster S.J., Svoboda J., Chong E.A., Nasta S.D., Mato A.R., Anak Ö., Brogdon J.L., Pruteanu-Malinici I., Bhoj V., Landsburg D. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J.H., Rivière I., Gonen M., Wang X., Sénéchal B., Curran K.J., Sauter C., Wang Y., Santomasso B., Mead E. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Stegen S.J., Hamieh M., Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 2015;14:499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlsson H., Svensson E., Gigg C., Jarvius M., Olsson-Strömberg U., Savoldo B., Dotti G., Loskog A. Evaluation of Intracellular Signaling Downstream Chimeric Antigen Receptors. PLoS ONE. 2015;10:e0144787. doi: 10.1371/journal.pone.0144787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee D.W., Gardner R., Porter D.L., Louis C.U., Ahmed N., Jensen M., Grupp S.A., Mackall C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esensten J.H., Helou Y.A., Chopra G., Weiss A., Bluestone J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity. 2016;44:973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croft M. The TNF family in T cell differentiation and function--unanswered questions and future directions. Semin. Immunol. 2014;26:183–190. doi: 10.1016/j.smim.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng Z., Wei R., Ma Q., Shi L., He F., Shi Z., Jin T., Xie R., Wei B., Chen J. In Vivo Expansion and Antitumor Activity of Coinfused CD28- and 4-1BB-Engineered CAR-T Cells in Patients with B Cell Leukemia. Mol. Ther. 2018;26:976–985. doi: 10.1016/j.ymthe.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long A.H., Haso W.M., Shern J.F., Wanhainen K.M., Murgai M., Ingaramo M., Smith J.P., Walker A.J., Kohler M.E., Venkateshwara V.R. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 2015;21:581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes-Silva D., Mukherjee M., Srinivasan M., Krenciute G., Dakhova O., Zheng Y., Cabral J.M.S., Rooney C.M., Orange J.S., Brenner M.K., Mamonkin M. Tonic 4-1BB Costimulation in Chimeric Antigen Receptors Impedes T Cell Survival and Is Vector-Dependent. Cell Rep. 2017;21:17–26. doi: 10.1016/j.celrep.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mueller K.T., Maude S.L., Porter D.L., Frey N., Wood P., Han X., Waldron E., Chakraborty A., Awasthi R., Levine B.L. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130:2317–2325. doi: 10.1182/blood-2017-06-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sotillo E., Barrett D.M., Black K.L., Bagashev A., Oldridge D., Wu G., Sussman R., Lanauze C., Ruella M., Gazzara M.R. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bollard C.M., Gottschalk S., Torrano V., Diouf O., Ku S., Hazrat Y., Carrum G., Ramos C., Fayad L., Shpall E.J. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J. Clin. Oncol. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leen A.M., Tzannou I., Liu H., Vera J.F., Gerdemann U., Kamble R.T., Ramos C.A., Grilley B., Gee A.P., Bollard C.M. Immunotherapy for Lymphoma Using T Cells Targeting Multiple Tumor-Associated Antigens. Biol. Blood Marrow Transplant. 2016;22(Suppl):S44–S45. [Google Scholar]
- 32.Cheson B.D., Pfistner B., Juweid M.E., Gascoyne R.D., Specht L., Horning S.J., Coiffier B., Fisher R.I., Hagenbeek A., Zucca E., International Harmonization Project on Lymphoma Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 33.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V., Dotti G., Huls M.H., Liu E., Gee A.P., Mei Z. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y., Zhang M., Ramos C.A., Durett A., Liu E., Dakhova O., Liu H., Creighton C.J., Gee A.P., Heslop H.E. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123:3750–3759. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vera J., Savoldo B., Vigouroux S., Biagi E., Pule M., Rossig C., Wu J., Heslop H.E., Rooney C.M., Brenner M.K., Dotti G. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]