Abstract
Locally advanced uterine cervical cancer continues to present a high number of pelvic relapses. Intraoperative radiation therapy (IORT) allows a precise therapeutic intensification in the surgical area in cases in which removal of the tumour recurrence is feasible. At the same time, IORT excludes the radiosensitive organs from the field of irradiation. While the first gynecological IORT took place in 1905, procedures have been limited over the years and the series are retrospective, including few patients. At the same, time recurrences are located at different pelvic areas. Both heterogeneity and the long recruiting time make it difficult to correctly interpret the published results. Despite this, we have reviewed the most relevant publications. Some institutions indicated IORT as a boost on the surgical bed of the excised tumor recurrence. In others, IORT permits an extra radiation dose after radical surgery of the primary tumor, usually in stage IIB. Most studies conclude that the addition of IORT increases the local control but probably with little impact on survival. On the other hand, there is a controversy in the indication of IORT in surgically resectable primary tumours. No clear advantage over the usual scheme of chemoradiation and brachytherapy has been detected. Randomized studies that allow a breakthrough in the conclusions are highly unlikely to be performed in this area.
Keywords: Intraoperative radiotherapy, Cervix cancer, Cervical cancer recurrences
1. Background
IORT (intraoperative radiation therapy) is a boosting technique which delivers a single high dose fraction of radiation directly to the resection bed during surgery. The goal is to selectively irradiate anatomical areas that have been identified as high risk of persistence of subclinical disease or even macroscopic inextirpable residual disease. IORT permits, at the same time, to protect or avoid surrounding organs or structures at risk (OAR) because they are radiosensitive. This allows a good protection of pelvic organs like the bladder, rectum, bowel, etc. and, consequently, a decrease in the incidence of enteritis, rectitis or cystitis. IORT can be delivered using a dedicated linear accelerator producing electron beams of different energies and penetration degrees. X-rays sources delivering low-energy radiation or high dose-rate brachytherapy sources can also be conveniently used for IORT procedures in gynaecological tumours, primary or recurrent.
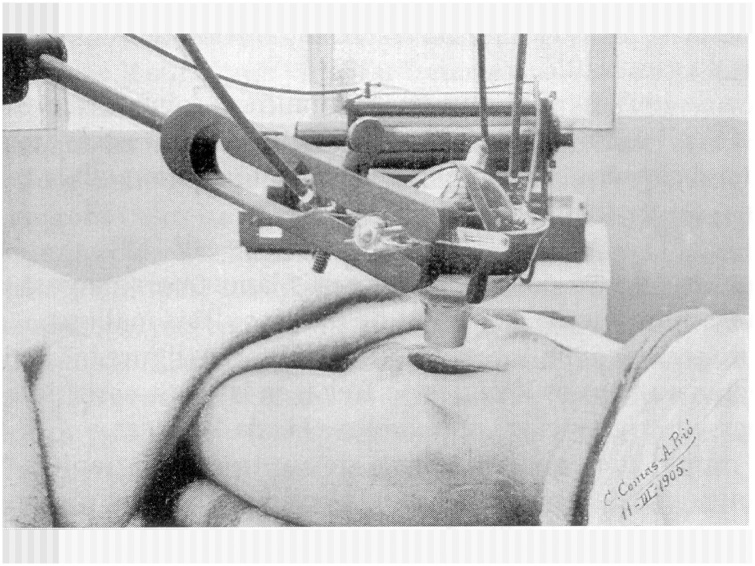
In the Radiation Oncology literature, the first description of an IORT procedure has been constantly attributed to Beck.1 Some years ago, an IORT treatment in the gynaecologic area has been documented in Barcelona by Casas et al.2 Comas and Prio3 reported the case of a 33 year old woman diagnosed with a cervical squamous cell carcinoma treated by radical surgery and intrapelvic roentgen therapy to the parametria. The patient survived at least 6 years after the treatment was completed (Fig. 1). Results were very limited for much of the century, but with the introduction of megavoltage linear accelerators and later specifically designed units, reports of IORT delivery procedures began to be published.
Fig. 1.
Original picture of the first published IORT treatment. The patient was irradiated to the distant parametrial area and survive at least seven years. The image is signed by Drs. C. Comas and A. Prio. Barcelona, 1905.
IORT has been used in the primary management, as well as in the salvage setting, for many solid tumours in different locations. It is estimated that the biological effect (RBE, relative biological efficacy) of this single large dose is equivalent to up to 2–3 times the dose delivered with conventional external beam radiotherapy. There probably exists an additional benefit of diminishing the release of cell-growth stimulating cytokines. This has been well reported by Belletti4 and later by Zaleska et al.5
Two reviews have previously been published on IORT in gynaecological tumours. The first one, from Backes and Martin,6 comprises all gynaecologic malignancies, including separate sections devoted to uterine primary tumours and recurrent cervical cancer. A total number of 276 cases of cervical cancer (primary and recurrent) were collected. The main conclusion is that if the surgical margins are positive or close, IORT appears to increase local control and has an acceptable toxicity profile. The second one, recently published by Krengli et al.,7 focuses on endometrial, cervical, renal, bladder and prostate cancers. A total amount of 153 patients (primary and recurrent cervical cancer) from 4 studies are analysed in detail. They conclude as follows: In recurrent cervical cancer from this studies, it emerged that the status of the margins is the most important risk factor for treatment and the association of IORT seems to improve the probability of local control. In contrast, Krengli et al.7 do not recommend surgery and IORT for primary tumours. They state: “The available data suggests that this aggressive strategy is not advantageous in particular for the risk of severe side effects and that concomitant radio-chemotherapy alone should be considered the best treatment strategy in this patient setting”.
In the present review we are going to try to separate the results for primary tumours from those for recurrent cervical tumours.
2. Intraoperative radiotherapy in cervical locally advanced primary tumours
For many years, locally advanced uterine cervical tumours have been treated by a chemoradiation approach, including, if possible, brachytherapy. Although in more than half of the patients the results are satisfactory, at least in one third of them the tumour persists or a local or nodal recurrence appears. For this reason, a therapeutic intensification associating radical surgery and IORT has been tested. This novel approach has been used mostly in FIGO stages IIB. (Table 1).
Table 1.
Selected studies of the use of IORT (intraoperative radiotherapy) for gynecologic malignancies. OS: overall survival; DFS: disease free survival; LC: local control.
| Year | Reference | N | Classification | IORT median dose and range | 5 y OS | 5 y DFS | 5 y LC |
|---|---|---|---|---|---|---|---|
| 1995 | Stelzer21 | 22 | 22 (14–27) Gy | 43% | – | 48% | |
| 1996 | Mahe15 | 70 | Recurrent | 18 (10–25) Gy | 8% (3 y) | – | 30% |
| 2001 | Martinez-Monge8 | 36 | Recurrent | 15 Gy | 14% | 16% | 42% |
| 31 | Primary | 12 Gy | 67% | 70% | 79% | ||
| 2002 | Liu12 | 97 | Primary (IIB) | 19 (18–20) Gy | 88% | – | – |
| 2007 | Tran18 | 17 | Recurrent | 11.5 (6–17.5) Gy | 76% | – | 44% |
| 2011 | Giorda9 | 35 | Primary | 11 (10–15) Gy | 49% | 46% | 89% |
| 2013 | Gao11 | 27 | Primary | 19 (18–20) Gy | 78% | 70% | 100% |
| 2013 | Barney16 | 73 | Recurrent | 15 (6–25) Gy | – | 31% (both) | 61% |
| 13 | Primary | 70% | |||||
| 2014 | Foley10 | 21 | Recurrent | 13.5 (10–22.5) Gy | 69% | 30% | 59% |
| 2014 | Backes19 | 21 | Recurrent | 17.5 (10–20) Gy | 30% | – | 59% |
| 2014 | Sole17 | 31 | Recurrent | 12.5 (10–15) Gy | 42% | 44% | 65% |
| 2016 | Arians20 | 18 | Recurrent | 15 (10–18) Gy | 6% | – | 44% |
Martinez-Monge et al.8 reported in 2001 a series of 31 patients recruited from 1986 to 1999 presenting a locally advanced but resectable cervix carcinoma. After chemoradiation with cisplatin and 5 Fu and 45 Gy RT (Radiotherapy) dose, surgery plus electron IORT was performed. The median IORT dose was 12 Gy (range 10–25 Gy) focused on higher risk areas (mainly pelvic walls). The mean field size was 6.5 cm (range 5–12 Gy) and the beam energies 9 or 12 MeV, The authors reported a 10 year 92.8% control rate “in field”. The 10-year probability of pelvic control reached 78.6%. Toxicity presumably attributable to IORT was detected in 14% of patients, mainly transient pelvic pain or neuropathy in one case. They concluded than IORT with electrons was a valuable radiation boost technique in advanced but still resectable cervix carcinoma.
Giorda et al.9 conducted a phase II study including 42 patients (stages IIB-IVA) between 2000 and 2007. They were treated with external pelvic radiotherapy (50.4 Gy/28 fr) and chemotherapy (cisplatin and 5FU weeks 1 and 5). No brachytherapy was added. Surgery with radical hysterectomy and pelvic lymphadenectomy was performed later (6–8 week). During the surgery, IORT was delivered to the parametria, pelvic sidewalls obturator fossa and iliac vessels. In case of positive nodes or macroscopic residual tumour, IORT was extended to treat the area. After chemoradiation, 83% of patients underwent radical surgery and IORT. At pathologic examination, 8/35 (23%) patients showed complete response, but in 10/35 cases gross residual disease persisted. IORT was delivered to the bilateral pelvic wall in 82% of patients, to the mono-lateral pelvic wall in 11%, to the bilateral pelvic wall and central pelvis in one case and to the bilateral pelvic wall and para-aortic area also in one patient. The mean delivered dose was 11 Gy (range from 10 to 15 Gy) and the mean diameter of irradiation field was 6.3 cm (range from 5.7 to 8.3 cm). It is not specified but we presume that IORT was administered using an electron beam from a linear accelerator. Five-year disease-free survival (DFS) was 46% and overall survival (OS) 49%. Median time to recurrence was 22 months. Chemoradiation was well tolerated but peri- and post-operative complications occurred frequently. 3/15 deaths were due to septic pelvic infections. There were no clearly related complications due to IORT administration, but it is hard to discriminate from other causes. Authors concluded that this treatment approach seemed to be active in a subgroup of patients with pathological complete response to treatment or partial response with residual tumour limited to the cervix. In our opinion, it is very difficult in this study to make any kind of statement about the role and efficacy of IORT.
It is also difficult to analyse it in the article by Foley et al.10 The authors presented a series of 32 patients treated with IORT over 17 years (from 1994 to 2011). Among them, we identify 21 (65.6%) with a diagnosis of cervical cancer. It is not clearly specified which cases are locally advanced primary tumours or recurrent after previous surgery. An important data is that in 84.4% of the patients, surgical resection margins were microscopically positive and in 15.6% macroscopically identifiable disease persisted. The mean IORT (electron beam) dose was 13.5 Gy (range, 10–22.5) increasing the dose according to the suspected or detected amount of local persistent disease. The mean cone size used was 6.6 cm (range, 4–10 cm). The irradiated areas were the pelvic side-wall in 59.4%, para-aortic region and central pelvis in 21.8% and 18.8% patients, respectively. The authors are not able to identify specific complications due to the IORT procedure. They insist that the low rate of neuropathy (only one case but grade III) is due to the limit in IORT dose delivered to major nerves of 12.5 Gy. As a general conclusion, Foley et al. suggest that IORT may benefit patients with locally advanced or locally recurrent gynaecologic cancers in whom complete resection of disease is feasible at the time of IORT. Nevertheless, as usual, they insist on the convenience to design and execute clinical trials permitting to adequately assess this suggested benefit.
A more specific study was conducted by Gao et al.11 published in 2013. The authors reported the results of a retrospective series of 27 women presenting an adenocarcinoma of the uterine cervix in stage II. The rationale is based on the worse prognosis of this histology in comparison to squamous cell carcinoma. Between 1999 and 2002, 27 women with cervix adenocarcinoma and FIGO stage IIB were enrolled and treated as follows: they underwent weekly intracavitary brachytherapy (two insertions) total dose HDR 12–14 Gy point A. After a break of 1–2 weeks, they underwent a simple hieterectomy and selective lymphadenectomy. A dose of 18–20 Gy was delivered intraoperatively using 12 MeV electron beam. A unique large field of 10–12 cm in diameter was used, shifting out or protecting the bladder, intestines, sigma and rectum. The portion of the obturator nerve in the pelvis was partially shielded. Two weeks after the IORT chemotherapy was implemented with cisplatin and 5FU completing a total of 4–6 courses. The surgical margins were positive in 2/27 cases and close in 6/27. The mean follow-up time was 81 months and the 5-year OS and DFS rates in all 27 patients were 77.8% and 70.4%, respectively. Local relapse was detected in 2/27 patients (7.4%), but none within the IORT field. Local control in field was 100% whether or not the resection margins were positive or close. IORT was well tolerated and the major complication observed was peripheral neuropathy which two patients (7.4%) developed after 8 and 17 months. The authors concluded that IORT was safe, feasible and appeared to confer a disease control benefit to surgical resection in locally advanced cervix adenocarcinoma.
A similar therapeutic approach to that previously described in adenocarcinoma was used some years before (2002) by a Chinese work published by the same authors.12 IORT was delivered in a series of 97 patients presenting cervical carcinoma. The electron energy selected was 12 MeV and the dose to the pelvic field ranged between 18 and 20 Gy. This schedule was applied to stage IIB patients and the 5-year survival rate achieved was 95%. Authors concluded that IORT provided a new therapy method for cervical carcinoma stage IIB, especially for adenocarcinoma.
3. IORT in recurrent carcinoma of the uterine cervix
Following the publication of two short series, Garton et al.13 and Kinney et al.,14 comprising 19 and 14 patients, the French multicentre series was the largest trial published until 1996.15 Between 1985 and 1993, 70 patients received IORT for pelvic recurrence of cervical carcinoma in seven institutions. In most of the patients (84%), the location of the pelvic failure was the side wall and the central pelvis alone in 16%. A great variability in surgical procedures occurred, from biopsy alone to pelvic exenteration. IORT was performed using 100 kV photons in 5 patients and electron beam in 65. Circular cones with 10–20° bevels and a mean diameter of 73 cm were used in most cases (95%). The mean follow-up after IORT was 15 months and at the time of the report, 78% of the patients had died: 43% with local failure and 27% with local failure and metastases. The overall survival (OS) at 1, 2 and 3 years was 47, 17 and 8%, respectively, and the local control rate 21%. Reported directly IORT-related toxicities were peripheral neuropathy (5/70 cases) and ureteral stenosis (4/70). Conclusions of the study are that IORT is feasible, but it cannot dramatically improve prognosis.
Barney et al.16 published in 2013 a study conducted at the Mayo Clinic reviewing the results of a retrospective series of 86 patients from 1983 to 2010. 13 (15%) were diagnosed of locally advanced and 73 (85%) of locally recurrent cervix carcinoma. All patients in this study were treated using high energy electrons from a linear accelerator. The median dose prescribed to the 90% isodose was 15 Gy (range: 6.25–25 Gy) and the most selected beam energies were 9 and 12 MeV. The median cone size diameter was 7 cm (range: 5–15 cm). Multivariate analysis revealed pelvic exenteration and perioperative IORT to be associated with improved local control. Directly related IORT toxicity was peripheral neuropathy. 16 patients (19%) experienced some degree of it, but only one grade 3. The authors concluded from this mixed study that, if possible, IORT should be strongly considered, even in previously irradiated patients, as the delivery of perioperative RT was associated with improved loco-regional control.
Sole et al.17 published a study on 62 oligometastatic gynaecologic tumours. Among them, we identified 31 cervical recurrent cancers. They were treated with surgery and electron IORT. The mean dose given was 12.5 Gy (range 10–15 Gy) using a median energy of 12 MeV. Lucite circular applicators ranged from 5 to 12 cm in diameter, bevelled if necessary. Reported 5-year OS, DFS and Local control were 42%, 44% and 65%, respectively. No IORT specifically related toxicity was reported.
Tran et al.18 conducted in 2007 a study reporting the results of IORT for recurrent gynaecologic malignancies. In this retrospective series, comprising 36 consecutive patients from 1986 to 2005, 17 (47%) were cervix tumours. IORT was delivered using an orthovoltage unit. X-rays 200–250 kV, 0.57–2.45-mm copper half value layer directly focused over the tumour bed. Circular cones with diameters of 2.5–10 cm and bevels from 0° to 45° were used. Median dose was 11.5 Gy (6–17.5). Location details for the cervix subgroup are not specifically reported. The mean follow-up of the living patients was 50 months. The 5 year probability of local control was 45% and disease free survival (DFS) was 46% for the cervix cancer recurrence subgroup. The local control rate was clearly superior in comparison to 21% reported by the French study, but one reasonable explanation is the large difference in the rate of pelvic sidewall location: 84% in the French study and 32% in the Stanford one. The sidewall location is well known and accepted to have the worst prognosis if compared to the central pelvis. They have a low incidence of IORT-related complications attributed to the policy of limiting the IORT dose to major nerves lower than 12.5 Gy and making every effort not to irradiate the ureters. The main reported conclusion is that survival for pelvic recurrence of gynaecological cancer is poor, but IORT after surgery seems to confer long-term local control in carefully selected patients.
Backes et al.19 published in 2014 an article investigating if the addition of IORT to pelvic exenteration for gynaecologic cancer recurrences improved survival. 32 patients were identified in this retrospective study. The majority of them had recurrent cervical cancer (21, 66%). The IORT doses ranged from 10 to 20 Gy (median 17.5 Gy). Electron energies ranged from 6 to 12 MeV and the dose was prescribed to the 90% isodose line. The main difficulty regarding the results of this series was that only 66% received IORT during the surgery and neither the amount of cervical tumours nor the specific local control rates achieved were reported. The broad conclusion is that IORT fails to change survival and recurrence outcomes. But, and it is very important, authors report that patients with clinical indications at the time of pelvic exenteration have a worse prognosis compared to those who do not require IORT. Then, it is reasonable to conclude that, as the local recurrence rate is similar in the two subgroups (36 and 31%), IORT contributes significantly to the rise in local control.
At our best knowledge, the most recent published article is a German one from Heidelberg (Arians et al.).20 A series of 36 patients with recurrent gynaecological malignancies, including 18 cervical cancers, were enrolled between 2002 and 2014. They underwent surgical resection combined with IORT. The median radiation dose was 15 Gy (range 10–18 Gy) with a median electron energy of 8 MeV (range 6–15 MeV) prescribed to the 90% isodose. IORT dose was usually restricted to 10–12 Gy, if major nerves had to be included in the radiation field. The median follow-up was 14 months. For patients with cervical cancer, 5-year OS was 6.4%, quite different from endometrial recurrences (50%). Local pelvic progression was documented in 10 of 18 cervix recurrences. IORT attributed toxicity consists in neuropathy in 11% of patients. No ureter stenosis was reported. The authors conclude that a radical procedure of resection combined with IORT seems to be a curative option for patients with recurrent endometrial carcinoma with 5-year survival rates of 50%. For patients with cervical or vulvar cancer this treatment should be considered a rather than palliative one.
Our institutional experience is short. Our IORT program started in 2013 with a mobile electron linear accelerator (LIAC) installed in a specifically designed operation room. Treatment objectives are mainly focused on conservative breast cancer but, until now, 7 patients were treated with surgery and IORT for recurrent cervix cancer. The median follow up is short, 13 months, but at the present 5 of 7 patients are living without recurrence and in complete remission. No neuropathy or ureteral toxicity have been detected until now.
4. Conclusions
The total number of cervix cancers collected from the referred studies is 512, of which 203 (39.6%) are primary tumours (most of them stage IIB) and 287 (60.4%) recurrent. An additional group of 22 is not clearly specified. In several publications it has been difficult to identify cervical tumours data from other locations and diagnoses, like the endometrium or vulva. We must recognize and advert about possible errors because of the difficulties explained above. The collected data comprises a long publishing period: 20 years (1996–2016). The median number of cervix patients per article is 42.6 and the median enrolment time per article is 14 (range 7–27). The median yearly collection rate is as low as 2.8 (range 0.9–5.1). From these data, we should conclude that we face a relatively small population, collected along a long time and in the French study (15) from multiple centres (7 institutions).
Additionally, there are other parameters of heterogeneity. Some of them are as follows: recurrence sites of different prognosis, like the pelvic walls or central pelvic recurrences, free margins on resection, tumour initial and residual burden, high level of heterogeneity according to different techniques, energies, fields, doses, etc. What is more, the conclusions of the referred studies are frequently different. Obviously, it is not easy to demonstrate the efficacy and the benefit of IORT in retrospective limited series. IORT is a radiation boost in a surgical procedure. In well designed aleatorized prospective studies it is frequently difficult to demonstrate the degree of local control benefit of postoperative radiotherapy. It is particularly difficult in IORT because it is necessarily associated with different degrees of radicality in surgery, from local resection to pelvic exenteration.
But most of the referred manuscripts agree that adding IORT to a surgical resection is a right strategy rising the local control rate. There are more doubts about the influence over the survival and probably there is a little impact. But in cervix cancer local control has a strong influence on the quality of life. We must keep in mind that half of the mortality in cervix cancer is due to a non controlled pelvic disease.
By contrast, the therapeutic approach in primary tumours, including surgery and IORT, is strongly debated. It seems, there is no clear advantage over the standard well established approach, including chemoradiotherapy and brachytherapy. But there is some agreement that, if surgery is a therapeutic option, IORT is an effective tool adding extra security and rising the local control rate.
Finally, we must point out the difficulty and the low probability to design and realize aleatorized prospective trials. Low accrual of a sufficient number of patients in a reasonable period of time and the heterogeneity of recurrences and surgical procedures are difficulties that are hard to overcome.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Beck C. On external roentgen treatment of internal structures. NY Med J. 1909;89:621–622. [Google Scholar]
- 2.Casas F., Ferrer C., Calvo F.A. European historical note of intraoperative radiation therapy (IORT). A case report from 1905. Radiother Oncol. 1997;43:323–324. doi: 10.1016/s0167-8140(97)00065-0. [DOI] [PubMed] [Google Scholar]
- 3.Comas C., Prio A. Ed Francisco Badia; Barcelona: 1907. Irradiation roentgen preventive intra-abdominale, après l’intervention chirurgicale dans un cas de cancer de l’uterus. Communication au III Congrés International d’Electro-radiologie. 1906. [Google Scholar]
- 4.Belletti B., Vaidya S., D’Andrea S. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res. 2008;14(5):1325–1331. doi: 10.1158/1078-0432.CCR-07-4453. [DOI] [PubMed] [Google Scholar]
- 5.Zaleska K., Suchorska W.M., Przybyla A., Murawa D. Effect of surgical wound fluids after intraoperative electron radiotherapy on the cancer stem cell phenotype in a panel of human breast cancer cell lines. Oncol Lett. 2016;12:3707–3714. doi: 10.3892/ol.2016.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backes F., Martin D. Intraoperative radiation therapy (IORT) for gynecologic malignancies. Gynecol Oncol. 2015;138:449–456. doi: 10.1016/j.ygyno.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Krengli M., Pisani C., Deantonio L. Intraoperative radiotherapy for gynaecological and genito-urinary malignancies: focus on endometrial, cervical, renal, bladder and prostate cancers. Radiat Oncol. 2017;12:18–27. doi: 10.1186/s13014-016-0748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez-Monge R., Jurado M., Aristu J.J. Intraoperative electron beam radiotherapy during radical surgery for locally advanced and recurrent cervical cancer. Gynecol Oncol. 2001;82:538–543. doi: 10.1006/gyno.2001.6329. [DOI] [PubMed] [Google Scholar]
- 9.Giorda G., Boz G., Gadducci A. Multimodality approach in extra-cervical locally advanced cervical cancer: chemoradiation, surgery and intra-operative radiation therapy. A phase II trial. EJSO. 2011;37:442–447. doi: 10.1016/j.ejso.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Foley O., Rauh-Hain J.A., Clark R. Intraoperative radiation therapy in the management of gynecologic malignancies. Am J Clin Oncol. 2016;39(4):329–334. doi: 10.1097/COC.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 11.Gao Y., Liu Z., Gao F., Chen X. Intraoperative radiotherapy in stage IIB adenocarcinoma of the uterine cervix: a retrospective study. Oncotargets Ther. 2013;6:1695–1700. doi: 10.2147/OTT.S53020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z., Chen X. Preliminary results of intraoperative radiation therapy for cervical carcinoma IIB. Zhongua Fu Chan Ke Za Zhi. 2002;37(9):553–555.. [PubMed] [Google Scholar]
- 13.Garton G.R., Gunderson L., Webb M. Intraoperative radiation therapy in gynecologic cancer: the Mayo Clinic experience. Gynecol Oncol. 1993;48(3):328–332. doi: 10.1006/gyno.1993.1057. [DOI] [PubMed] [Google Scholar]
- 14.Kinney W., Koh W., Schray M. Survival following intraoperative radiotherapy for recurrent early stage carcinoma of the cervix (abstract). Proccedings of the seventy-second annual American Radium Society meeting; Scottsdale, AZ, April 21–25; 1990. p. 13. [Google Scholar]
- 15.Mahe M.A., Gerard J.P., Dubois J.B. Intraoperative radiation therapy in recurrent carcinoma of the uterine cervix: report of the French intraoperative group on 70 patients. Int J Radiat Oncol Biol Phys. 1996;34:21–26. doi: 10.1016/0360-3016(95)02089-6. [DOI] [PubMed] [Google Scholar]
- 16.Barney B., Petersen I., Dowdy S. Intraoperative electron beam radiotherapy (IOERT) in the management of locally advanced or recurrent cervical cancer. Radiat Oncol. 2013;8:80–89. doi: 10.1186/1748-717X-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sole C.V., Calvo F.A., Lozano M.A. External-beam radiation therapy after surgical resection and intraoperative electron beam radiation therapy for oligorecurrent gynecological cancer. Long-term outcome. Strahlenther Onkol. 2014;190(2):171–180. doi: 10.1007/s00066-013-0472-5. [DOI] [PubMed] [Google Scholar]
- 18.Tran P., Su Z., Hara W. Long-term survivors using intraoperative radiotherapy for recurrent gynecological malignancies. Int J Radiat Oncol Biol Phys. 2007;69(2):504–511. doi: 10.1016/j.ijrobp.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Backes F., Billingsley C., Martin D. Does intra-operative radiation at the time of pelvic exenteration improve survival for patients for recurrent previously irradiated cervical, vaginal or vulvar cancer? Gynecol Oncol. 2014;135:95–99. doi: 10.1016/j.ygyno.2014.07.093. [DOI] [PubMed] [Google Scholar]
- 20.Arians N., Foerster R., Rom J. Outcome of patients with local recurrent gynecological malignancies after resection combined with intraoperative electron radiation therapy (IOERT) Radiat Oncol. 2016;11:44–54. doi: 10.1186/s13014-016-0622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stelzer K.J., Koh W.J., Greer B.E. The use of intraoperative radiotherapy in radical salvage for recurrent cervical cancer: outcome and toxicity. Am J Obst Gynecol. 1995;172:1881–1886. doi: 10.1016/0002-9378(95)91427-7. [DOI] [PubMed] [Google Scholar]