Abstract
Background
Most publications about low-calorie sweeteners (LCSs) focus on person-level intake prevalence.
Objective
We assessed LCS distribution in foods, beverages, and food and beverage additions (FBAs), e.g., mayonnaise, in the US adult diet as reported in the NHANES (2007-2012).
Methods
Dietary items reported in the first 24-h recall were coded for LCS and/or nutritive sweeteners (NSs) with the use of USDA What We Eat in America food files. We calculated the number of times items were reported and LCS/NS content.
Results
Of reported items, 56.1% were foods, 29.1% were beverages, and 14.8% were FBAs. LCS was contained in 0.7% of foods, 8.1% of beverages, and 10.4% of FBAs. This food-level analysis identified FBAs as a significant source of LCSs in the US diet.
Conclusion
Identifying the diversity of LCS and NS sources will enhance exposure classification for examining diet and health relations, including body weight management.
Keywords: low-calorie sweeteners, nutritive sweetener, food items, adults, NHANES, dietary, United States
Introduction
Low-calorie sweeteners (LCSs; also referred to as artificial sweeteners, non-nutritive sweeteners, non-nutritive artificial sweeteners, high-intensity sweeteners, or sugar substitutes) are incorporated into foods, beverages, and self-added tabletop sweeteners as an alternative to sugars or nutritive sweeteners (NSs). Compared with NSs, LCSs are associated with lower calorie intake and have been shown to facilitate weight loss in clinical trial settings (1–5). Additionally, LCS consumption has been associated with healthier lifestyle choices, such as increased physical activity, greater fruit and vegetable intake, and higher overall Health Eating Index scores (6). Compared with water consumption, equal or better dietary intake and glycemic response have also been observed for LCS-containing beverages (7). However, the potential health effects of LCSs remain controversial (8) as various animal, epidemiologic, and prospective studies have reported associations of LCSs with weight gain, cardiometabolic risk factors, and alteration of the gut microbiota (9–12).
The prevalence of consumption of beverages, foods, and recently “packets” containing LCSs by US adults (13) has been reported; however, other sources of LCSs exist within the US diet. Recent studies have analyzed LCS intake based on beverages alone, and then examined the dietary patterns of LCS beverage consumers (14, 15). Often missing in these analyses are sources of LCSs from the intake of the broader array of foods themselves. Also often excluded, or grouped with foods or beverages, are those items that are added to beverages and foods, such as self-added tabletop sweeteners, relishes, toppings, sauces, and other items that do not meet the USDA Food Surveys Research Group (FSRG) definition of dietary items consumed on their own, that represent
“… a major contributor to the daily volume or weight of food or beverage intake” (16). We have grouped these latter items separately as food and beverage additions (FBAs). Omitting or not understanding the impact of these frequently consumed items may lead to misinterpretations regarding the implications of consuming LCSs.
The objective of this study was not to focus on the prevalence of consumption of LCSs by the US population (which we present elsewhere), but rather to focus on the foods, beverages, and FBAs that were reported as consumed, and conduct a “food-level examination” of LCS- and NS-containing items from all dietary sources. We employed data from the NHANES 2007–2012 for this study. To our knowledge, this is the first food-level analysis of LCSs and NSs in foods, beverages, and FBAs to use population-based dietary data.
Methods
Sample
Dietary items described in this study were based on food, beverages, and FBAs reported in the first 24-h interviewer-based NHANES dietary recall. Food-level results were generated and representative of items consumed by US adults during a single day through the use of NHANES 2007–2012 study participant data.
This study assessed food, beverages, and FBAs reported by all adult survey participants (≥19 y of age) for whom NHANES obtained the first 24-h dietary recall. The pooled NHANES interview 2007–2012 included 30,442 people, of whom 18,191 were adults. The final number of interviewees used was further limited to 16,087 adults: 50.8% (7996) women and 49.2% (8091) men with a complete 24-h recall according to the USDA What We Eat in America food files (16), after exclusion of reports by individuals in which the dietary data were marked as unreliable by the NHANES interviewer (n = 1627) (see https://www.cdc.gov/nchs/tutorials/dietary/SurveyOrientation/DietaryDataStructureContents/Info2.htm), and reports by pregnant or lactating women, whose intake is often altered during this life stage (n = 477). All interviewees self-reported their intake, with the exception of individuals whose responses required the support of a proxy as identified by NHANES procedure (17, 18).
This study, as a secondary analysis of de-identified data, was determined exempt from the full Human Subjects Institutional Review Board procedures by the Medical University of South Carolina.
Food, beverage, and FBA item coding
We used standard USDA FSRG-defined food group codes (16) to categorize foods and beverages (see Table 1). All beverages (e.g., fluid milk and similar beverages from Milk and Milk Products and all beverages from Sugar, Sweets, and Beverages) were separated from the food categories and coded through the use of a system based on 12 specific beverage types available in the USDA Food and Nutrient Database for Dietary Studies (FNDDS) 5.0 (16).
TABLE 1.
Percentages of foods and beverage categories that contain no or any LCSs based on reported items consumed by US adults in the first 24-h interviewer-based dietary recall: NHANES (2007–2012)1
| Foods2 | No LCS, % | Any LCS, % |
|---|---|---|
| Milk3 and milk products | 94.6 | 5.4 |
| Meat, poultry, fish, and mixtures | 100.0 | — |
| Eggs | 100.0 | — |
| Legumes, nuts, seeds | 99.8 | 0.2 |
| Grain products | 99.5 | 0.5 |
| Fruits | 100.0 | 0.04 |
| Vegetables | 100.0 | — |
| Fats, oils, salad dressings | 92.9 | 7.1 |
| Sugars, sweets, beverages3 | 80.4 | 19.6 |
| Beverages | ||
| Milk and milk products (replacements) | 99.8 | 0.2 |
| Coconut beverages | 100.0 | — |
| Fruit juices | 100.0 | — |
| Tomato juices, 100% vegetable juice blends | 100.0 | — |
| Coffee | 99.7 | 0.3 |
| Tea | 89.2 | 10.8 |
| Soft drinks, carbonated | 64.3 | 35.7 |
| Fruit drinks, not juice—includes sports drinks | 77.2 | 22.8 |
| Non-fruit drinks—includes energy drinks | 90.9 | 9.1 |
| Non-alcoholic beer, wine, cocktails | 100.0 | — |
| Alcoholic beer, wine, cocktails | 100.0 | — |
| Water | 99.3 | 0.7 |
USDA food categories. LCS, low-calorie sweeteners.
Rows may not add up to 100 due to rounding.
We reclassified fluid milk and beverages into the separate beverage groups as shown.
If the item typically was consumed on its own, it was categorized as a beverage or food following USDA definitions (16). If the item was primarily used as an addition to a beverage or food it was categorized as an FBA (e.g., ketchup, sauces, relishes, dips, sugar, jams, creamer, individually packaged tabletop sweeteners). A total of 5464 unique food, beverage, and FBA items were reported by adult NHANES participants who were included in the current analysis: 4689 food items, 393 beverages, and 382 FBAs. Two experienced nutrition professionals (DMDV and BPM) coded all items as food, beverages, or FBAs, for which there was 99.7% agreement with a κ of 0.989 (95% CI: 0.984, 0.995).
All unique items were categorized as including any LCS if the USDA item description included “with low/no calorie sweetener”, “sugar-free”, or “dietetic/low sugar” or if review of the Nutrition Facts Panel ingredients list included any of the FDA-approved LCSs. Items were categorized as including NS if the USDA item was known to contain a NS, as defined by the USDA Food Patterns Equivalent Database (19) or if review of the Nutrition Facts Panel revealed an NS as an ingredient. Items which included both LCSs and NSs were categorized accordingly. DMDV and BPM categorized all foods, beverages, and FBAs for LCS and/or NS content, for which there was 90.3% agreement with a kappa of 0.785 (95% CI: 0.768, 0.802). Whenever uncertainty existed regarding the appropriate coding for an item and as part of final coding determinations, the item’s sweetener content was verified based on online product labels.
Statistical analysis
Each continuous NHANES cycle (2007–2008, 2009–2010, 2011–2012) was designed to be representative of the US civilian non-institutionalized population through the use of a complex multistage probability sample. Descriptive statistics, such as proportions and SEM, were conducted to summarize dietary data available from NHANES. All analyses incorporated appropriate sampling weights to account for the NHANES clustered sampling design, oversampling, and differential noncoverage and nonresponse across the 3 continuous NHANES cycles included in the analysis (17, 18). No statistical comparisons are presented. Food-level results were generated and were representative of items consumed by US adults in a single day in the US during our study time frame through the use of the Statistical Analysis System (SAS) version 9.3 (SAS Institute) and its complex survey-specific procedures.
Results
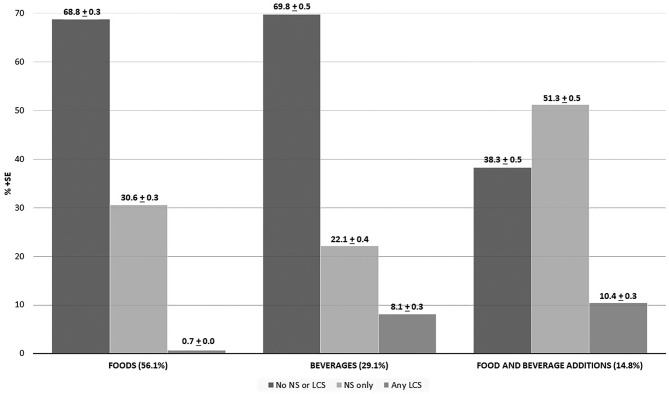
The percentages of food and beverage items containing any LCS based on USDA food categories are displayed in Table 1. As shown in Figure 1, of the 258,471 total dietary items (weighted n = 3,623,222,694) that were reported in the initial 24-h recall across the 3 NHANES cycles (2007–2012), 56.1% were foods, 29.1% were beverages, and 14.8% were FBAs. Of these dietary items 0.7% of food items, 8.1% of beverage items, and 10.4% of FBAs contained any LCS; whereas an estimated 30.6% of reported food items, 22.1% of beverage items, and 51.3% of FBAs contained some NS. Among these dietary reports, there were 5464 unique items, only 180 of which contained any LCS: 72 food items, 61 beverage items, and 47 FBAs.
FIGURE 1.
Percentages of reported items containing nutritive sweetener (NS) and any low-calorie sweetener (LCS) among all items consumed in a single day by US adults (>19 y of age): NHANES 2007–2012. Reported items were categorized into foods, beverages, and food and beverage additions (FBAs). Denominator is “food”-level data. Weighted percentage of reported consumed food, beverages, or FBAs.
Examining the foods, beverages, and FBAs by USDA food code categorization (which groups FBAs within foods and beverages) along with our beverage categorization (Table 1), the food categories with the greatest proportion of LCS reported were “Sugars and sweets”, followed by “Fats, oils, salad dressings”. The beverage category with the greatest proportion of LCS reported was “Soft drinks, carbonated”. The 10 most-commonly reported foods, beverages, and FBAs containing LCS are shown in Table 2. The most-commonly reported LCS-containing food item was “Yogurt (fruit-variety, low-fat milk with low calorie sweetener)”, beverage item was “Soft drink, cola-type (sugar-free)”, and FBA was “Sucralose-based sweetener”.
TABLE 2.
Most commonly reported consumed foods, beverages, and FBAs containing any LCS in a single day by US adults (≥19 y of age): NHANES 2007–20121
| Foods | % ± SEM2 | Beverages | % ± SEM2 | FBAs | % ± SEM2 | |
|---|---|---|---|---|---|---|
| 1. | Yogurt: fruit variety, lowfat milk, w/LCS | 20.1 ± 1.7 | Soft drink, cola-type, sugar-free | 40.7 ± 1.4 | Sucralose-based sweetener, sugar substitute | 28.2 ± 1.4 |
| 2. | Yogurt: fruit variety, nonfat milk, w/LCS | 6.9 ± 1.2 | Soft drink, cola-type, decaffeinated, sugar-free | 10.5 ± 0.8 | Sugar substitute, saccharin-based, dry powder and tablets | 21.1 ± 1.3 |
| 3. | Kashi GoLean | 5.3 ± 1.3 | Fruit-flavored drink made from powder, low calorie | 6.1 ± 0.6 | Sugar substitute, aspartame-based, dry powder | 9.2 ± 0.8 |
| 4. | Yogurt: vanilla, lemon, or coffee; lowfat milk; w/LCS | 4.9 ± 1.0 | Soft drink, fruit-flavored, w/caffeine, sugar-free | 5.8 ± 0.9 | Mayonnaise, light | 5.5 ± 0.7 |
| 5. | Gelatin dessert: dietetic, w/LCS | 4.0 ± 0.9 | Soft drink, fruit-flavored, sugar-free, caffeine-free | 5.6 ± 0.5 | Creamy dressing, light | 4.2 ± 0.5 |
| 6. | Yogurt: vanilla, lemon, or coffee; nonfat milk; w/LCS | 3.9 ± 1.3 | Tea, presweetened w/LCS | 4.6 ± 0.7 | Cream substitute, light, powdered (incl CoffeeMate) | 3.8 ± 0.6 |
| 7. | Yogurt: frozen, not chocolate; nonfat milk; w/LCS | 3.7 ± 1.0 | Soft drink, pepper-type, sugar-free | 4.0 ± 0.4 | Mayonnaise-type salad dressing, light | 3.3 ± 0.6 |
| 8. | Ice cream: no sugar added, flavors other than chocolate | 2.8 ± 0.9 | Cranberry juice drink or cocktail, low calorie, w/high vit c | 2.3 ± 0.3 | Cream substitute, sugar-free, flavored, liquid | 2.9 ± 0.4 |
| 9. | Light ice cream, bar/stick, w/LCS, chocolate coating | 2.6 ± 0.7 | Tea, leaf, presweetened w/LCS | 2.1 ± 0.4 | Cream substitute, light, liquid | 2.7 ± 0.5 |
| 10. | Light ice cream, no sugar added, not chocolate | 2.4 ± 0.8 | Tea, made from powdered instant, w/LCS | 1.8 ± 0.4 | Italian dressing, light | 2.7 ± 0.4 |
Cumulative frequency is 56.6% for foods, 83.5% for beverages, and 83.5% for FBAs. FBA, food and beverage addition; incl, including; LCS, low-calorie sweetener; vit c, vitamin c.
Does not add up to 100 due to inclusion of the top 10 foods.
Discussion
Many recent reports have focused on the prevalence of LCS in beverages (14, 15). In our study, we provide a summary count of the LCS-containing foods, beverages, and FBAs representative of items consumed by US adults in a single day during 2007–2012. Overall, we found that only 180 (3.3%) of the 5464 unique dietary items contained any LCS with much higher percentages of beverages (15.5%) and FBAs (12.3%) compared with foods (1.5%) containing LCS. However, of the dietary items reported consumed during a single day, a higher proportion of FBAs contained any LCS (10.4%) and NS (51.3%) compared with foods and beverages (respectively: LCS: 0.7%; 8.1%; NS: 30.6%; 22.1%). Prevalence studies have underscored LCS sweetener packets as a major source of LCSs in the United States (6, 13, 20, 21). From the food perspective, we similarly found that of the most commonly reported FBAs containing LCSs, 58.5% were sweetener packets, with salad dressings (15.7%) and cream substitutes (9.4%) constituting other major FBA sources of LCSs.
Studies examining relations among the prevalence of consumption of LCS in beverages alone or beverages and sweetener packets with body weight have largely found a direct association between LCS consumption and body weight (5, 22). However, these studies also typically identify an association of LCS consumption with healthier eating patterns and lifestyle choices at the person level, but these authors did not break out FBAs (6, 15). Therefore, the inclusion of FBAs in prevalence analyses could potentially provide additional insight into dietary behaviors, as well as additional items as targets for nutrition education. For example, 5.4% of LCS-containing food items that we reported in the current study were found in the Milk and Milk Products category (e.g., yogurts, light ice cream, and other frozen desserts) and it would be important to know how this impacts prevalence of LCS consumption at the person level.
The results from our study were based on the dietary information from a single 24-h recall, and are subject to some limitations. Our use of the dietary data from NHANES 2007–2012 as well as the USDA's FNDDS 5.0 may not accurately reflect either current (2018) LCS intake, or market availability of food, beverages, and FBAs for individuals in the United States. Further, our ability to identify the specific type of LCS and other sweeteners in this analysis was limited by the current FNDDS database contents and the product manufacturers’ websites.
Despite the potential limitations of NHANES and the FNDDS, these sources are the most comprehensive nationally representative data for dietary intake studies in the United States. The Automated Multiple-Pass Method used in the NHANES is designed to minimize misreporting of dietary data (23, 24). In addition to the FNDDS descriptive labeling, which indicated if LCSs are included in items, for coding we also assessed food and beverage labels to confirm LCS and NS presence. Extending previous population-level prevalence reports of LCS consumption (4, 14, 24, 25), we provide a food-level analysis of all foods, beverages, and FBAs reported in a single 24-h period in this nationally representative sample. This food-level analysis underscores that not only foods and beverages but also what people add to their foods and beverages are important considerations in understanding LCS and NS products consumed in the United States.
In conclusion, our analysis shows that LCSs are contained in relatively few items reported in the US diet. Of these items, not only beverages reported by US adults but also significant numbers of additions to food and beverages contain LCSs. Although the reported intake of LCSs in beverages has been the focus of many reports, characterizing all sources of LCSs will enhance exposure classification for examining diet and health relations, including body weight management.
Acknowledgments
We acknowledge the early data management contributions of Myla Ebeling and study operational support of Chris Fink to this work. The extensive administrative contributions of Samantha Wise throughout this project are much appreciated. The authors’ responsibilities were as follows—BPM, KJH, DMDV, DG, and JVS: formulated the research question and designed the study; BPM, KJH, DMDV, and AMM: carried out the research including acquiring data; KJH and AMM: analyzed the data; AMM, BPM, and KJH: wrote the manuscript draft; BPM and AMM: revised the manuscript; AMM, KJH, and BPM: had responsibility for final content; as corresponding author, BPM: had full access to the data in the study and final responsibility for the decision to submit for publication; and all authors: contributed to, read, and approved the final manuscript.
Notes
Supported as a collaborative project by the PepsiCo R&D Research Fellows Program of PepsiCo, Inc. (Medical University of South Carolina contract no. 2013—2129806). Scientists from the sponsor were involved in the study design; interpretation of data; writing of the report; and in the decision to submit the article for publication.
JVS and DG are full-time employees of PepsiCo, Inc. DMDV, AMM, KJH, and BPM, no conflicts of interest.
The opinions expressed in this report are those of the authors and do not necessarily represent the position or policy of PepsiCo, Inc.
Abbreviations used:
- FBA
food and beverage addition
- FNDDS
food and nutrient database for dietary studies
- LCS
low-calorie sweetener
- NS
nutritive sweetener
References
- 1. Peters JC, Beck J. Low calorie sweetener (LCS) use and energy balance. Physiol Behav 2016;164(Pt B):524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rogers PJ, Brunstrom JM. Appetite and energy balancing. Physiol Behav 2016;164(Pt B):465–71. [DOI] [PubMed] [Google Scholar]
- 3. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014;100(3):765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson GH, Foreyt J, Sigman-Grant M, Allison DB. The use of low-calorie sweeteners by adults: impact on weight management. J Nutr 2012;142(6):1163S–9S. [DOI] [PubMed] [Google Scholar]
- 5. Tate DF, Turner-McGrievy G, Lyons E, Stevens J, Erickson K, Polzien K, Diamond M, Wang X, Popkin B. Replacing caloric beverages with water or diet beverages for weight loss in adults: main results of the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2012;95(3):555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drewnowski A, Rehm CD. Consumption of low-calorie sweeteners among U.S. adults is associated with higher Healthy Eating Index (HEI 2005) scores and more physical activity. Nutrients 2014;6(10):4389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leahy M, Ratliff JC, Riedt CS, Fulgoni VL. Consumption of low-calorie sweetened beverages compared to water is associated with reduced intake of carbohydrates and sugar, with no adverse relationships to glycemic responses: results from the 2001–2012 National Health and Nutrition Examination Surveys. Nutrients 2017;9(9):928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav 2016;164(Pt B):446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fowler SP, Williams K, Resendez RC, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008;16(8):1894–900. [DOI] [PubMed] [Google Scholar]
- 10. Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514(7521):181–6. [DOI] [PubMed] [Google Scholar]
- 11. Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116(5):480–8. [DOI] [PubMed] [Google Scholar]
- 12. Swithers SE, Sample CH, Davidson TL. Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. Behav Neurosci 2013;127(2):262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sylvetsky AC, Jin Y, Clark EJ, Welsh JA, Rother KI, Talegawkar SA. Consumption of low-calorie sweeteners among children and adults in the United States. J Acad Nutr Diet 2017;117(3):441–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Piernas C, Mendez MA, Ng SW, Gordon-Larsen P, Popkin BM. Low-calorie- and calorie-sweetened beverages: diet quality, food intake, and purchase patterns of US household consumers. Am J Clin Nutr 2014;99(3):567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Piernas C, Tate DF, Wang X, Popkin BM. Does diet-beverage intake affect dietary consumption patterns? Results from the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr 2013;97(3):604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. USDA Food Surveys Research Group. Dietary methods research: overview of What We Eat in America food categories. FSRG-defined food groups. Beltsville, MD: US Department of Agriculture, 2014[updated October 2014; cited 2014 November]. Available from: http://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/fndds/fndds5_doc.pdf#page=64. [Google Scholar]
- 17. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: analytic guidelines, 2011-2012. National Center for Health Statistics, editor. Atlanta, GA: Division of Health and Nutrition Examination Surveys, 2013. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/analytic_guidelines_11_12.pdf; p. 13. [Google Scholar]
- 18. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. National Center for Health Statistics, editor. Atlanta, GA: Division of Health and Nutrition Examination Surveys, 2013. Available from: https://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf; p. 17. [PubMed] [Google Scholar]
- 19. Bowman SA, Clemens JC, Friday JE, Thoerig RC, Moshfegh AJ. Food Patterns Equivalents Database 2011–12: methodology and user guide [Internet].Beltsville, MD: Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, US Department of Agriculture, 2014[cited 2015 November 10]. Available from: http://www.ars.usda.gov/nea/bhnrc/fsrg. [Google Scholar]
- 20. Drewnowski A, Rehm CD. The use of low-calorie sweeteners is associated with self-reported prior intent to lose weight in a representative sample of US adults. Nutr Diabetes 2016;6:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sylvetsky AC, Welsh JA, Brown RJ, Vos MB. Low-calorie sweetener consumption is increasing in the United States. Am J Clin Nutr 2012;96(3):640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Griffioen-Roose S, Smeets PA, Weijzen PL, van Rijn I, van den Bosch I, de Graaf C. Effect of replacing sugar with non-caloric sweeteners in beverages on the reward value after repeated exposure. PLoS One 2013;8(11):e81924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. USDA Food Surveys Research Group. USDA Automated Multiple-Pass Method (AMPM). Beltsville, MD: US Department of Agriculture, 2014. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=7710. [Google Scholar]
- 24. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr 2008;88(2):324–32. [DOI] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention. National Center for Health Statistics. National Health and Nutrition Examination Survey: survey methods and analytic guidelines. Atlanta, GA: Centers for Disease Control and Prevention, 2014[updated May 2014; cited 2014 November]. Available from: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx. [Google Scholar]