Abstract
The stem cell factor (SCF)/c-KIT axis plays an important role in the hematopoietic differentiation of human pluripotent stem cells (hPSCs), but its regulatory mechanisms involving microRNAs (miRs) are not fully elucidated. Here, we demonstrated that supplementation with SCF increases the hematopoietic differentiation of hPSCs via the interaction with its receptor tyrosine kinase c-KIT, which is modulated by miR-221 and miR-222. c-KIT is comparably expressed in undifferentiated human embryonic and induced pluripotent stem cells. The inhibition of SCF signaling via treatment with a c-KIT antagonist (imatinib) during hPSC-derived hematopoiesis resulted in reductions in the yield and multi-lineage potential of hematopoietic progenitors. We found that the transcript levels of miR-221 and miR-222 targeting c-KIT were significantly lower in the pluripotent state than they were in terminally differentiated somatic cells. Furthermore, suppression of miR-221 and miR-222 in undifferentiated hPSC cultures induced more hematopoiesis by increasing c-KIT expression. Collectively, our data implied that the modulation of c-KIT by miRs may provide further potential strategies to expedite the generation of functional blood cells for therapeutic approaches and the study of the cellular machinery related to hematologic malignant diseases such as leukemia.
Keywords: c-KIT, hematopoiesis, hPSCs, miR-221/222, SCF
INTRODUCTION
Human pluripotent stem cells (hPSCs) offer an attractive alternative to adult hematopoietic stem and progenitor cells due to their unlimited potential to self-renew and their pluripotency (Takahashi et al., 2007; Thomson et al., 1998). It is critical to utilize the hematopoietic growth factors (hGFs) that are involved in early embryonic hematopoietic development with a precise understanding of their stage-specific functions. Among the most commonly used hGFs, stem cell factor (SCF) is known as an early inducer that plays an important role in the regulation of embryonic and adult hematopoiesis (Carow et al., 1991; Pick et al., 2007). SCF interacts with other essential cytokines, such as bone morphogenetic protein 4 (BMP4) and vascular endothelial growth factor (VEGF), during the emergence of bipotent hemogenic precursors from hPSCs by binding to its receptor tyrosine kinase c-KIT (Ding et al., 2012; Perry et al., 2007). SCF/c-KIT signaling has a conserved role in both hPSC and mouse PSC-derived hematopoiesis. Mouse embryonic stem cells (mESCs) with c-KIT deficiencies are not able to self-renew without supplementation with leukemia inhibitory factor, and they exhibit defects in the survival of the differentiated cells due to a reduction in anti-apoptotic protein (Bashamboo et al., 2006; Guo et al., 2006; Kimura et al., 2011). These studies indicate that the SCF/c-KIT signaling pathway is implicated in the functionality of hPSCs in terms of maintaining their state or differentiating into hematopoietic lineage cells. We previously found that c-KIT is comparably expressed in undifferentiated human embryonic stem cells (hESCs) and that the c-KIT+ fraction within hESCs is biased toward the hematopoietic lineage compared with c-KIT− cells (Hong et al., 2011). In undifferentiated mESC cultures, the c-kithi population has a greater ability to self-renew, can differentiate into embryoid bodies and express higher levels of Bmp4 and Nanog than can either the unfractionated or c-kit− populations (Lu et al., 2007; Marshall et al., 2007). These findings suggest that the ESC population expressing c-KIT has greater abilities for hematopoietic differentiation and self-renewal. Although c-KIT can be a useful indicator that is capable of predicting hematopoietic potenti al., its regulatory mechanisms related to microRNAs (miRs) during the hematopoietic development of hPSCs remain to be investigated.
Most miRs are small non-coding RNA molecules that contain small nucleotides and function in the regulation of gene expression via pleiotropic effects during biogenesis (Ambros, 2004; Bartel, 2004). Among the many miRs, miR-221 and miR-222 are two highly homologous miRs that are well known to be involved in angiogenic events and hematologic malignancies (Moses et al., 2016; Poliseno et al., 2006; Ramon et al., 2012; Rommer et al., 2013). Almost all blood lineage cells differentiate from the hemogenic endothelium, which possesses bipotent precursor cells that can generate endothelial cells and blood lineage cells (Gits et al., 2013; Lancrin et al., 2009; Lis et al., 2017). Based on this concept, we postulated that miR-221 and miR-222 may function as regulators that target c-KIT to initiate the differentiation of hematopoietic lineage cells.
In this study, we demonstrated that supplementation with SCF increases hematopoietic differentiation from hPSCs via an interaction with c-KIT and that this interaction is modulated by miR-221 and miR-222. The inhibition of SCF signaling with the kinase inhibitor imatinib (Im) during hPSC-derived hematopoiesis resulted in a reduction of both the yield and multi-lineage potential of hematopoietic progenitors. Furthermore, the suppression of miR-221 and miR-222 in undifferentiated hPSC cultures induced increased hematopoiesis by increasing c-KIT expression. Our study reinforces the pivotal role of the SCF-c-KIT signaling pathway and is the first to demonstrate the roles of miR-221 and miR-222 that are relevant to the SCF/c-KIT axis during hPSC-derived hematopoiesis, which could expedite the generation of functional blood products for therapeutic use.
MATERIALS AND METHODS
Maintenance of hESCs and hiPSCs
Human PSCs (CHA15-ESC and iPS-NT4-S1) were kindly provided by CHA University, South Korea. The cells were maintained in mTeSR1® serum-free medium (Stem Cell Technologies, Canada) on Matrigel (BD Bioscience, USA)-coated dishes. They were subcultured at 80% confluence and passaged every 5 days by mechanical dissociation. Human PSC cultures were carried out at 37°C incubator.
Hematopoietic differentiation
Hematopoietic differentiation was performed as previously described (Hong et al., 2011). Briefly, undifferentiated hPSC colonies were prepared with low density (approximately 5–7 colonies in a 35 mm culture dish). Cells were grown to 1 mm size, media were changed to Stemline II serum-free medium (Sigma) supplemented with Insulin-Transferrin-Selenium and BMP4 (20 ng/ml) for 4 days, followed by treatment with SCF (50 ng/ml) and VEGF (40 ng/ml) for 2 days. On day 6, the cultures were given fresh hematopoietic induction medium supplemented with hGFs cocktail (50 ng/ml SCF, 10 ng/ml Thrombopoietin, 50 ng/ml Interleukin-3, 50 ng/ml FMS-like tyrosine kinase 3 ligand and 50 ng/ml Granulocyte colony-stimulating factor, R&D Systems) with and without 10 μM Im and cultured for 11 days. The hematopoietic differentiation was assessed by the frequencies of CD34+CD45+ and CD34−CD45+ populations on day 17.
Colony-Forming units assay
Colony-Forming Unit (CFU) assay was performed as previously described (Kim et al., 2010). Briefly, 10,000 hematopoietic progenitor cells were plated into methylcellulose H4434 (Stem Cell Technologies) and incubated for 7–10 days at 37°C in 5% CO2.
Flow cytometric analysis
Single cell suspensions were harvested from undifferentiated and differentiated hPSCs and v-hESCs. The undifferentiated cells were dissociated with cell dissociation medium (CDM, Invitrogen) for 20 min at 37°C. After collagenase IV treatment, the cells were filtered through a 70 μm cell strainer and incubated for 30 min at 4°C with following anti-human antibodies: c-KIT (BD Biosciences), CD31 (BD Biosciences), CD34 (Miltenyi) andCD45 (BD Biosciences). Dead cells were excluded by 7-aminoactinomycin Dstaining. FACS was performed using FACSCanto II flow cytometer (BD Biosciences) and data was analyzed by FlowJo software.
Immunofluorescence staining
Undifferentiated hPSCs were fixed with 4% paraformaldehyde (Alfa Aesar) for 20 min at room temperature. The hPSCs were subjected to immunostaining using rabbit antic-KIT (1:200, BD Pharmingen) and Alexa 488 (Invitrogen) donkey anti-rabbit IgG (H+L) after blocking step (10% donkey serum). Nuclei were counterstained with DAPI (Sigma) for 5 min, and images were captured with a fluorescence microscope (IX-51, Olympus, Japan) and confocal microscope (Carl Zeiss LSM 880, Germany).
miRNA expression
Expression levels of miRs were analyzed as previously described (Kim et al., 2017). Briefly, RNA was isolated from the serum using the miRNeasy Kit (Qiagen, Germany) and reverse transcription was performed using the miScript II RT Kit (Qiagen) according to the manufacturer’s instructions. Subsequently, cDNAs were amplified from has-miR-221 and has-miR-222 using the custom miScriptmiRNAPCR Array (CMIHS02261C; Qiagen). The data were analyzed using PCR array data analysis tools (Qiagen).
In vitro transfection experiments of miR-221 and -222 inhibitors
Unless otherwise indicated, all materials for miRNA study were purchased from Qiagen. For a transient transfection approach with the aim to inhibit the miR-221 and -222 function, cells were transfected with anti-miRs oligos using the fast forward transfection protocol as suggested by the HiPerFect Transfection Reagent protocol according to the manufacturer’s instructions. A specific miR-221 and -222 inhibitors were commercially purchased. For the reference to normalize the findings, we used the miScript inhibitor negative Control under the same concentrations and conditions as used for the inhibitor (100 nM). Transfected hPSCs were incubated under their normal conditions and the effect of miR-221 and -222 manipulations on changes in gene expression levels were measured by quantitative RT PCR after 24 h as described above.
Statistical analysis
All results are presented as mean ± S.D. Data was generated from at least three independent experiments. Statistical significance was determined using the Student’s t-test with p < 0.05 as the cutoff.
RESULTS
SCF augments hematopoietic differentiation from hPSCs via interaction with c-KIT
We first investigated the expression of c-KIT in undifferentiated hPSCs, including hESCs and hiPSCs, using flow cytometry. As shown in Fig. 1A, the c-KIT protein was present in 24.8% of hESCs and 28.8% of hiPSCs, whereas somatic human dermal fibroblasts (hdFs) exhibited no expression of c-KIT (Fig. 1A). The confocal images also clearly showed the presence of c-KIT+ cells in both hPSCs (Fig. 1B), suggesting their putative relevance with the ligand, SCF, when SCF is supplemented in culture conditions. To determine the implications of c-KIT expression during hPSC hematopoietic differentiation, we employed a stepwise induction strategy that was divided into two phases. First, the specification phase is characterized by the emergence of bipotent hemogenic precursors. Second, the commitment phase is characterized as the period in which committed hematopoietic progenitors (CD34+CD45+) and mature blood (CD34−CD45+) cells are detected (Fig. 2A). During embryonic development, hematopoietic cells have been found to arise from aortic hemogenic precursors that can maintain the properties of hematopoietic and endothelial lineage cells. Based on this developmental concept, the specification of hemogenic precursors is required to generate hematopoietic cells. Thus, we successfully induced hematopoietic progenitors and mature blood cells from hemogenic precursors over 17 days via the application of the proper induction conditions. Flow cytometric analysis showed that the proportion of the CD34+CD45+ populations was synergistically increased with statistical significance when hPSCs were treated with hGFs and SCF compared to SCF alone and hGFs alone treatments (Fig. 2B). Additionally, the proportions of both populations were significantly decreased by c-KIT antagonist (Im) treatment (Fig. 2C), which suggests the pivotal roles of c-KIT in hematopoietic lineage differentiation. The SCF/c-KIT axis is known as an important factor for survival and differentiation into blood lineage cells. Consistent with previous papers (Bashamboo et al., 2006; Rojas-Sutterlin et al., 2014), our data also addressed the effects of c-KIT in the differentiation of PSCs into hematopoietic lineage cells. We further investigated whether SCF/c-KIT signaling influences the ability of hematopoietic progenitors to produce mature myeloid lineage cells, including erythrocytes (CFU-E), granulocytes (CFU-G), megakaryocytes (CFU-M) and granulocytes-megakaryocytes (CFU-GM). CFU assays showed that Im treatment significantly decreased the number of each CFU subtype as well as the total number of CFUs (Figs. 2D and 2E). Among the CFU subtypes, CFU-E production was unarguably blocked by treatment with Im. In addition, a decreased proportion of CFU-E colonies was also observed when the function of c-KIT was inhibited (68.8 ± 2.8% vs 50.7 ± 2.8%), suggesting the positive regulatory mechanism of c-KIT to erythrocyte differentiation. In contrast, treatment of Im increased the number of CFU-M, while decreasing its proportion (9.1 ± 1.9% vs 22.5 ± 1.7%) (Fig. 2F). These results suggest critical roles of the SCF/c-KIT signaling pathway at the point of cell fate commitment to the hematopoietic lineage as well as at the level of hematopoietic lineage development.
Fig. 1. c-KIT is expressed in undifferentiated hPSCs.
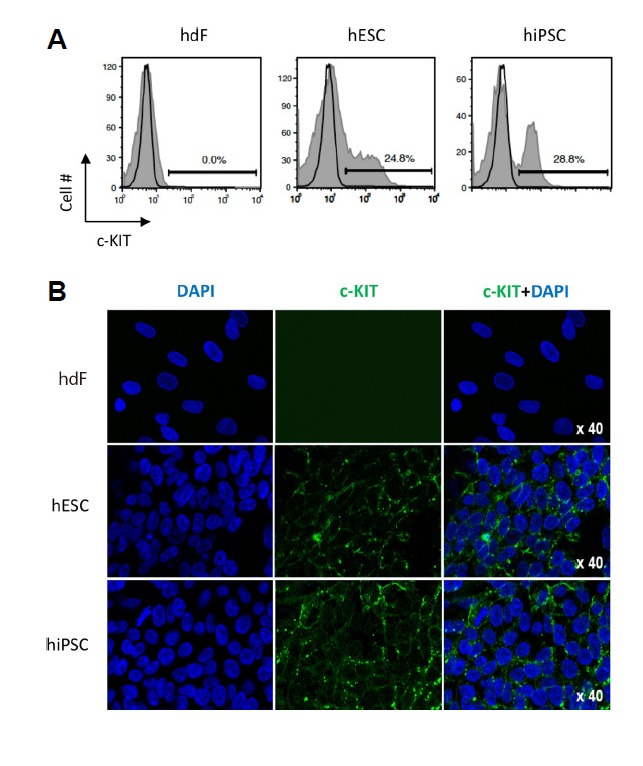
(A) Flow cytometry analysis for c-KIT in undifferentiated hESCs and hiPSCs. (B) Immunocytochemistry staining for c-KIT (green) in feeder-free hPSC cultures. Nuclei were counterstained with DAPI (blue). Scale bar 100 μm. hdF, human dermal fibroblast; hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell. DAPI (blue) was used for nuclear staining and c-KIT+ cells presented green colors. Magnification X40.
Fig. 2. SCF augments the hematopoietic differentiation of hPSCs via an interaction with c-KIT.
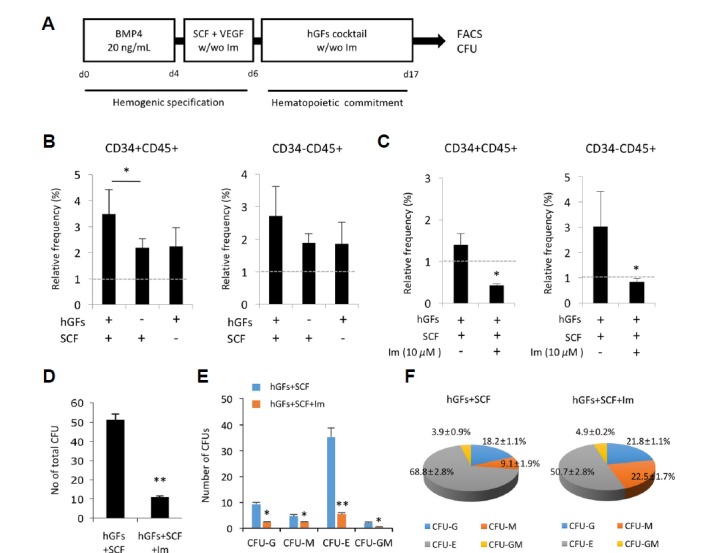
(A) A schematic diagram of the stepwise hematopoietic differentiation of hPSCs showing the hemogenic specification phase and the hematopoietic commitment phase. (B) Effects of SCF on the production efficiencies of hematopoietic progenitors (CD34+CD45+) and mature hematopoietic cells (CD34−CD45+) at day 17 of differentiation. The relative frequency is set to 1 (dotted line) for cells cultured in hematopoietic differentiation medium alone. (C) Effects of c-KIT inhibitor on hematopoietic differentiation. On day 6, the cells were cultured in the absence and presence of 10 μM Im for 11 days. The relative frequency is set to 1 (dotted line) for cells cultured in hGFs without SCF. (D and E) Differentiation capacities of hematopoietic progenitors differentiated under various treatments for 17 days. Percentages of the total number of CFUs (D) and each CFU subunit (E). (F) Pie diagrams showing the distribution of CFU types. Im, Imatinib; CFU-E, erythroid; CFU-M, megakaryocyte; and CFU-G, granulocytes. All results are presented as the means ± S.D. *p < 0.05, **p < 0.01.
SCF/c-KIT signaling increases the hematopoietic differentiation of hPSCs in a BMP4-dependent manner
BMP4 is a multifunctional protein that regulates a broad spectrum of functions during embryonic differentiation and is known to promote the hematopoietic differentiation of hPSCs. To examine the interaction between SCF and BMP4 during the hematopoietic differentiation of hPSCs, hPSCs were cultured under hematopoietic induction conditions in the presence and absence of BMP4. A hemogenic endothelium-like structure was observed in the hPSCs at approximately 6–8 days of differentiation in the presence of BMP-4. The morphological changes of hPSCs are restricted to BMP4 treatment, regardless of whether SCF is added (Fig. 3A). Flow cytometric analysis clearly demonstrated that the frequencies of CD34+CD45+ and CD34−CD45+cells were significantly increased in the BMP4-treated groups in the presence and absence of SCF compared with the SCF-only treated group (Fig. 3B). The BMP4 and SCF co-treated cells displayed the highest frequencies of both CD34+CD45+ and CD34−CD45+ cells. These data indicate that SCF/c-KIT signaling increases the hematopoietic differentiation of hPSCs in a BMP4-dependent manner.
Fig. 3. Effects of SCF on hPSC-derived hematopoiesis in the presence and absence of BMP4.
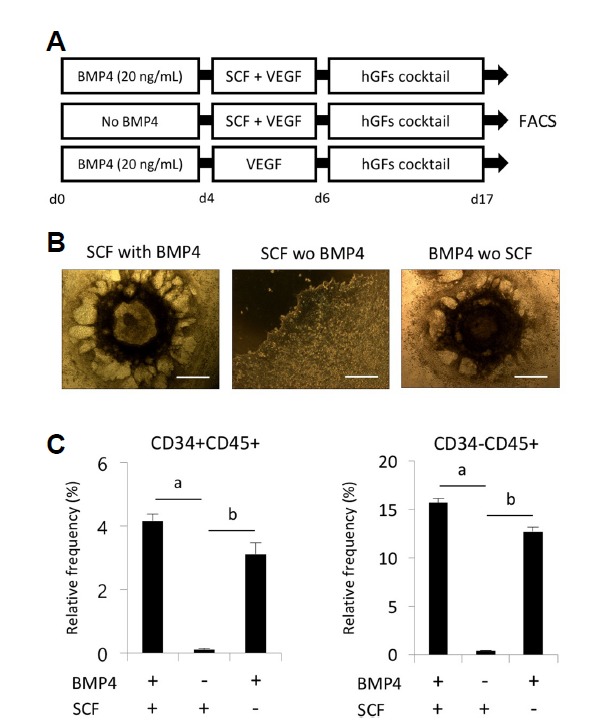
(A) A schematic diagram for determining the temporal effects of BMP4 on hematopoietic differentiation. (B) Representative image of hematopoietic cells derived from hPSCs. The scale bar is 100 μm. (C) Effects of SCF (50 ng/ml) and BMP4 (20 ng/ml) on the generation of hematopoietic progenitors (CD34+CD45+) and mature hematopoietic cells (CD34−CD45+) at day 17 of differentiation. hPSCs were cultured in hematopoietic differentiation medium supplemented with different combinations of SCF and BMP4. All results are presented as the means ± S.D. ap < 0.05, bp < 0.01.
Suppression of miR-221 and miR-222 increases the hematopoietic differentiation of hPSCs
Because miR-221 and miR-222 are known to regulate angiogenic activity and erythropoiesis via the down-modulation of c-KIT (Felli et al., 2005), we postulated that the hematopoietic differentiation of hPSCs might be augmented by the down-modulations of miR-221 and miR-222. To address the effects of miR-221 and miR-222 on the hematopoietic differentiation of hPSCs, we repressed their expression with anti-miR oligos. Based on their transcript levels, the expression levels of both miR-221 and miR-222 were significantly decreased in the hPSCs compared to that of hdF (Fig. 4A). The transfection of miR-221 and miR-222 specific inhibitors successfully upregulated the expression level of c-KIT in the undifferentiated hPSCs (Fig. 4B), suggesting that miR-221 and miR-222 target the c-KIT receptor in hPSCs. Furthermore, the suppression of miR-221 and miR-222 significantly increased the frequencies of CD34+CD45+ and CD34−CD45+ cells (Figs. 4C and 4D). Dual treatment with the miR-221 and miR-222 specific inhibitors elicited greater inductions of CD34+CD45+ and CD34−CD45+ cells; the frequencies of these cells were nearly doubled compared with those of the control group. Increased c-KIT, which is relevant to hematopoietic lineage cells, is modulated by miR-221 and miR-222 inhibitors, which lead to the effective differentiation of hPSCs into hematopoietic lineage cells.
Fig. 4. Effects of the knockdown of miR-221 and miR-222 on hPSC-derived hematopoiesis.
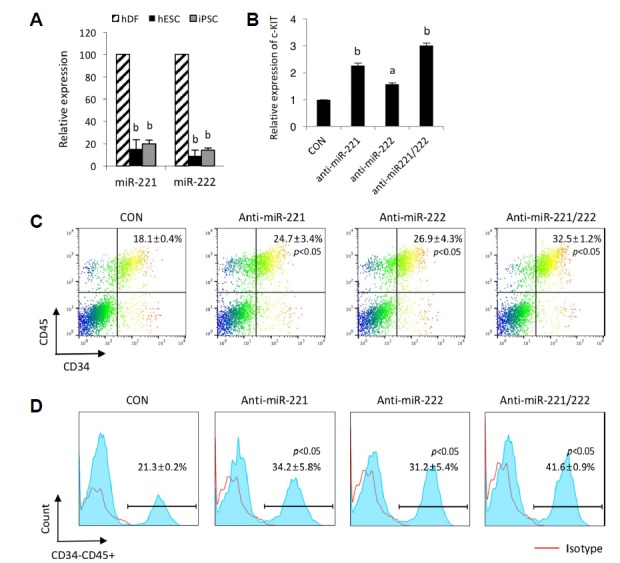
(A) Expression levels of miR-221 and miR-222 in hdFs and undifferentiated hESCs and hiPSCs. All results are presented as the means ± S.D. ap < 0.05, bp < 0.01. (B) The inhibition of miR-221 and miR-222 in undifferentiated hPSCs. Measurement of the c-KIT transcript levels after the knockdown of miR-221 and miR-222 in undifferentiated hPSCs. Bars indicate the means ± S.D. ap < 0.05, bp < 0.01. (C and D) Representative FACS plots and histograms showing the effects of the knockdown of miR-221 and miR-222 on the generation of hematopoietic progenitors (CD34+CD45+) (C) and mature hematopoietic cells (CD34−CD45+) (D) at day 15 of differentiation. Frequencies indicate the average and standard deviation. p < 0.05 (vs. control group).
DISCUSSION
In our previous work, the dissection of hESC cultures based on the expression of c-KIT revealed a greater hematopoietic differentiation potential of the c-KIT+ population compared with the c-KIT− population as well as differential clonogenic capacities of the c-KIT+ and c-KIT− populations (Hong et al., 2011). The predisposition of the c-KIT+ subset to differentiation toward a hematopoietic cell fate is encoded by modifications of the activation (H3K4me3) and repression (H3K27me3) of histone methylations at mesodermal lineage-associated genes, such as Brachyury and MIXL1. c-KIT+ cells were detected at approximately 30% in the undifferentiated hESC cultures. Interestingly, the c-KIT gene is activated during human somatic cell reprogramming to induce pluripotency. Our results suggest the possibility of improving hematopoietic differentiation by upregulating c-KIT expression in undifferentiated hPSCs. Numerous studies have also reported that c-KIT could be a tempting target for cancer treatment due to the presence of gain-of-function mutations and the overexpression of c-KIT in various cancer types (Gao et al., 2015; Han et al., 2011; Ohwada et al., 2006). These findings suggest that the balanced expression of c-KIT in undifferentiated hPSCs might be important for sustaining the hematopoietic differentiation potential.
Functional studies have demonstrated that miR-221 and miR-222 are involved in regulating cell proliferation and differentiation by targeting c-KIT. Transfection with miR-221 and miR-222 oligonucleotides causes impaired proliferation and erythropoiesis in CD34+ hematopoietic progenitors derived from cord blood via the down-modulation of c-KIT (Spinello et al., 2009). The transfection of miR-221 and miR-222 into human umbilical vein endothelial cells modulates the angiogenic activity of SCF by reducing c-KIT expression (Poliseno et al., 2006). The inhibition of both miR-221 and miR-222 with synthetic inhibitors in primary cultures of THY1+ mouse spermatogonia impairs the maintenance of an undifferentiated state by increasing the proportion of the c-KIT+ population (Yang et al., 2013). These findings indicate that miR-221 and miR-222 regulate the self-renewal and differentiation of stem cells by modulating c-KIT expression. Our study is the first to show the function of miR-221 and miR-222 and their effect on c-KIT expression during hPSC-derived hematopoietic differentiation. Our data also showed that the dual inhibition of miR-221 and miR-222 seems to more efficiently induce c-KIT expression and augment hematopoietic differentiation. Corresponding to the activation of the c-KIT gene in the pluripotent state, the expression levels of miR-221 and miR-222 were significantly decreased in the pluripotent state compared with those in somatic fibroblasts, suggesting that the direct interaction between miR-221/222 and c-KIT is likely to be part of the molecular machinery that determines the ability to differentiate toward the hematopoietic lineage. However, we cannot rule out the possibility that transient inhibition of miR-221/222 can target to c-KIT as well as other targets, because mammal miRs can down-regulate a greater number of transcripts (Lim et al., 2005).
BMP4 is also known as inducer hematopoietic cells as well as mesodermal lineage cells from hPSCs (Bhatia et al., 1999; Chadwick et al., 2003; Hong et al., 2011; Zhang et al., 2008). Especially, differentiation into hematopoietic linage cells in aorta-gonad-mesonephros (AGM) region is strongly involved and it can modulate c-KIT expression in vitro condition. To examine the effects of SCF and BMP4 in HPCs and HCs differentiation from hPSCs, hPSCs were cultured in various combined medium after BMP4 exposure. As expected, we observed that morphology of hPSCs can readily differentiate into specialized cells with hemogenic endothelium like structure and confirmed strong effect of BMP4 to differentiate into hematopoietic cells, regardless of additive SCF.
Taking our results together, we showed that SCF/c-KIT signaling enhances the hematopoietic differentiation of hPSCs in the presence of BMP4, and this molecular and cellular shift toward the differentiation of hematopoietic lineage cells is the result of the enhancement of c-KIT due to the inhibition of miR-221 and miR-222. Our data also suggest that the frequency of c-KIT+ cells in undifferentiated hPSC cultures can be a useful indicator of hematopoietic potential. Furthermore, the identification of upstream molecules that regulate the expression of c-KIT could help improve the quality and quantity of hematopoietic progenitors derived from hPSCs.
ACKNOWLEDGEMENTS
This work was supported by grants from the Ministry of Science, ICT and Future Planning (2015R1A4A1038666 and 2017M3A9B4029250), the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03031406).
REFERENCES
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bashamboo A, Taylor AH, Samuel K, Panthier JJ, Whetton AD, Forrester LM. The survival of differentiating embryonic stem cells is dependent on the SCF-KIT pathway. J Cell Sci. 2006;119:3039–3046. doi: 10.1242/jcs.03038. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, Dick JE. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med. 1999;189:1139–1148. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carow CE, Hangoc G, Cooper SH, Williams DE, Broxmeyer HE. Mast cell growth factor (c-kit ligand) supports the growth of human multipotential progenitor cells with a high replating potential. Blood. 1991;78:2216–2221. [PubMed] [Google Scholar]
- Chadwick K, Wang L, Li L, Menendez P, Murdoch B, Rouleau A, Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, Liuzzi F, Lulli V, Morsilli O, Santoro S, et al. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. P Nat Acad Sci USA. 2005;102:18081–18086. doi: 10.1073/pnas.0506216102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Lin J, Gao L, Deng A, Lu X, Li Y, Wang L, Yu L. High expression of c-kit mRNA predicts unfavorable outcome in adult patients with t(8;21) acute myeloid leukemia. PloS one. 2015;10:e0124241. doi: 10.1371/journal.pone.0124241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gits CM, van Kuijk PF, Jonkers MB, Boersma AW, van Ijcken WF, Wozniak A, Sciot R, Rutkowski P, Schöffski P, Taguchi T, et al. MiR-17–92 and miR-221/222 cluster members target KIT and ETV1 in human gastrointestinal stromal tumours. British J Cancer. 2013;109:1625–35. doi: 10.1038/bjc.2013.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Graham-Evans B, Broxmeyer HE. Murine embryonic stem cells secrete cytokines/growth modulators that enhance cell survival/anti-apoptosis and stimulate colony formation of murine hematopoietic progenitor cells. Stem Cells. 2006;24:850–856. doi: 10.1634/stemcells.2005-0457. [DOI] [PubMed] [Google Scholar]
- Han CP, Lin WL, Wang PH, Yang SF, Lewis JS, Jr, Chen CK, Ruan A, Kuo JF, Lee MY, Chiang H. Overexpression of c-KIT (CD117) occurs infrequently in squamous cell carcinoma of the uterine cervix. Histopathology. 2011;58:988–990. doi: 10.1111/j.1365-2559.2011.03849.x. [DOI] [PubMed] [Google Scholar]
- Hong SH, Rampalli S, Lee JB, McNicol J, Collins T, Draper JS, Bhatia M. Cell fate potential of human pluripotent stem cells is encoded by histone modifications. Cell Stem Cell. 2011;9:24–36. doi: 10.1016/j.stem.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, Sohn YD, Lee MY, Houge MA, Yoon YS. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circulation Res. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Lim JH, Hong Y, Hong SH, Bang CY, Lee JS, Oh YM, Kim JH. Altered miRNA expression in lung tissues of patients with chronic obstructive pulmonary disease. Mol Cell Toxicol. 2017;13:207–212. [Google Scholar]
- Kimura Y, Ding B, Imai N, Nolan DJ, Butler JM, Rafii S. c-Kit-mediated functional positioning of stem cells to their niches is essential for maintenance and regeneration of adult hematopoiesis. PloS one. 2011;6:e26918. doi: 10.1371/journal.pone.0026918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lee JE, Kang MS, Park MH, Shim SH, Yoon TK, Chung HM, Lee DR. Evaluation of 28 human embryonic stem cell lines for use as unrelated donors in stem cell therapy: implications of HLA and ABO genotypes. Cell Transplantation. 2010;19:1383–1395. doi: 10.3727/096368910X513991. [DOI] [PubMed] [Google Scholar]
- Lis R, Karrasch CC, Poulos MG, Kunar B, Redmond D, Duran JGB, Badwe CR, Schachterle W, Ginsberg M, Xiang J, et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature. 2017;545:439–445. doi: 10.1038/nature22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Glover CH, Tien AH, Humphries RK, Piret JM, Helgason CD. Involvement of tyrosine kinase signaling in maintaining murine embryonic stem cell functionality. Exp Hematol. 2007;35:1293–1302. doi: 10.1016/j.exphem.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Marshall CJ, Sinclair JC, Thrasher AJ, Kinnon C. Bone morphogenetic protein 4 modulates c-Kit expression and differentiation potential in murine embryonic aorta-gonad-mesonephros haematopoiesis in vitro. British J Haematol. 2007;139:321–330. doi: 10.1111/j.1365-2141.2007.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses BS, Evans R, Slone WL, Piktel D, Martinez I, Craig MD, Gibson LF. Bone Marrow Microenvironment Niche Regulates miR-221/222 in Acute Lymphoblastic Leukemia. Molecular Cancer Research: MCR. 2016;14:909–919. doi: 10.1158/1541-7786.MCR-15-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwada M, Wada T, Saga Y, Tsunoda S, Jobo T, Kuramoto H, Konno R, Suzuki M. C-kit overexpression in neuroendocrine small cell carcinoma of the uterine cervix. European J Gynaecological Oncol. 2006;27:53–55. [PubMed] [Google Scholar]
- Perry JM, Harandi OF, Paulson RF. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood. 2007;109:4494–4502. doi: 10.1182/blood-2006-04-016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick M, Azzola L, Mossman A, Stanley EG, Elefanty AG. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells. 2007;25:2206–2214. doi: 10.1634/stemcells.2006-0713. [DOI] [PubMed] [Google Scholar]
- Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- Ramon LA, Braza-Boils A, Gilabert J, Chirivella M, Espana F, Estelles A, Gilabert-Estelles J. microRNAs related to angiogenesis are dysregulated in endometrioid endometrial cancer. Human Reproduction. 2012;27:3036–3045. doi: 10.1093/humrep/des292. [DOI] [PubMed] [Google Scholar]
- Rojas-Sutterlin S, Lecuyer E, Hoang T. Kit and Scl regulation of hematopoietic stem cells. Curr Opin Hematol. 2014;21:256–264. doi: 10.1097/MOH.0000000000000052. [DOI] [PubMed] [Google Scholar]
- Rommer A, Steinleitner K, Hackl H, Schneckenleithner C, Engelmann M, Scheideler M, Vlatkovic I, Kralovics R, Cerny-Reiterer S, Valent P, et al. Overexpression of primary microRNA 221/222 in acute myeloid leukemia. BMC Cancer. 2013;13:364. doi: 10.1186/1471-2407-13-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinello I, Quaranta MT, Pasquini L, Pelosi E, Petrucci E, Pagliuca A, Castelli G, Mariani G, Diverio D, Foa R, et al. PLZF-mediated control on c-kit expression in CD34(+) cells and early erythropoiesis. Oncogene. 2009;28:2276–2288. doi: 10.1038/onc.2009.87. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Werbowetski-Ogilvie TE, Bosse M, Stewart M, Schnerch A, Ramos-Mejia V, Rouleau A, Wynder T, Smith MJ, Dingwall S, Carter T, et al. Characterization of human embryonic stem cells with features of neoplastic progression. Nat Biotechnol. 2009;27:91–97. doi: 10.1038/nbt.1516. [DOI] [PubMed] [Google Scholar]
- Yang QE, Racicot KE, Kaucher AV, Oatley MJ, Oatley JM. MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells. Development. 2013;140:280–290. doi: 10.1242/dev.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, Yong J, Jiang W, Sun X, Du L, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]


